Published online Mar 26, 2022. doi: 10.12998/wjcc.v10.i9.2908
Peer-review started: October 5, 2021
First decision: December 2, 2021
Revised: December 15, 2021
Accepted: February 20, 2022
Article in press: February 20, 2022
Published online: March 26, 2022
Processing time: 168 Days and 11.8 Hours
Laparoscopic hepatectomy has recently become popular because it results in less bleeding than open hepatectomy. However, CO2 embolism occurs more frequently. Most CO2 embolisms during laparoscopic surgery are self-resolving and non-symptomatic; however, severe CO2 embolism may cause hypotension, cyanosis, arrhythmia, and cardiovascular collapse. In particular, paradoxical CO2 embolisms are highly likely to cause neurological deficits. We report a case of paradoxical CO2 embolism found on transesophageal echocardiography (TEE) during laparoscopic hepatectomy, although the patient had no intracardiac shunt.
A 71-year-old man was admitted for laparoscopic left hemihepatectomy. During left hepatic vein ligation, the inferior vena cava was accidentally torn. We observed a sudden drop in oxygen saturation to 85%, decrease in systolic blood pressure (SBP) below 90 mmHg, and reduction in end-tidal CO2 to 24 mmHg. A “mill-wheel” murmur was auscultated over the precordium. The fraction of inspired oxygen was increased to 100% with 5 cmH2O of positive end-expiratory pressure (PEEP) and hyperventilation was maintained. Norepinephrine infusion was increased to maintain SBP above 90 mmHg. A TEE probe was inserted, revealing gas bubbles in the right side of the heart, left atrium, left ventricle, and ascending aorta. The surgeon reduced the pneumoperitoneum pressure from 17 to 14 mmHg and repaired the damaged vessel laparoscopically. Thereafter, the patient’s hemodynamic status stabilized. The patient was transferred to the intensive care unit, recovering well without complications.
TEE monitoring is important to quickly determine the presence and extent of embolism in patients undergoing laparoscopic hepatectomy.
Core Tip: CO2 embolism is a rare but potentially life-threatening complication of laparoscopic surgery. In particular, paradoxical CO2 embolisms are highly associated with postoperative neurologic deficits. We report a case of paradoxical CO2 embolism identified using transesophageal echocardiography (TEE) during laparoscopic hepatectomy even though the patient did not have an intracardiac shunt. Generally, EtCO2 monitoring is a sensitive method for detecting gas embolism, but it is difficult to identify the extent or detailed status, such as paradoxical embolism. Intraoperative TEE can help quickly identify the presence and extent of CO2 embolism, which will help provide appropriate treatment and predicting postoperative complications.
- Citation: Jeon S, Hong JM, Lee HJ, Kim Y, Kang H, Hwang BY, Lee D, Jung YH. Paradoxical carbon dioxide embolism during laparoscopic hepatectomy without intracardiac shunt: A case report. World J Clin Cases 2022; 10(9): 2908-2915
- URL: https://www.wjgnet.com/2307-8960/full/v10/i9/2908.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i9.2908
Laparoscopic surgery is an alternative to conventional laparotomy and is widely used because of its advantages including less postoperative pain, earlier recovery after surgery, and reduced postoperative complications such as infection or incisional hernia[1]. In particular, laparoscopic hepatectomy has recently become popular because it leads to less bleeding than open hepatectomy. However, laparoscopic hepatectomy requires low central venous pressure, high pneumoperitoneum pressure, and Pringle maneuvers, all of which increase the likelihood of CO2 embolism[2]. Therefore, CO2 embolism occurs more frequently in laparoscopic hepatectomy than in general laparoscopic surgery. Most CO2 embolisms during laparoscopic surgery are microembolisms and may be self-resolving without any symptoms or clinical signs due to the high solubility of CO2 in blood. However, severe CO2 embolism may cause hypotension, cyanosis, arrhythmia, cardiovascular collapse, neurologic deficits, or even arrest[3]. In particular, paradoxical CO2 embolisms are highly likely to cause neurological deficits[4].
We describe a case of paradoxical CO2 embolism found using transesophageal echocardiography (TEE) during laparoscopic hepatectomy even though the patient did not have an intracardiac shunt.
A 71-year-old man (height, 169.4 cm; weight, 80.5 kg) presented with sudden hypoxia, hypotension, and decreased end-tidal carbon dioxide (EtCO2) during laparoscopic left hemi-hepatectomy.
The patient was diagnosed with hepatocellular carcinoma and was admitted to our hospital for laparoscopic left hemi-hepatectomy. Preoperative ECG, chest radiography, and laboratory findings were within the normal range except renal function test results [blood urea nitrogen (BUN): 20.4 mg/dL, creatinine: 1.44 mg/dL, and glomerular filtration rate: (GFR) 48.2 mL/min/1.73 m2]. Preoperative transthoracic echocardiography (TTE) revealed degenerative moderate/severe aortic stenosis [aortic valve peak velocity: 3.2 m/s, peak pressure gradient (PG), 41 mmHg; mean PG, 20 mmHg]. For further evaluation, TEE was performed, and the aortic valve area on three dimensional multiplanar reconstruction planimetry was 1.13 cm2. The biventricular and other valves were normal or had mild degenerative changes.
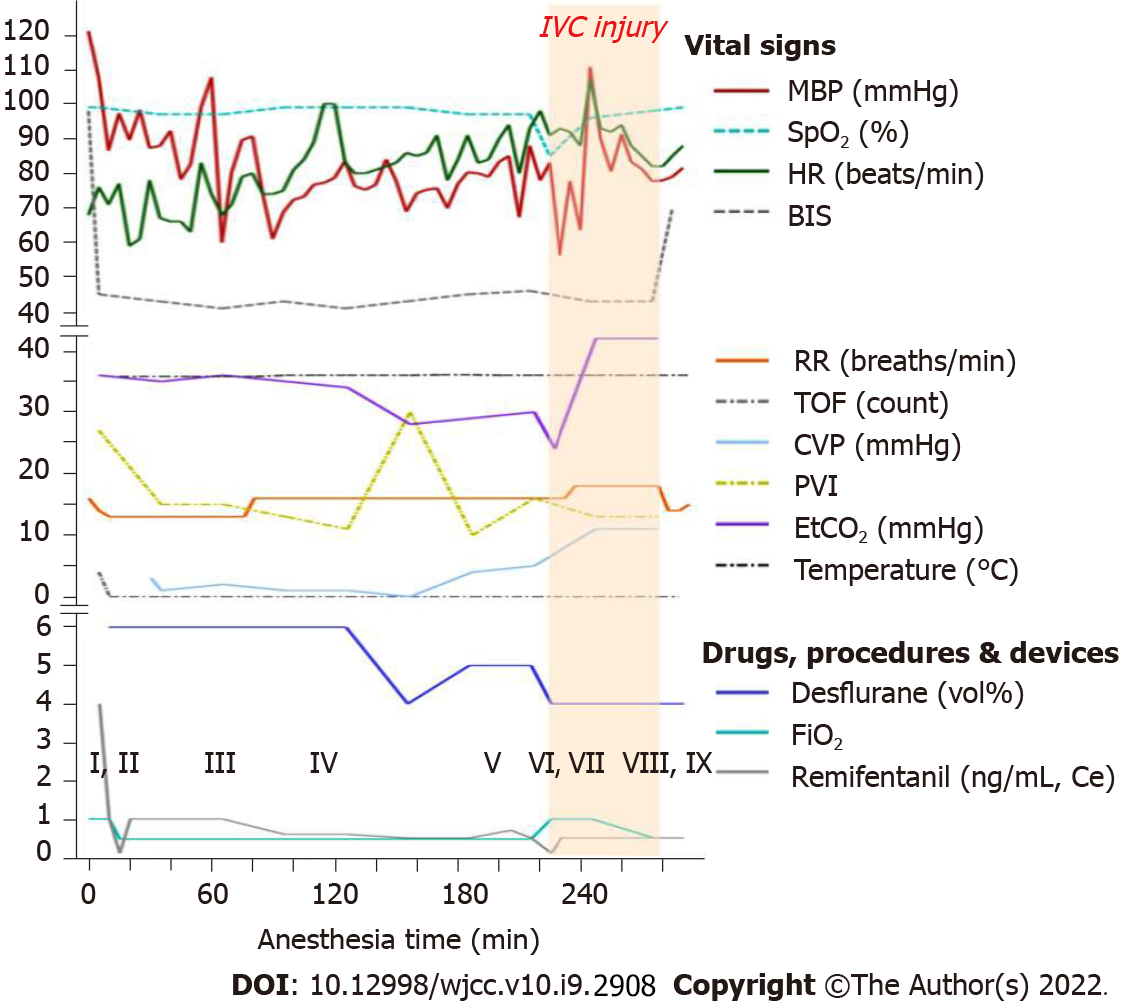
Glycopyrrolate 0.2 mg was administered as premedication. In the operating room, standard monitoring [ECG, pulse oximetry (SpO2), noninvasive blood pressure, end-tidal CO2 (EtCO2), and esophageal stethoscope temperature], bispectral index (BIS), train of four, pleth variability index (PVI), and urine output monitoring were performed, and the patient’s initial (pre-induction) heart rate (HR), SpO2, systolic blood pressure (SBP), diastolic blood pressure (DBP), EtCO2, and respiratory rate (RR) were 68 beats/min, 99%, 186 mmHg, 89 mmHg, 36 mmHg, and 16 breaths/min, respectively. The vital signs, administered drugs, and arterial blood gas analysis (ABGA) values during surgery are shown in Figure 1. The operation was performed under general anesthesia. Before induction, arterial catheterization of the left radial artery was performed under local anesthesia for real-time blood pressure monitoring. Anesthesia was induced using propofol (70 mg), remifentanil [4.0 ng/mL; effect-site concentration (Ce), target-controlled infusion (TCI) based on the Minto Model], and atracurium (25 mg). After anesthesia induction, a central line was cautiously placed via the right internal jugular vein. To prevent hypotension, phenylephrine infusion was administered at 0.5 µg/kg/min. Anesthesia was maintained with 4-6 vol% desflurane, and continuous infusion of remifentanil (0.1-4.0 ng/mL). Atracurium (4 µg/kg/min) was infused to induce muscle relaxation during surgery. Volume-controlled ventilation with the following parameters was adopted: tidal volume, 425 mL; respiratory rate, 12-14 breaths/min, inspiratory-to-expiratory time (I:E) ratio 1:2; and FiO2 0.5, without positive end-expiratory pressure (PEEP). Intraoperative fluid was restricted to lower central venous pressure (CVP) during surgery, and intermittent boluses of fluid were administered to maintain urine output (0.5 mL/kg/h). When the patient was draped, hydrocortisone (100 mg) was administered via slow intravenous injection. After three trocars were inserted, the patient was placed in the reverse Trendelenburg position with a left tilt, and CO2 was insufflated to a pressure of 17 mmHg. During the surgical procedure, hypotension (SBP/DBP, 90/45 mmHg) was observed despite continuous phenylephrine infusion; therefore, norepinephrine infusion was administered at 0.1-0.2 µg/kg/min and phenylephrine was tapered. After administration of norepinephrine, the patient’s vital signs remained stable. During ligation of the left hepatic vein, the inferior vena cava (IVC) was accidentally torn. The patient’s SpO2 suddenly dropped from 97% to 85%. His blood pressure decreased to 89/40 mmHg (SBP/DBP). The EtCO2 dropped from 30 to 24 mmHg. A “mill-wheel” murmur was auscultated over the precordium.
He had a history of hypertension, diabetes, and stage 3a chronic kidney disease. He was taking aspirin, lercanidipine, gemigliptin, insulin glargine, kalimate (polystyrene sulfonate calcium), and renamezin (spherical adsorptive carbon).
There was no remarkable personal and family history.
The “mill-wheel” murmur was auscultated over precordium at the same time as sudden hypoxia, hypotension, and decrease in EtCO2 (from 30 mmHg to 24 mmHg, Figure 1).
When paradoxical CO2 embolism occurred, ABGA was performed and revealed a pH of 7.112, PCO2 of 78.8mmHg, and PO2 of 73.3 mmHg.
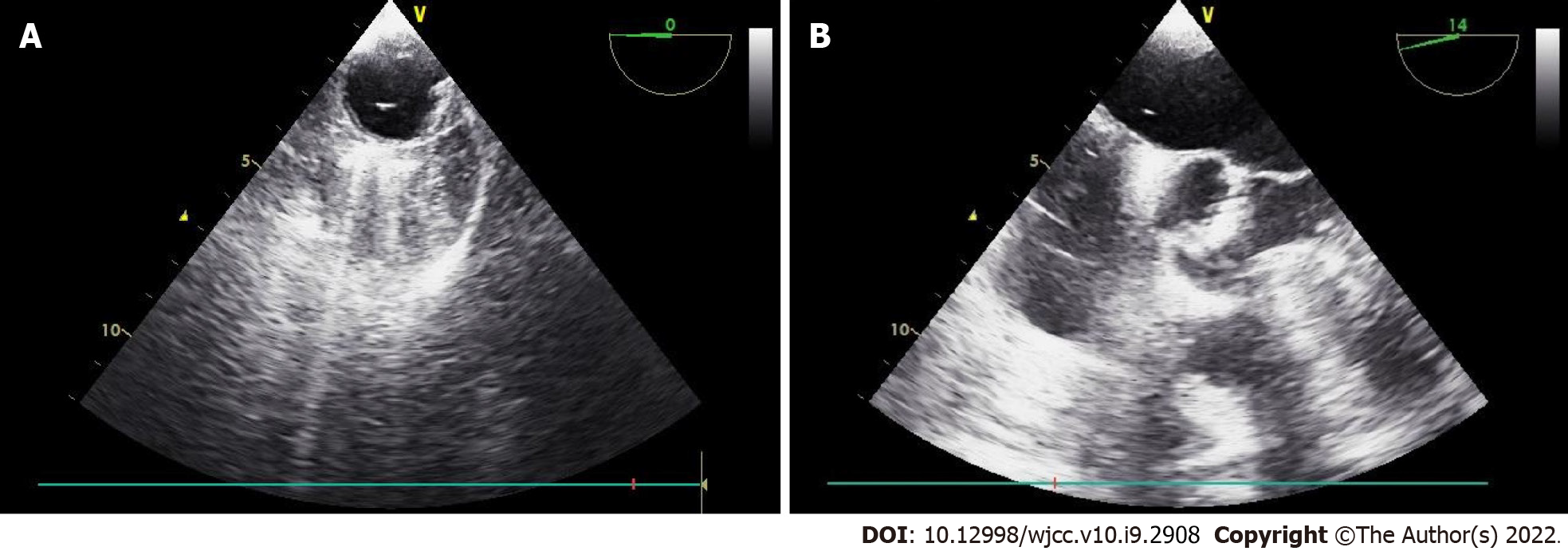
Given the clinical signs, we suspected that CO2 gas had entered the injured vessel. However, since the patient also had moderate aortic valve stenosis, TEE was immediately performed for accurate differentiation and diagnosis. TEE revealed that gas bubbles were scattered in the right side of the heart, and a few were detected in the left atrium, left ventricle, and ascending aorta (Figure 2). Intracardiac shunts, such as a patent foramen ovale (PFO) or ventricular septal defect (VSD), were not observed.
The patient was diagnosed with paradoxical CO2 embolism developed during laparoscopic hepatectomy.
Fluid resuscitation for blood loss due to the torn IVC was initiated. To correct the sudden onset hypoxia, the infused oxygen concentration was increased immediately from 50% to 100% with 5 cmH2O of PEEP, and hyperventilation was maintained to wash out CO2. To correct the hypotension, the infusion of norepinephrine was increased to maintain systolic blood pressure above 90 mmHg. Immediately after confirming paradoxical CO2 embolism using TEE, we informed the surgeon of the situation, and the surgeon then reduced the insufflation pressure from 17 mmHg to 14 mmHg. After the torn IVC was repaired laparoscopically and the bleeding was controlled, the size of the gas bubble on TEE reduced, and the patient’s hemodynamic state stabilized. No more air was observed on the TEE performed 30 min later (Figure 3). The norepinephrine infusion was gradually tapered. The SpO2 increased to 99%, and FiO2 reduced to 0.5. At the end of the operation, the ABGA revealed a pH of 7.282, PCO2 of 50.5 mmHg, and PO2 of 111.1 mmHg. The total anesthesia time and operation time were 290 min and 265 min, respectively. The estimated blood loss was 1000 mL, and the total intraoperative urine output was 560 mL. Crystalloid (1700 mL), 5% albumin (250 mL), and packed red blood cells (1 unit) were administered during the operation.
The patient was transferred to the surgical intensive care unit for close monitoring and ventilator care under sedation. Bedside TTE confirmed that definite CO2 bubbles had disappeared. Brain computed tomography was performed on postoperative day (POD) 1 to exclude embolic stroke, and it revealed no abnormalities. Ventilator weaning and extubation were performed on POD2 and the patient was transferred from the intensive care unit to the general ward on POD3 without any cardiovascular or neurologic complications. The postoperative laboratory examination results on POD3 were within normal range, except for ALT, 59 U/L; BUN/Cr, 46.2/1.31 mg/dL; GFR, 53.8 mL/min/1.73 m2; PT, 14.1 s; and INR, 1.25. This study was approved by the Institutional Review Board of Pusan National University Hospital, Republic of Korea (ID: 2105-009-102).
Gas embolism during laparoscopic surgery is reported as a rare complication, with an overall incidence of 0.014%-0.6%[3,5]. However, in cases of clinically significant CO2 embolism, mortality is reported to be 28%[6]. CO2 embolism is mainly caused by incorrect insertion of a Veress needle, and surgical sites with many venous channels or the generation of pressure gradients for gas entry into circulation also increase the risk of CO2 embolism during laparoscopic surgery[7]. Particularly, the risk may increase in patients with primary biliary cirrhosis by upregulated angiogenesis or with unanticipated anatomical variations such as patent paraumbilical veins[8,9]. The incidence of gas embolism is further increased in laparoscopic hepatectomy (1.2%-4.6%)[10]. Laparoscopic hepatectomy, which has recently become a popular surgical method, is performed as it is associated with less bleeding, less postoperative pain, and faster recovery. Low CVP, high-pressure pneumoperitoneum, and the Pringle maneuver are required for successful laparoscopic hepatectomy. However, these methods are believed to be associated with an increased risk of CO2 embolism. High-pressure pneumoperitoneum with low CVP creates a pressure gradient, which increases the likelihood of CO2 embolism. The Pringle maneuver is a method of controlling bleeding by inhibiting blood flow through the portal vein and hepatic artery. When this maneuver is implemented, the intrahepatic pressure becomes equal to the CVP, which may contribute to the increased risk of CO2 embolism[2,10].
All these methods were used in this case. In particular, a higher pneumoperitoneum pressure (17 mmHg) was used. Typically, pressures of 12 mmHg or less are used to reduce the risk of gas embolism, and a pressure of 12-15 mmHg is used for bleeding control in some institutions[10]. Chiu et al[11] reported that the incidence of CO2 embolism was higher in a high insufflation pressure group than in a low pressure group. According to animal studies, intraoperative bleeding is lower when a high pneumoperitoneum pressure is used; however the risk of CO2 embolism increases[12,13]. Although the biggest cause of embolism in this case was the rapid inflow of CO2 gas into the injured vessel, it is presumed that all these factors may have increased the effect.
Clinical manifestations of CO2 embolism include hypotension, tachycardia, arrhythmia, dyspnea, cyanosis, and cardiovascular collapse[3]. CO2 embolism can be caused by unintended placement of a Veress needle or trocar into a blood vessel. Additionally, CO2 entering injured blood vessels or surgical sites during surgery may lead to embolism. The CO2 bubbles can move to the vena cava and right ventricle (RV), which may limit venous return and cause RV outflow obstruction[14]. The “mill-wheel” murmur, which is described as loud, harsh, splashing, or machine-like sound, can be auscultated when a large amount of gas is entrapped. Moreover, a sudden decrease in EtCO2 is also a clinical sign of gas embolism, which is caused by an obstruction of the pulmonary circulation[15]. In this case, hypoxia, hypotension, decreased EtCO2, and mill-wheel murmur occurred after IVC injury; therefore, we suspected CO2 embolism and confirmed it using TEE. Furthermore, we confirmed that paradoxical CO2 embolism had occurred when TEE was used.
Paradoxical air embolism is generally known to occur in the presence of an intracardiac shunt, such as patent foramen ovale (PFO), ventricular septal defect (VSD), and patent ductus arteriosus[16]. However, in this case, no intracardiac shunt was found on preoperative or intraoperative echocardiographic examinations. Similar to our case, several reports have described paradoxical air embolism that occurred during laparoscopic surgery even though the patients did not have an intracardiac shunt[4,17-19]. In these reports, the authors described three possible mechanisms. First, it may be caused by temporarily opened PFO; second, it may be caused by pulmonary vascular dilation and arteriovenous communication in patients with liver cirrhosis; and third, CO2 embolism may be caused by the overflow of venous gas bubbles into the arterial circulation through the lung[4]. In our patient, no VSD or PFO was observed during the preoperative and intraoperative TEE. Furthermore, the patient did not have liver cirrhosis. The existence of communication of pulmonary arterioles and venous cannot be completely excluded in this patient. For confirmation, the agitated saline test may be helpful. If the left side gas bubble appears after 3 to 8 heartbeats, the existence of the abnormal communication of pulmonary arterioles and venous may be suspected[20]. However, considering the improvement of symptoms after repairing damaged blood vessels, we presumed that the overflow of a large amount of venous gas bubbles entering the damaged blood vessel was the main mechanism.
Although our patient fortunately did not exhibit any neurological deficit after surgery, previous reports have indicated that paradoxical gas embolism may cause neurological deficits such as cerebral infarction[4]. Considering this point, it is important to quickly identify paradoxical CO2 embolisms and to pay attention to complications such as neurological deficits after surgery.
We confirmed paradoxical CO2 embolism using immediate TEE. TEE, the most sensitive diagnostic tool, can detect a much smaller amount of gas, as little as 0.2 mL/kg, than can be detected using EtCO2[15,16]. CO2 embolism is usually diagnosed with clinical symptoms alone when TEE cannot be used. Therefore, most reported cases of CO2 embolism are accompanied by serious symptoms, such as hemodynamic deterioration. However, although not reported, it is assumed that many cases of CO2 embolism occurred without symptoms and then disappeared without being recognized by anesthesiologists and surgeons. Derouin reported that the incidence of CO2 embolism was 69% during laparoscopic cholecystectomy when TEE was pre-inserted[21]. Furthermore, Kim reported that venous air embolism was observed in all patients during laparoscopic hysterectomy, and 37.5% of these emboli were present in significant amounts[22]. However, neither study showed any hemodynamic instability. Therefore, it is not sufficient to diagnose CO2 embolism based on symptoms alone. Moreover, it is difficult to identify paradoxical CO2 embolism, as in this case, only based on clinical symptoms without the use of TEE. In particular, cases in which the absence of an intracardiac shunt is confirmed by preoperative examination are more likely to be excluded. TEE can also diagnose CO2 embolism quickly and accurately, so it can differentiate it from other causes of hemodynamic deterioration. The patient in this case had moderate-to-severe aortic stenosis. Because symptoms such as hypotension, hypoxia, and decreased EtCO2 may also occur due to aortic stenosis, it is difficult to make an accurate diagnosis based on clinical symptoms alone. Therefore, if CO2 embolism is suspected or if it is necessary to differentiate it from other causes, we recommend quick identification using TEE. Kim et al[22] described that although most venous air embolism is asymptomatic, caution is needed because fatal air embolism can occur in patients with cardiopulmonary disease or intracardiac shunt. Therefore, pre-insertion of TEE may be helpful for these patients.
When CO2 embolism is suspected, the anesthesiologist should promptly inform the surgeon, and the CO2 insufflation should be stopped to prevent additional gas entrainment. Hyperventilation with 100% oxygen was used to eliminate CO2. Further, PEEP can be applied to prevent additional gas entrapment. A Trendelenburg or left lateral decubitus position is helpful in preventing the progression of CO2 embolism[3]. CO2 aspiration through the central venous catheter may also be considered. In this case, the surgeon did not switch to open surgery and only reduced the peritoneum pressure and sutured the damaged vessel. This is because it was judged that if the pneumoperitoneum was stopped and there was a conversion to open surgery, the bleeding could become more severe and the vital signs would worsen during the conversion. Although the Trendelenburg position is helpful, the position was not changed to ensure optimal viewing of the surgical field during laparoscopic repair in this case. Therefore, communication with the surgeon is essential for such a complex surgery, and a dedicated and well-trained team is important.
Herein, we report a case of paradoxical CO2 embolism during laparoscopic hepatectomy. Anesthesiologists should be aware that CO2 embolism can occur during laparoscopic surgery, particularly during laparoscopic hepatectomy. Additionally, paradoxical embolism should be considered even in the absence of an intracardiac shunt. Therefore, TEE is important to quickly confirm the presence and extent of CO2 embolism and to prevent complications.
Laparoscopic surgery, especially laparoscopic hepatectomy, inevitably carries the risk of CO2 embolism, including paradoxical CO2 embolism. Paradoxical CO2 embolism can occur even without an intracardiac shunt, and it is difficult to diagnose based on symptoms alone. Intraoperative TEE can help quickly identify the presence and extent of CO2 embolism, which will help with appropriate treatment and prediction of postoperative complications.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anesthesiology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Costa T, Li BB S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Buia A, Stockhausen F, Hanisch E. Laparoscopic surgery: A qualified systematic review. World J Methodol. 2015;5:238-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 143] [Article Influence: 14.3] [Reference Citation Analysis (4)] |
| 2. | Takechi K, Ito M, Peng Y, Daizen W, Shimizu I. Laparoscopic image of carbon dioxide embolism during laparoscopic hepatectomy: a case report. JA Clin Rep. 2020;6:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Park EY, Kwon JY, Kim KJ. Carbon dioxide embolism during laparoscopic surgery. Yonsei Med J. 2012;53:459-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Kawahara T, Hagiwara M, Takahashi H, Tanaka M, Imai K, Sawada J, Kunisawa T, Furukawa H. Cerebral Infarction by Paradoxical Gas Embolism During Laparoscopic Liver Resection with Injury of the Hepatic Vessels in a Patient without a Right-to-Left Systemic Shunt. Am J Case Rep. 2017;18:687-691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Burcharth J, Burgdorf S, Lolle I, Rosenberg J. Successful resuscitation after carbon dioxide embolism during laparoscopy. Surg Laparosc Endosc Percutan Tech. 2012;22:e164-e167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Cottin V, Delafosse B, Viale JP. Gas embolism during laparoscopy: a report of seven cases in patients with previous abdominal surgical history. Surg Endosc. 1996;10:166-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | de Jong KIF, de Leeuw PW. Venous carbon dioxide embolism during laparoscopic cholecystectomy a literature review. Eur J Intern Med. 2019;60:9-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Cadis AS, Velasquez CD, Brauer M, Hoak B. Intraoperative management of a carbon dioxide embolus in the setting of laparoscopic cholecystectomy for a patient with primary biliary cirrhosis: A case report. Int J Surg Case Rep. 2014;5:833-835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Mattei P, Tyler DC. Carbon dioxide embolism during laparoscopic cholecystectomy due to a patent paraumbilical vein. J Pediatr Surg. 2007;42:570-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Otsuka Y, Katagiri T, Ishii J, Maeda T, Kubota Y, Tamura A, Tsuchiya M, Kaneko H. Gas embolism in laparoscopic hepatectomy: what is the optimal pneumoperitoneal pressure for laparoscopic major hepatectomy? J Hepatobiliary Pancreat Sci. 2013;20:137-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Chiu KM, Lin TY, Wang MJ, Chu SH. Reduction of carbon dioxide embolism for endoscopic saphenous vein harvesting. Ann Thorac Surg. 2006;81:1697-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Kobayashi S, Honda G, Kurata M, Tadano S, Sakamoto K, Okuda Y, Abe K. An Experimental Study on the Relationship Among Airway Pressure, Pneumoperitoneum Pressure, and Central Venous Pressure in Pure Laparoscopic Hepatectomy. Ann Surg. 2016;263:1159-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Eiriksson K, Fors D, Rubertsson S, Arvidsson D. High intra-abdominal pressure during experimental laparoscopic liver resection reduces bleeding but increases the risk of gas embolism. Br J Surg. 2011;98:845-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Smith HJ. Carbon dioxide embolism during pneumoperitoneum for laparoscopic surgery: a case report. AANA J. 2011;79:371-373. [PubMed] |
| 15. | O'Sullivan DC, Micali S, Averch TD, Buffer S, Reyerson T, Schulam P, Kavoussi LR. Factors involved in gas embolism after laparoscopic injury to inferior vena cava. J Endourol. 1998;12:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Muth CM, Shank ES. Gas embolism. N Engl J Med. 2000;342:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 605] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 17. | Hou W, Zhong J, Pan B, Huang J, Wang B, Sun Z, Miao C. Paradoxical carbon dioxide embolism during laparoscopic surgery without intracardiac right-to-left shunt: two case reports and a brief review of the literature. J Int Med Res. 2020;48:300060520933816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Hieber C, Ihra G, Nachbar S, Aloy A, Kashanipour A, Coraim F. Near-fatal paradoxical gas embolism during gynecological laparoscopy. Acta Obstet Gynecol Scand. 2000;79:898-899. [PubMed] |
| 19. | Huang YY, Wu HL, Tsou MY, Zong HJ, Guo WY, Chan KH, Ting CK. Paradoxical carbon dioxide embolism during pneumoperitoneum in laparoscopic surgery for a huge renal angiomyolipoma. J Chin Med Assoc. 2008;71:214-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Saboo SS, Chamarthy M, Bhalla S, Park H, Sutphin P, Kay F, Battaile J, Kalva SP. Pulmonary arteriovenous malformations: diagnosis. Cardiovasc Diagn Ther. 2018;8:325-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 21. | Derouin M, Couture P, Boudreault D, Girard D, Gravel D. Detection of gas embolism by transesophageal echocardiography during laparoscopic cholecystectomy. Anesth Analg. 1996;82:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Kim CS, Kim JY, Kwon JY, Choi SH, Na S, An J, Kim KJ. Venous air embolism during total laparoscopic hysterectomy: comparison to total abdominal hysterectomy. Anesthesiology. 2009;111:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |