Published online Mar 26, 2022. doi: 10.12998/wjcc.v10.i9.2864
Peer-review started: September 10, 2021
First decision: October 25, 2021
Revised: October 29, 2021
Accepted: February 12, 2022
Article in press: February 12, 2022
Published online: March 26, 2022
Processing time: 193 Days and 0.3 Hours
Neuraminidase inhibitor-associated acute hemorrhagic colitis is rare. We report a case of ischemic enterocolitis that was likely caused by laninamivir.
A 54-year-old female patient with influenza type A was administered 40 mg of laninamivir via inhalation once. On the same day, the patient experienced bloody stools and lower abdominal pain. A contrast-enhanced abdominal computed tomography showed edema-like changes from the descending colon to the sigmoid colon, which suggested ischemic enterocolitis.
We treated a patient with ischemic enterocolitis caused by laninamivir, a rare but similar symptom following the administration of oseltamivir.
Core Tip: Although a clear mechanism underlying oseltamivir-associated hemorrhagic enteritis/ischemic enterocolitis has not been elucidated, the simultaneous occurrence of the following two factors has been considered responsible for the condition: (1) Local angiospasm and vasculitis due to the effect of an allergic reaction to oseltamivir; and (2) Hypoperfusion in the intestinal tract due to the effect of dehydration caused by influenza. Laninamivir is considered to demonstrate the same mechanism as oseltamivir.
- Citation: Suzuki C, Kenzaka T. Laninamivir-induced ischemic enterocolitis: A case report. World J Clin Cases 2022; 10(9): 2864-2870
- URL: https://www.wjgnet.com/2307-8960/full/v10/i9/2864.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i9.2864
Influenza is an infectious disease with a repetitive winter cycle. According to a report from the National Institute of Infectious Diseases, Japan, of a total population of 126 million in the country, an estimated 12 million individuals (population ratio, 9.5%) were affected by influenza during the 2018–2019 season, and 7.28 million individuals were affected during the 2019–2020 season (population ratio: 5.7%)[1]. In 2018, 3325 deaths were reportedly due to influenza[1]. In a meta-analysis of placebo-controlled clinical studies of oseltamivir in all adults, the oseltamivir group was observed to have a shortened duration of seasonal influenza and a reduced risk of hospitalization[2]. Additionally, a decrease in the influenza-associated mortality rate has been reported with the administration of neuraminidase inhibitors as the first-line of treatment[3].
Neuraminidase inhibitors approved in Japan include oral oseltamivir, inhaled zanamivir and laninamivir, and intravenous peramivir. Adverse reactions to neuraminidase inhibitors are usually mild; however, they can cause bronchospasm in patients with asthma or chronic obstructive pulmonary disease[4] and other relatively common adverse reactions such as nausea/vomiting and diarrhea. Similarly, rare occurrences of acute hemorrhagic colitis (hemorrhagic enterocolitis/ischemic enterocolitis) associated with neuraminidase inhibitors have been reported[5].
Here, we report the case of a patient with ischemic enterocolitis that was considered to have been caused by laninamivir.
Lower abdominal pain and bloody stools.
During winter, a 54-year-old female patient with no significant medical history presented with general malaise and a fever of 39.2°C. The next day (the second day of symptom onset), the patient visited a nearby medical institution.
Her blood pressure was 115/60 mmHg, and her pulse rate was regular with 77 beats/min.
At the same institution, the patient was diagnosed with influenza type A using a rapid antigen kit (Quick Navi-Flu2 Influenza Diagnostic Kit, Otsuka Pharmaceutical Co., Ltd. Tokushima, Japan), and she was administered a dose of 40 mg laninamivir via inhalation. Approximately three hours after inhalation on the same day, the patient reportedly experienced bloody stools and lower abdominal pain while sleeping at night. On the third day of symptom onset, the patient visited our institution and was hospitalized due to persistent intermittent lower abdominal pain every 20 min and bloody stools at a frequency of ten times a day. No oral medication, including any anti-platelets/anticoagulants, was prescribed.
Nothing in particular.
Nothing in particular.
Upon clinical examination, the patient appeared lucid. Her blood pressure was 125/67 mmHg, pulse rate was regular with 67 beats/min, body temperature was 36.7°C, respiratory rate was 16 breaths/min, and SpO2 was 97% at room air. No pallor was observed on the palpebral conjunctiva. Heart sounds were regular, and there were no cardiac murmurs. Breath sounds were clear. The abdomen was flat and soft, with tenderness on the left flank. There was no rebound tenderness or muscle guarding. Bowel peristaltic sounds did not increase or decrease, and no palpable mass or hemorrhage was observed on rectal examination. There was no edema in the extremities, and no signs of cyanosis were observed. Her mouth was dry, and the capillary refilling time was four seconds, which suggested dehydration.
Blood test results revealed the following: white blood cell count, 7810/μL; hemoglobin, 12.9 g/dL; platelet count, 20.7 × 104/μL; L-dimer, 1.0 μg/mL; blood urea nitrogen, 17.7 mg/dL; creatinine, 0.47 mg/dL; creatinine kinase, 75 U/L; aspartate aminotransferase, 17 U/L; alanine aminotransferase, 13 U/L; lactate dehydrogenase, 155 U/L; alkaline phosphatase, 210 U/L; C-reactive protein, 3.08 mg/dL; and glycated hemoglobin A1c, 5.9%. She had no abnormalities, such as diabetes mellitus or liver dysfunction, which could manifest in ischemic episodes. Stool culture tests were performed for normal intestinal flora alone, and Salmonella growth was not observed. Polymerase chain reaction was not performed to detect bacteria in stools, and the stool was negative for Clostridium difficile toxin A and B.
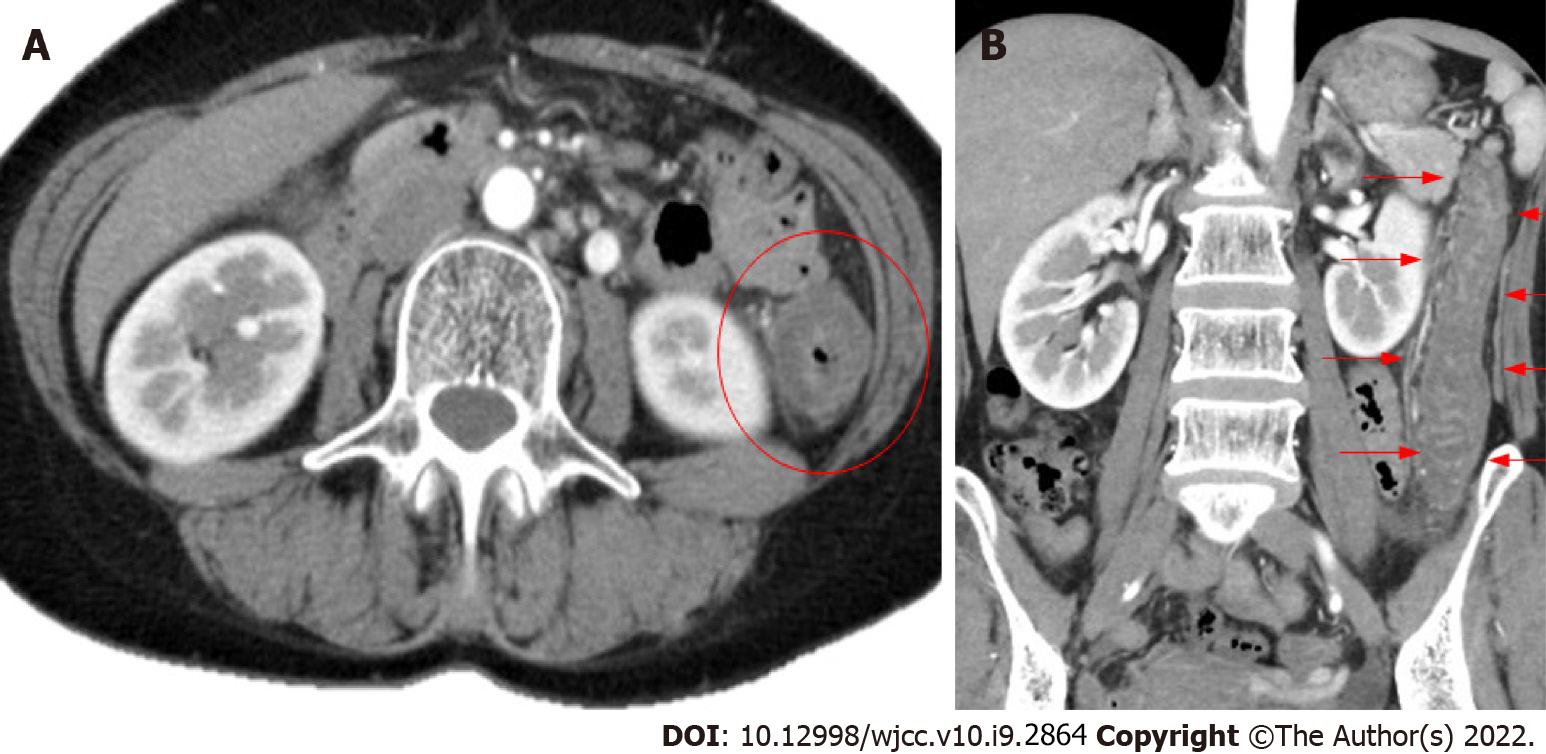
Abdominal contrast-enhanced computed tomography showed edema-like changes that extended from the descending colon to the sigmoid colon (Figure 1).
Based on the patient’s clinical symptoms and imaging, her condition was diagnosed as ischemic enterocolitis.
Fluid replacement was administered for bowel rest, and food and drink intake was withheld. For influenza type A, laninamivir had been administered via inhalation two days ago, and the patient was kept in a private room and followed up with symptomatic treatment. On the second day of hospitalization, fresh blood in the stool disappeared, and abdominal pain improved. On the third day of hospitalization, the patient started eating again, which did not worsen the symptoms. The patient was discharged on the fifth day of hospitalization.
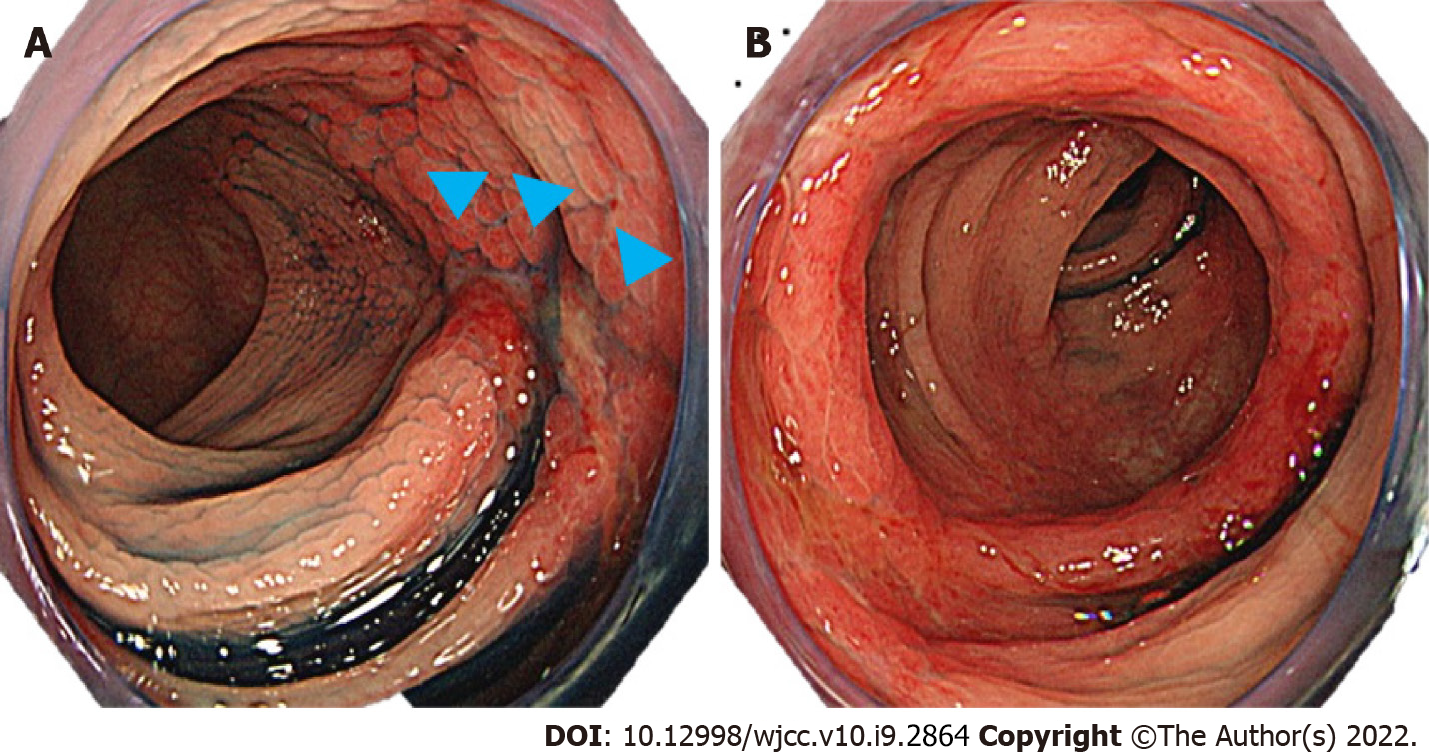
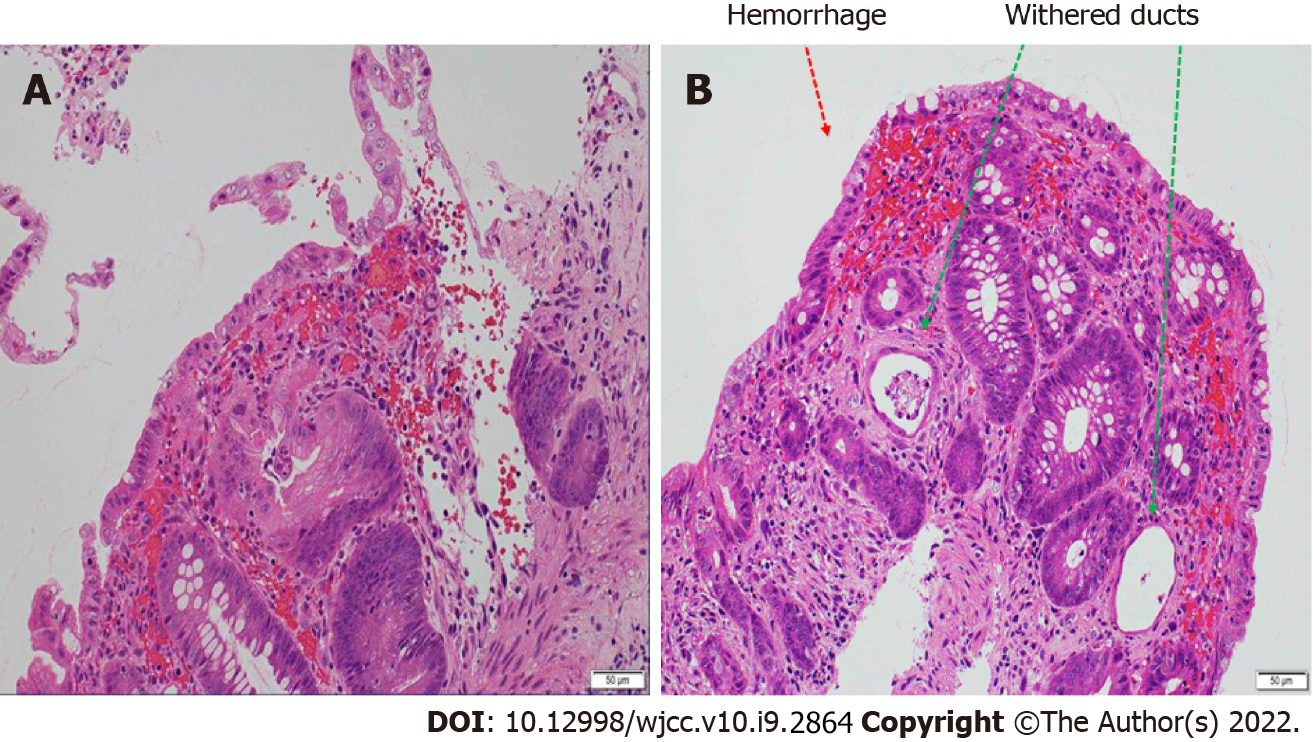
Lower gastrointestinal endoscopy performed following hospital discharge (8 d after admission) showed a longitudinal ulcer extending from the descending colon to the sigmoid colon and circumferential edema-like changes (Figure 2). Histopathological examination of the same region (Figure 3) revealed stromal edema, hemorrhage, and inflammatory cell infiltration, with a prominent tendency for detachment of the superficial epithelium. These findings were consistent with those observed in ischemic enterocolitis. She had a score of seven points on the Naranjo algorithm for the adverse drug reaction causality assessment scale[6]. Therefore, the patient was diagnosed with laninamivir-induced ischemic enterocolitis. No recurrence of ischemic enterocolitis has been observed in the last five years since the patient presented to us.
We present a case of ischemic enterocolitis that was considered to be caused by laninamivir. Although there have been a few reports of acute hemorrhagic colitis caused by neuraminidase inhibitors, to the best of our knowledge, this is the first reported case of acute hemorrhagic colitis caused by laninamivir.
In total, 13 reports of acute hemorrhagic colitis associated with neuraminidase inhibitors, which were published between 2005 and 2020, are shown in Table 1[5,7-14]. All previous cases have been attributed to oseltamivir. The age of onset ranged from 12 to 61 years, and there have been no reports of onset among the elderly. Among the 13 patients, nine were female individuals. The time of onset was four days following oral administration of oseltamivir in one patient; however, in the remaining patients, acute onset was observed immediately-to-within two days following the oral administration of oseltamivir. The descending colon was the most common site of onset. The outcome was favorable after discontinuation of the drug and conservative treatment; however, in patient 13, the hemoglobin level decreased to 3.8 mg/dL, which necessitated red blood cell transfusions.
| Patient | Ref. | Publication year | Age (yr) | Sex | Influenza type | Medication used | Time from use of medication to onset | Symptoms | Endoscopic findings |
| 1 | [5] | 2005 | 58 | Female | A | Oseltamivir | 2 d | Diarrhea, abdominal pain, and bloody stools | Longitudinal ulcer from the descending colon to the left side of the transverse colon |
| 2 | [7] | 2006 | 28 | Female | B | Oseltamivir | 2 d | Bloody stools | Erosion, edema, and hemorrhage on the left side of the transverse colon |
| 3 | [8] | 2007 | 20 | Female | Unknown | Oseltamivir | 2 d | Melena and abdominal pain | Erosion, redness, and edema in the descending colon |
| 4 | [8] | 2007 | 50 | Female | Unknown | Oseltamivir | 2 d | Diarrhea, bloody stools, abdominal pain | Erosion and suspicion of ischemic enterocolitis on the left side of the colon |
| 5 | [8] | 2007 | 30 | Male | Unknown | Oseltamivir | 2 d | Abdominal pain, diarrhea, and melena | Edema-like necrotic material and suspicion of ischemic enterocolitis from the sigmoid to the descending colon |
| 6 | [8] | 2007 | 28 | Female | Unknown | Oseltamivir | 2 d | Abdominal pain and bloody stools | Hemorrhage, erosion, edema, and redness on the left side of the transverse colon |
| 7 | [8] | 2007 | 50 | Female | A | Oseltamivir | 2 d | Bloody stools | Redness and ulceration in the descending colon |
| 8 | [9] | 2013 | 32 | Female | A | Oseltamivir | 2 d | Bloody stools and tenesmus | Redness, ulcer, and mucosal edema in the rectum |
| 9 | [10] | 2007 | 61 | Male | A | Oseltamivir | 1 d | Abdominal pain, diarrhea, and bloody stools | Mucosal edema, hemorrhage, and longitudinal ulcer in the entire colon |
| 10 | [11] | 2011 | 40 | Female | A | Oseltamivir | 4 h | Abdominal pain, diarrhea, and bloody stools | Redness, edema, erosion, and hemorrhage in the descending colon |
| 11 | [12] | 2015 | 31 | Female | A | Oseltamivir | 2 d | Abdominal pain and bloody diarrhea | Not performed |
| 12 | [13] | 2016 | 12 | Male | Unknown | Oseltamivir | 4 d | Bloody stools | Not performed |
| 13 | [14] | 2020 | 14 | Male | A | Oseltamivir | Immediately after (time not specified) | Abdominal pain, nausea, and bloody stools | Findings of ischemic enterocolitis (details not specified) |
Our patient was a middle-aged female individual without any risk factors; however, she developed ischemic enterocolitis within half a day after the administration of laninamivir via inhalation. Consistent with the previously reported patient who experienced hemorrhagic enteritis/ischemic enterocolitis after the oral administration of oseltamivir[5,7], this patient was diagnosed with ischemic enterocolitis induced by laninamivir, which is a neuraminidase inhibitor similar to oseltamivir.
Although a clear mechanism underlying oseltamivir-associated hemorrhagic enteritis/ischemic enterocolitis has not been elucidated, simultaneous occurrence of the following two factors has been considered responsible for the condition: (1) Local angiospasm and vasculitis due to the effect of an allergic reaction to oseltamivir; and (2) Hypoperfusion in the intestinal tract due to the effect of dehydration caused by influenza[9,12]. Laninamivir is considered to demonstrate the same mechanism as oseltamivir. Approximately three hours after inhalation, she reportedly experienced bloody stools and lower abdominal pain. In addition, she developed certain symptoms shortly after inhalation and a possible allergic reaction. Similarly, she was dehydrated based on physical findings. Therefore, the mechanism underlying oseltamivir-associated ischemic enterocolitis in this patient is considered related to these factors.
During treatment for influenza, attention should be paid not only to respiratory tract symptoms such as pyrexia, cough, and sputum but also to abdominal symptoms.
In conclusion, we treated a patient with ischemic enterocolitis that was considered to be caused by laninamivir. The onset of ischemic enterocolitis, although rare, has been reported following the administration of oseltamivir. Similarly, laninamivir may cause similar abdominal symptoms through the same mechanism; thus, caution should be exercised during the administration of this medication.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pichon M, Vunnam SR S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | National Institute of Infectious Diseases. Infectious Agents Surveillance Report (IASR). Influenza 2019/20 season. IASR; 41: 191-193. [cited January 31, 2021] Available from: https://www.niid.go.jp/niid/ja/flu-m/flu-iasrtpc/9961-489t.html. |
| 2. | Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385:1729-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 405] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 3. | Muthuri SG, Venkatesan S, Myles PR, Leonardi-Bee J, Al Khuwaitir TS, Al Mamun A, Anovadiya AP, Azziz-Baumgartner E, Báez C, Bassetti M, Beovic B, Bertisch B, Bonmarin I, Booy R, Borja-Aburto VH, Burgmann H, Cao B, Carratala J, Denholm JT, Dominguez SR, Duarte PA, Dubnov-Raz G, Echavarria M, Fanella S, Gao Z, Gérardin P, Giannella M, Gubbels S, Herberg J, Iglesias AL, Hoger PH, Hu X, Islam QT, Jiménez MF, Kandeel A, Keijzers G, Khalili H, Knight M, Kudo K, Kusznierz G, Kuzman I, Kwan AM, Amine IL, Langenegger E, Lankarani KB, Leo YS, Linko R, Liu P, Madanat F, Mayo-Montero E, McGeer A, Memish Z, Metan G, Mickiene A, Mikić D, Mohn KG, Moradi A, Nymadawa P, Oliva ME, Ozkan M, Parekh D, Paul M, Polack FP, Rath BA, Rodríguez AH, Sarrouf EB, Seale AC, Sertogullarindan B, Siqueira MM, Skręt-Magierło J, Stephan F, Talarek E, Tang JW, To KK, Torres A, Törün SH, Tran D, Uyeki TM, Van Zwol A, Vaudry W, Vidmar T, Yokota RT, Zarogoulidis P; PRIDE Consortium Investigators, Nguyen-Van-Tam JS. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med. 2014;2:395-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 480] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 4. | Relenza dear doctor letter in EU follow U.S. advisory. In: "The Pink Sheet" F-D-C Reports, Chevy Chase, MD. January 31, 2000, p20. |
| 5. | Nara N, Oshimoto H, Hirouchi K, Toyoda M, Katakai K, Masuda J, Matsumoto J, Arai T. A case of ischemic colitis induced by oseltamivir phosphate. Prog Dig Endosc. 2005;67:114-115 (article in Japanese). |
| 6. | Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7061] [Cited by in RCA: 8165] [Article Influence: 185.6] [Reference Citation Analysis (0)] |
| 7. | Yoneda S, Kobayashi Y, Nunoi T, Takeda K, Matsumori A, Andoh M, Tsujinoue H, Nishimura K, Fukui H. [Acute hemorrhagic colitis induced by the neuraminidase inhibitor oseltamivir]. Nihon Shokakibyo Gakkai Zasshi. 2006;103:1270-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Abe A, Sugiura N, Akiike T, Ito K, Aruga A, Kaneda S, Nakano M. A case of colon ulcer induced by oseltamivir phosphate. Prog Dig Endosc. 2007;70:110-111 (article in Japanese). |
| 9. | Chen YH, Lai HJ. Acute hemorrhagic colitis after oral administration of oseltamivir for influenza. Gastrointest Endosc. 2013;77:976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Matsushita M, Nishihara H, Nishiyama R, Kobayashi Y. Acute hemorrhagic colitis associated with oral administration of oseltamivir for the treatment of influenza A. J Infect Chemother. 2007;13:267-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Nakagawa Y, Nagai T, Okawara H, Nakashima H, Hisamatsu A, Shuto M, Yamauchi M, Kai S, Yokoyama S, Murakami K, Fujioka T. Acute hemorrhagic colitis induced by the oral administration of oseltamivir used for influenza A treatment. Endoscopy. 2011;43 Suppl 2 UCTN:E261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Manickam P, Mogrovejo E, Cappell MS. Oseltamivir-induced acute hemorrhagic colitis. Minerva Gastroenterol Dietol. 2015;61:299-301. [PubMed] |
| 13. | Beraldi-Magalhães F, Batista V, Cordeiro-Santos M. Oseltamivir as a cause of acute enterorrhagia. Braz J Infect Dis. 2016;20:521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Abe Y, Tsukano S, Izumita Y, Yamanaka T. Hemorrhagic colitis can be a life-threatening side effect of oseltamivir. Pediatr Int. 2020;62:634-635. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |