Published online Mar 26, 2022. doi: 10.12998/wjcc.v10.i9.2851
Peer-review started: September 2, 2021
First decision: November 19, 2021
Revised: November 24, 2021
Accepted: February 12, 2022
Article in press: February 12, 2022
Published online: March 26, 2022
Processing time: 201 Days and 9.9 Hours
Congenital intestinal malrotation (CIM) is a common malformation in neonates. Early diagnosis and surgical intervention can improve the prognosis. CIM combined with congenital gastric wall defect is a potentially fatal condition. We present a severe case of CIM with gastric wall defect causing extensive gut necrosis and short gut syndrome. After three operations, the neonate survived and subsequently showed normal growth and development during infancy.
A male neonate (age: 4 d) was hospitalized due to bloody stools and vomiting for 2 d, and abdominal distention for 1 d. Emergent exploratory laparotomy revealed black purplish discoloration of the bowel loops. Bowel alignment was abnormal with congestion and dilatation of the entire intestine, and clockwise mesentery volvulus (720°). The posterior wall of the gastric body near the greater curvature showed a defect in the muscularis layer (approximately 5.5 cm), and a circular perforation (approximately 3 cm diameter) at the center of this defect. Ladd’s procedure was performed and gastric wall defect was repaired. Third operation performed 53 d after birth revealed extensive adherence of small intestine and peritoneum, and adhesion angulated between many small intestinal loops. We performed intestinal adhesiolysis, resection of necrotic intestine, and small bowel anastomosis.
This case highlights that prolonged medical treatment may help improve intestinal salvage after surgical removal of necrotic intestines, and improve patient prognosis.
Core Tip: In cases of extensive intestine necrosis, adequate delay in the timing of the second laparotomy can offer more time for medical treatment allowing recovery of intestines and improving treatment outcomes.
- Citation: Wang Y, Gu Y, Ma D, Guo WX, Zhang YF. Congenital intestinal malrotation with gastric wall defects causing extensive gut necrosis and short gut syndrome: A case report. World J Clin Cases 2022; 10(9): 2851-2857
- URL: https://www.wjgnet.com/2307-8960/full/v10/i9/2851.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i9.2851
Congenital intestinal malrotation (CIM) is a common malformation of the digestive tract with 80% of the cases presenting in the neonatal period[1]. The condition is caused by defective embryonic development due to midgut volvulus and fixation disorders, resulting in abnormal bowel position or unfixed mesentery[2]. The main clinical manifestations are bilious vomiting, abdominal pain, distension, obstruction in superior intestines and intestinal necrosis due to volvulus. The incidence rate of intestinal necrosis due to volvulus may be as high as 44%[3]. CIM is one of the causes of digestive tract obstruction in the neonatal period[4]. Early diagnosis and surgical intervention are essential to improve the prognosis of these patients. In such cases, intestinal malrotation can be the sole congenital anomaly or it may occur in combination with other anomalies such as duodenal diaphragm, paraduodenal hernia, or Meckels diverticulum[5].
Congenital gastric wall defect (CGWD) is the most common cause for spontaneous gastric perforation in neonates, with the underlying mechanisms being unknown[6]. It is generally believed that CGWD is associated with developmental defects of the gastric wall musculature in the embryonic stage[6]. Some scholars have attributed this to perinatal hypoxia[7]. These defects most commonly occur near the greater curvature of the stomach and frequently result in adverse consequences and mortality[7].
To the best of our knowledge, there are no documented cases of CIM with gastric wall defect accompanied by septic shock or multiple organ failure or with extensive necrosis of the small intestine where the patient has survived. Moreover, there are no reported cases where a patient suffering from the above diseases was successfully treated with three operations. In the present case report, we describe a severe case of CIM with gastric wall defect resulting in extensive necrotic gut and short gut syndrome. After three operations, the neonate survived and subsequently showed normal growth and development during infancy.
A male neonate aged 4 d was brought to the Department of Pediatrics of our hospital with the chief complaints of passage of bloody stool accompanied by vomiting for 2 d, and abdominal distension for 1 d.
The patient was delivered by caesarean section at the gestational age of 38 wk due to premature rupture of membranes for 6 h. The weight of the neonate was 3200 g.
The patient had a free previous medical history.
The family history of the patient was unremarkable.
At admission, the vital parameters were: Body temperature 36.0 °C; pulse 150/min; respiratory rate 66/min; blood pressure 75/52 mmHg. The general condition of the patient was poor and he exhibited skin cyanosis and shortness of breath. The three concave sign was positive. The abdomen was distended with prominent abdominal varicose veins. On palpation, the abdomen was firm and tender. Bowel sounds were diminished.
Blood parameters: moderate leukocytosis (white blood cell count: 26.1 × 109/L; neutrophils 17.96 × 109/L), hemoglobin 120 g/L, and platelet count 198.0 × 109/L.
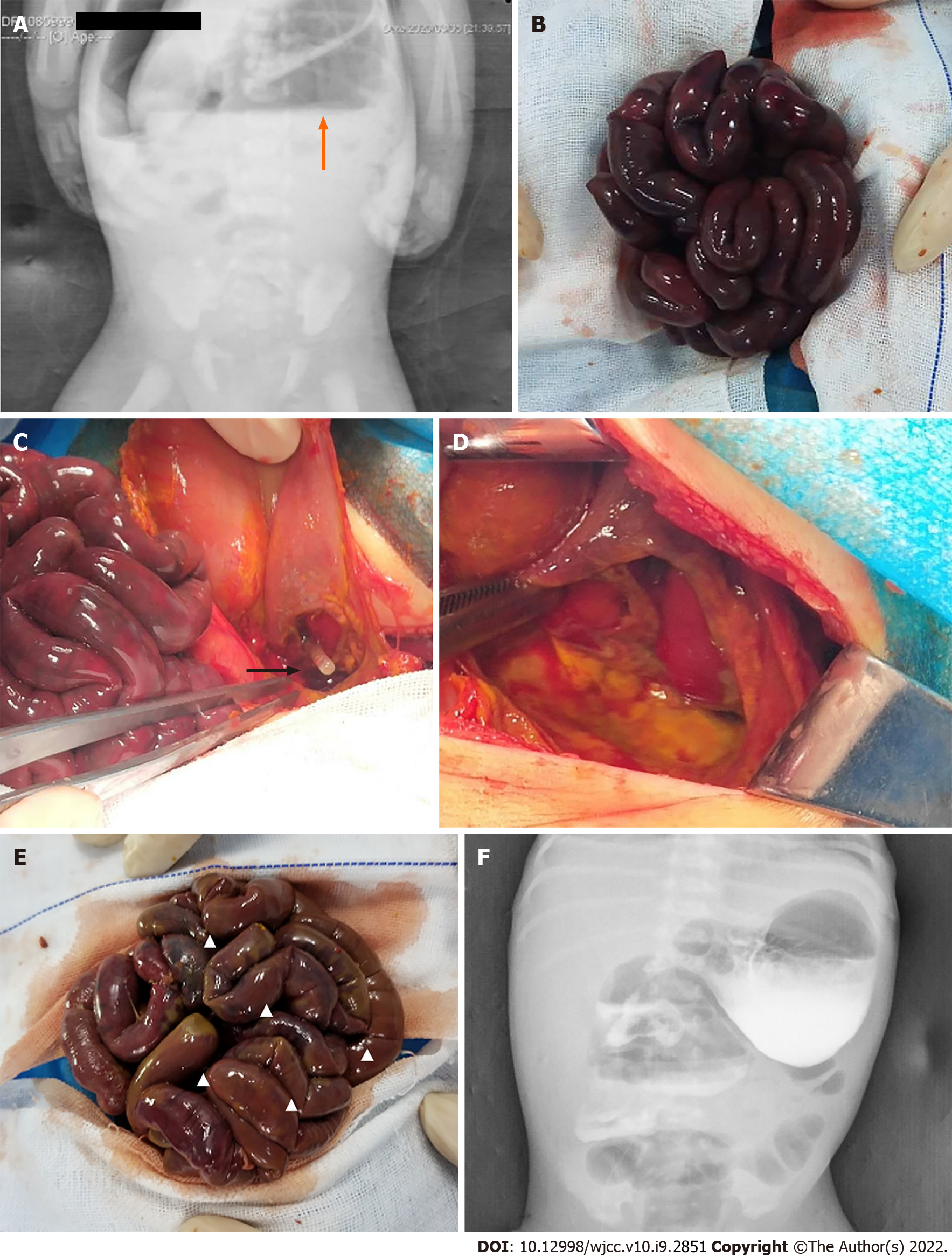
Upright abdominal plain film revealed free gas and liquid under the diaphragm (Figure 1A).
Congenital gastric wall rupture due to the muscular wall defects, CIM with associated midgut volvulus and small intestine necrosis.
After ensuring that the vital signs were stable, an emergent exploratory laparotomy was performed. Intraoperative examination revealed a large amount of gas and yellow feces accompanied with dark red bloody fluid in the peritoneal cavity. The bowel loops appeared black purplish in color (Figure 1B). We applied warm saline wet gauze dressing to the bowels. Bowel alignment was abnormal with intestinal congestion and dilatation, and mesentery volvulus (clockwise 720°). The posterior wall of the gastric body near the greater curvature had an approximately 5.5 cm defect in the gastric muscularis, with a circular perforation, approximately 3 cm in diameter, at the center of the defect. The tissue at the edges of the perforation was irregular, cyan colored, and the muscle layer was interrupted at the junction of the normal stomach wall (Figure 1C and D).
We performed Ladd’s procedure and stitched the gastric wall defect. We also observed mild intermittent reddish discoloration of the small intestine after the application of wet saline gauze dressing. Since the color of the small intestine showed poor viability, resection would have led to short bowel syndrome (SBS). Therefore, the small intestine was returned to the abdominal cavity.
Second surgical exploration performed 32 h after the first surgery showed massive small intestine necrosis. The gut appeared unviable, with plaque-like appearance and loss of elasticity; parts of the intestinal canal were left with serosa layer only (Figure 1E). The total length of viable intestine wall with fair blood supply was less than 20 cm. After the condition of the patient was explained to the family, they did not provide consent for resection of the necrotic intestinal tract. The intestinal tract was again returned to the abdominal cavity for palliative care. We adopted the following treatment strategies: supportive treatment to stabilize the interior milieu, anti-infection, and parenteral nutrition (PN). After the second surgery, approximately 100-200 mL green-colored fluid was extracted on gastrointestinal decompression. Seventeen days after the second surgery, a glycerin enema was administered to the patient, and yellow mucosa was excreted. Thereafter the patient started to pass yellow colored stool every day. At the same time the color of the gastrointestinal de
A third operation was performed 53 d after birth. Intraoperative examination revealed close adherence of many parts of the small intestinal wall and peritoneum, and adhesion angulated between much of the small intestines. From the starting point of the jejunum approximately 15 cm of the intestinal tract and approximately 25 cm away from the ileocecal junction, the bowel was intact with good blood supply and no narrowing, obstruction, or damage. Between the two sites, many parts of the small intestinal tract were narrow and devitalized.
Perioperative diagnosis was intestinal stenosis and intestinal necrosis. We performed intestinal adhesiolysis, resection of necrotic intestine, and small bowel anastomosis.
Histopathological examination of the surgical specimen showed mucosal and submucosal infiltration of a large number of acute and chronic inflammatory cells. Ischemia, necrosis, and cellulosic exudation were present. Abundance of dilated blood vessels in submucosa, congestion, and lymphoid follicles were also observed. Serosa layer showed vasodilation, congestion, fibrous tissue hyperplasia, and extensive acute inflammatory cell infiltration. One month after the third operation, the mother started micro breastfeeding (one time per hour, each time 3-5 mL); in addition, intermittent oral probiotics were administered to the patient to improve the intestinal environment along with amoxicillin to achieve bacteriostasis.
PN was stopped 137 d after birth and restoration of complete feeding was achieved [(breast milk, milk volume 334 mL/(kg·d)]; however, the infant showed poor weight gain, achieving only 4 kg at the age of 5 mo. There was improvement in diarrhea after addition of food supplements and the infant showed gradual weight gain. At the age of 9 mo, the body height and head circumference were both about 50th percentile of similar aged children and the weight was between 3rd-10th percentile of similar aged children. Abdominal ultrasound showed no signs of intestinal stenosis. At the age of 13 mo, the weight had increased to > 25th percentile of similar age children, and total protein, albumin, hemoglobin, and electrolyte levels were all within the normal range.
Neonatal SBS refers to the effects of extensive small intestine resection, which results in the remaining intestine tract being too short and leading to the impaired water and electrolyte balance and malabsorption syndrome[8]. The advances in PN technology has helped improve the survival of children with SBS and their quality of life. Studies have shown that children in whom the ileal valve is retained and the length of the remaining small intestine is at least 11 cm, or those in whom the ileal valve is not retained but the remaining small intestine length is at least 25 cm can be eventually weaned off PN with good survival outcomes[9]. The intestinal adaptation process of the remaining intestinal tract is vital and some children may die because of malnutrition[10].
Our patient was a full-term neonate and had no history of intrauterine distress. He suffered congenital intestinal malrotation with stomach wall defect. Pei et al[11] believe that intestinal malrotation during the fetal period results in intrauterine duodenal ileus which leads to gastric hypertonia; the consequent hypoxia and ischemia cause dysplasia of the stomach wall leading to gastric musculature defects. Moreover, postnatal digestive tract obstruction leads to increased intragastric pressure, which may cause perforation of the defective gastric wall[11]. Our patient already had bloody stools and abdominal distension for 2 d at the time of admission. We observed rupture of gastric wall defect accompanied by extensive small intestine necrosis, a critical condition with a high risk of death. During the first laparotomy, we observed dilation of the small intestine and the entire small intestine appeared dark purple. After an application of wet dressing for hydropathic compress, the color of the small intestine intermittently turned slightly red. Thirty-six hours after the bowel was returned to the abdominal cavity and abdominal closure, there was further aggravation of intestinal necrosis and part of the intestinal tract was left with only a serosal layer. In this setting, short intestine syndrome was bound to have appeared even if the neonate had survived. Yang et al[12] reported 48 cases of neonatal intestinal malrotation which were admitted to the Neonatology Department of the Kaifeng Children’s Hospital in Henan Province between 2012-2016. Among these 48 cases, 4 cases suffered necrosis of a large part of intestine, surgical reduction was performed, and bowels were returned to abdominal cavity. In 2 cases, a second laparotomy performed after 48 h showed resumption of blood circulation in most of the intestinal tract, and these two cases were finally cured after intestinal resection and entero-anastomosis. The remaining two cases had massive necrosis of the intestine and died of shock and multiple organ failure. There was also a postoperative death case because of severe defect in the stomach wall.
In our patient, less than 20 cm of the intestinal tract was viable after the two abdominal operations and the patient had severe systemic infection combined with septic shock. After fifty days of supportive treatment, we found more viable intestinal tract at the third laparotomy compared to that at the previous operation. We assumed that the necrotic and perforated intestinal tract was encapsulated and absorbed.
In the domestic literature, there are no reported cases of neonates with gastric perforation and intestinal malrotation accompanied with septic shock or multiple organ failures or those with extensive small intestine necrosis that survived. In most such cases, death is attributable to the severity of the disease or the abandonment of all hope by the parents, thereby not permitting exploration of further options. Moreover, there have been no reported cases of neonates who have received three operations and survived.
Our patient was administered broad-spectrum antibiotics plus Meropenem and supportive treatment. The systemic infection was gradually treated and the intestinal necrotic tissue was encapsulated and absorbed, which offered an opportunity for the third operation. After the third surgery, even though the length of the remaining intestinal tract was only 40 cm, the patient’s growth and development have been comparable to those of children of similar age.
In our patient, intravenous nutrition was stopped 4 mo after birth, but there was no significant weight gain. A significant weight gain was observed only after initiation of complementary nutrition at the age of 5 mo. It is not clear if the weight gain was because of the effective compensation of the remaining intestinal tract or whether the transition from a liquid to a solid diet prolonged the retention time of the diet in the intestinal tract.
To sum up, early diagnosis and surgery for neonatal acute abdomen is critical and can help improve the survival rate and prognosis. Perioperative diagnosis of extensive intestinal necrosis does not necessarily imply a dismal prognosis. Whether to extend the time of medical intervention or delay the second laparotomy needs to be discussed. Integrated medical management after surgery can significantly improve the quality of life.
The typical approach for perioperative detection of extensive intestine necrosis entails removal of the necrosed intestine and closure of the abdomen. A repeat laparotomy is then performed after 24 to 48 h to assess any improvement in blood circulation. In the present case, no improvement of the necrosed intestinal tract was observed at second laparotomy. After a period of effective medical treatment, third laparotomy was performed which showed that portions of the intestinal tract were viable. Therefore, in cases of extensive intestine necrosis, the second laparotomy can be delayed to allow more time for the recovery of the intestinal tract with medical treatment. This approach may help improve the survival outcomes and decrease the time for intestine adaptation.
We would like to thank Ms. Gao YX for her support and help on the care of the patient.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Awad AK, Le PH S-Editor: Li X L-Editor: A P-Editor: Li X
| 1. | Strouse PJ. Disorders of intestinal rotation and fixation ("malrotation"). Pediatr Radiol. 2004;34:837-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 132] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | Ma YY, Ping S, Niu HZ, Zhang PJ, Dong YQ, Liu F, Ren H. Related factors influencing the enteral nutrition toler-ance in neonates with congenital intestinal malrotation after operation. Hebei Yiyao. 2019;41:2117-2121. |
| 3. | Zhu WJ, Wu RZ. Practical surgical operation study. People's Health Press, 1997: 573-574. |
| 4. | Ren HX, Wu XX. Application of laparoscope in the treatment of the neonates with malrotation and intestinal twist. Linchuang Waike Zazhi. 2017;25:888-889. |
| 5. | van der Zee DC, Bax NM. Laparoscopic repair of acute volvulus in a neonate with malrotation. Surg Endosc. 1995;9:1123-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Wang K, Chen YW, Cai SY, He YJ, Guo WH, Hou DW, Peng CH, Pang WB, Chen YJ. Study on the characteristics and prognosis of neona-tal gastric perforations. Zhonghua Xiaoer Waike Zazhi. 2018;39:274-278. |
| 7. | Terui K, Iwai J, Yamada S, Takenouchi A, Nakata M, Komatsu S, Yoshida H. Etiology of neonatal gastric perforation: a review of 20 years' experience. Pediatr Surg Int. 2012;28:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Cai W, Chen B, Chen ZS, Chi Q, Fan HN, Li YX, Wang XD, Wei L, Wu XT, Xu PY, Yin L, Zhang XQ, Zhao QC, Zhou YB, Mao Q, Li YS, Li JS. Consensus on the diagnosis and treatment of short bowel syndrome in China. Zhonghua Weichang Waike Zazhi. 2017;20:1-8. |
| 9. | Goulet O. Short bowel syndrome in pediatric patients. Nutrition. 1998;14:784-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Squires RH, Duggan C, Teitelbaum DH, Wales PW, Balint J, Venick R, Rhee S, Sudan D, Mercer D, Martinez JA, Carter BA, Soden J, Horslen S, Rudolph JA, Kocoshis S, Superina R, Lawlor S, Haller T, Kurs-Lasky M, Belle SH; Pediatric Intestinal Failure Consortium. Natural history of pediatric intestinal failure: initial report from the Pediatric Intestinal Failure Consortium. J Pediatr. 2012;161:723-8.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 349] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 11. | Pei HG, Mao JX, Zhang C, Li SY. Analysis of clinical characteristics of neonatal gastric perforation with intestinal malrotation. Zhonghua Xiaoer Waike Zazhi. 2013;34:259-261. |
| 12. | Yang GW, Guo YF, Gong YX, Jing DP. Clinical analysis of 48 cases of intestinal malrotation in newborns. Zhongguo Shiyong Yiyao Zazhi. 2016;11:81-82. |