Published online Mar 26, 2022. doi: 10.12998/wjcc.v10.i9.2844
Peer-review started: August 23, 2021
First decision: November 17, 2021
Revised: December 11, 2021
Accepted: February 19, 2022
Article in press: February 19, 2022
Published online: March 26, 2022
Processing time: 211 Days and 2.8 Hours
Gitelman syndrome (GS) is an autosomal recessive renal tubular disorder characterized by renal wasting hypokalemia, metabolic alkalosis, hypomagnesemia, and hypocalciuria. It is usually caused by mutations in the gene SLC12A3, which encodes the thiazide-sensitive Na-Cl cotransporter. GS is not usually diagnosed until late childhood or adulthood.
Here, we report the case of a one-year-old girl who was brought to the emergency department due to persistent vomiting for two days. On admission to our hospital, generalized weakness was observed, and laboratory investigations revealed severe hypokalemia (1.9 mmol/L). However, persistent hypokalemia was observed during outpatient follow-up. Suspicion of the GS phenotype was assessed via the patient’s clinical presentation, family history, and biochemical analysis of blood and urine. Further genetic analysis was performed for her and her family by exon-wide sequencing analysis of the gene SLC12A3. The genetic diagnosis of GS was established in the Taiwanese family with three affected individuals, two of whom were children (7 years/17 years) without obvious symptoms, with the youngest being only one year old (patient in our case).
We successfully demonstrated the early diagnosis of GS using family genetic analysis. Any instances of hypokalemia should not be neglected, as early detection of GS with suitable treatment can prevent patients from potentially life-threatening complications.
Core Tip: In this study, the genetic diagnosis of Gitelman syndrome (GS) was established in a Taiwanese family with three affected individuals, two of them being young children without obvious symptoms, with the youngest child being only one year old. We further describe the case of the one-year-old girl brought to the emergency department due to persistent vomiting for two days. We believe that our report makes a significant contribution to the literature because we successfully demonstrated the early diagnosis of a case of GS using family genetic analysis. The early diagnosis and treatment of this condition can help prevent potentially life-threatening conditions.
- Citation: Wu CY, Tsai MH, Chen CC, Kao CH. Early diagnosis of Gitelman syndrome in a young child: A case report . World J Clin Cases 2022; 10(9): 2844-2850
- URL: https://www.wjgnet.com/2307-8960/full/v10/i9/2844.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i9.2844
Hypokalemia is defined as a serum potassium level of less than 3.5 mmol/L and may develop into life-threatening complications in some patients[1]. It can be caused either by inadequate potassium intake, increased potassium excretion in the urine, or increased potassium excretion through the gastrointestinal tract. Gastrointestinal losses of potassium are the most common, secondary to conditions such as prolonged diarrhea, vomiting, diuretic use, or infections[2]. However, genetic causes of hypokalemia, such as the inherited renal tubular disorders, Bartter (BS) and Gitelman (GS) syndromes, are relatively rare; and their diagnosis is challenging especially in children[3]. Most cases of BS are noted in neonates due to polyuria or maternal polyhydramnios; in contrast, the symptoms of GS are milder and it is commonly diagnosed during adulthood[4].
GS is an inherited renal tubular disorder characterized by hypokalemia, metabolic alkalosis, hypomagnesemia, and hypocalciuria. It is recessively inherited and caused by mutations in the gene SLC12A3, which encodes for the thiazide-sensitive Na-Cl cotransporter. The estimated GS prevalence is 1:40000. More than 180 different mutations in the gene SLC12A3 have been identified[5].
GS is often diagnosed in adults with muscle weakness and cramps associated with frequent fatigue or reduction in daily activity. Patients with GS do not show symptoms throughout infancy and preschool years, and they are often discovered incidentally[4,6]. Although GS is described as an asymptomatic or benign disorder, it may develop life-threatening complications, such as ventricular arrhythmia, in some cases[7]. Early detection with suitable treatment may prevent potentially dangerous complications. In this study, we successfully demonstrated an early diagnosis of GS using family genetic analysis.
A one-year-and-10-month-old Taiwanese girl was admitted to our hospital because of acute gastroenteritis with severe vomiting and diarrhea for two days.
The index patient was referred to our genetic clinic because of unusually severe hypokalemia. She had been recently admitted to our hospital because of acute gastroenteritis with severe vomiting and diarrhea for 2 d. On admission, we noted generalized weakness. Laboratory investigations showed severe hypokalemia (1.9 mmol/L) (Table 1). The patient was treated with intravenous potassium supplementation (potassium chloride, 0.15 mEq/kg of body weight per hour) under the diagnosis of hypokalemia related to acute gastroenteritis. The patient’s symptoms gradually improved after treatment. Therefore, the patient was discharged, and post-discharge follow-up was suggested.
| ED | Admission | OPD | Unit (normal range) | ||
| Blood | |||||
| Cr | 0.18 | 0.12 | mg/dL | 0.5-0.9 | |
| Na | 136 | 138 | 131 | mmol/L | 136-146 |
| K | 1.9 | 3.1 | 2.9 | mmol/L | 3.5-5.1 |
| Ca | 9.1 | 9.7 | mg/dL | 8.6-10.3 | |
| Cl | 94 | 91 | mmol/L | 101-109 | |
| Mg | 1.9 | 1.9 | mg/dL | 1.9-2.7 | |
| Osmolarity | 271 | mOsmol/Kg | 280-305 | ||
| TSH | 4.0 | μIU/mL | 0.27-4.2 | ||
| Free T4 | 1.94 | ng/dL | 0.8-2.0 | ||
| ACTH | 12.65 | pg/mL | 10-60 | ||
| Cortisol (8AM) | 8.77 | μg/dL | 5-25 | ||
| Aldosterone | 6.1 | ng/dL | 0.75-15 | ||
| Renin | 168.0 | pg/mL | |||
| VBG | |||||
| pH | 7.558 | 7.442 | 7.545 | 7.31-7.41 | |
| PCO2 | 31.2 | 41.4 | 28.3 | mmHg | 41-57 |
| HCO3- | 27.2 | 27.6 | 23.9 | mmol/L | 23-30 |
| Spot urine | |||||
| Na | 74 | 81 | mmol/L | ||
| K | 23 | 39 | mmol/L | 25-120 | |
| Cl | 64 | mmol/L | 110-250 | ||
| Cr | 28.17 | mg/dL | |||
| Osmolarity | 412 | mOsmol/Kg | 200-1200 | ||
| K/Cr ratio | 15.6 | mmol/mmol | |||
| TTKG | 8.9 | ||||
| 24 Hrs Urine | |||||
| Total Volume | 210 | ml | |||
| Na (Sodium) urine | 95 | mmol/L | |||
| K(Potassium) urine | 34 | mmol/L | 25-120 | ||
| Cl (Chloride) Urine | 442 | mmol/L | 110-250 | ||
| Ca (Calcium) Urine | 0.4 | mg/dL | 6.8-21.3 | ||
| Ca/Cr ratio | 0.01 | ||||
Based on the birth history, the girl was previously healthy and denied any systemic diseases.
During a consultation held at our hospital, the patient’s mother said that her 7-year-old daughter was also diagnosed with hypokalemia at another hospital, but no further investigations were performed. In addition, her 17-year-old daughter had been experiencing muscle cramps and muscle pain associated with a severe reduction in daily activities for several years.
No history of salt-craving, tetany, motor developmental delay, arthralgia, or arthritis was observed. Her growth parameters and blood pressure were also within the normal range.
During outpatient follow-up, recurrent severe hypokalemia (2.6 mmol/L) was observed. Subsequent laboratory investigations, including blood analysis, showed metabolic alkalosis with hypokalemia (2.9 mmol/L) and borderline hypomagnesemia (1.9 mg/dL). Normal thyroid, adrenal function, plasma renin, and aldosterone levels were noted. Urinalysis revealed renal potassium wasting (urine potassium 39 mmol/L, urine potassium-creatinine ratio 15.6 mmol/mmol, transtubular potassium gradient (TTKG) 8.9), increased level of urine chloride (64 mmol/day), and hypocalciuria (0.4 mg/dL, urine calcium to creatinine ratio 0.01). Based on the above laboratory findings, a renal tubular disorder was highly suspected. Further, family history revealed that the hypokalemia might have been due to genetic processes.
Laboratory investigations showed that potassium levels were as follows: eldest daughter, 1.9 mmol/L; second eldest daughter, 4.1 mmol/L; third eldest daughter, 2.1 mmol/L; and index patient, 2.4 mmol/L. In addition, the potassium levels of the mother and father were 4.5 mmol/L and 4.2 mmol/L, respectively. To date, some autosomal recessive tubular disorders presenting with hypokalemia, metabolic alkalosis, and hypocalciuria have been established in the family.
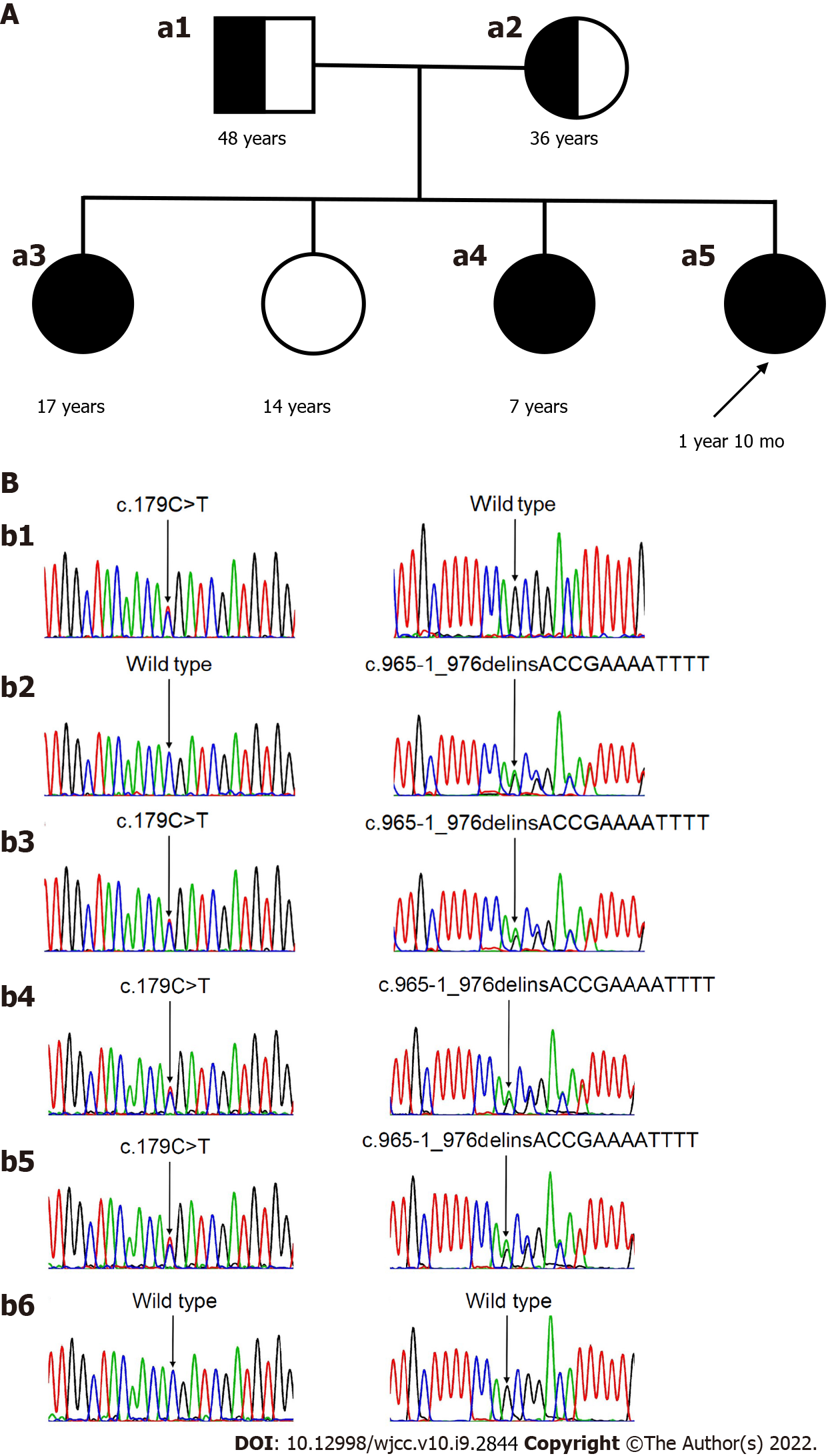
Venous blood samples were obtained from the three affected individuals with severe hypokalemia and their parents after detailed informed consent. Blood samples were sent to the DNA diagnostics laboratory (SOFIVA GENOMICS). According to their established protocols, the laboratory performed an exon-wide sequencing analysis of the gene SLC12A3. Genetic analysis of the gene SLC12A3 in the three affected individuals with severe hypokalemia revealed compound heterozygous mutations of the gene SLC12A3: c.179C>T in exon1 and c.965-1_976delinsACCGAAAATTTT in exon8, respectively. These two mutations are known to be associated with GS (Figure 1A). Both parents were heterozygous carriers of GS-associated mutations found in their daughters. The mother was the carrier of c.179C>T, and the father was the carrier of c.965-1_976delinsACCGAAAATTTT (see Figure 1B).
GS, with compound heterozygous mutations of the gene SLC12A3: c.179C>T in exon1 and c.965-1_976delinsACCGAAAATTTT in exon8.
In this study, GS was diagnosed genetically in a Taiwanese family with three affected individuals presenting with severe hypokalemia (less than 2.5 mmol/L), two of whom were young children without obvious symptoms, with the youngest being a one-year-old female toddler. These three girls started treatment of oral potassium supplementation (potassium gluconate) and nonsteroidal anti-inflammatory drugs (NSAIDs) (indomethacin, daily dose of 1 mg/kg/day). Due to gastrointestinal side effects being common (especially diarrhea) and serum magnesium level above 1.6 mg/dL, magnesium supplementation was withheld. Because of the side effects of NSAIDs and their stable condition, oral medications were shifted to potassium-sparing diuretics (spironolactone) 2 years later.
These patients were followed up in the outpatient department for regular monitoring of their serum potassium/ magnesium levels.
Hypokalemia is a common clinical problem, especially in hospitalized patients. Blood and urine tests, such as venous blood gas, serum concentrations of sodium, chloride, magnesium, aldosterone, renin, and urine concentrations of calcium, should be obtained to determine the etiology, such as decreased intake, increased intracellular uptake, gastrointestinal loss, or increased urinary loss[8,9].
Genetic tubular disorders are a source of hypokalemia caused by increased urinary potassium loss. Because of mutations in genes encoding tubular transport proteins involved in sodium reabsorption, patients’ sodium absorption is disrupted, leading to increased distal delivery of sodium, which results in metabolic alkalosis and hypokalemia. These disorders, known as BS or GS, are autosomal recessive diseases involved in sodium-chloride reabsorption in the loop of Henle or the distal tubule, respectively. However, both conditions are rare. The prevalence of GS and BS is estimated to be 1 in 40,000 and 1 in one million, respectively[10]. The heterozygous carrier rate is approximately 1 in 100 patients[11]. In contrast to the typical prenatal or neonatal clinical presentation of many patients with BS, GS is usually not diagnosed until late childhood or adulthood, although presentation in infancy has been described in some cases[12].
The diagnosis of BS or GS has primarily been based on clinical presentations and biochemical analysis of blood and urine. Measurement of urinary calcium excretion can help differentiate between BS and GS. Urine calcium excretion is high or elevated in BS and below normal in GS[13]. Hyperreninemia and hyperaldosteronism may present in BS and GS. However, metabolic alkalosis and renin-angiotensin system activation are not obvious in GS, unlike BS. Genetic analysis for many of the suspected gene mutations is possible and available commercially; for example, screening for recurrent hot spot SLC12A3 mutations may provide an early diagnosis of GS[14].
In this report, we describe the case of a female toddler who initially presented with acute gastrointestinal symptoms. Acute gastroenteritis in toddlers is a common epidemic disease in which patients frequently experience abdominal pain, vomiting, or diarrhea. If these symptoms are persistent or progressive for a few days, dehydration will occur, and IV treatment is needed. However, unusual, persistent, and severe hypokalemia (1.9 mmol/L) was noted in our case, and a genetic tubular disorder was suspected due to the hypokalemia. After a review of the patient’s family history, detailed examinations, including blood and urine tests, and genetic analysis were performed for her family. Finally, this female toddler (one year) and two of her elder sisters (7 years/17 years) were diagnosed with GS due to mutations in the SLC12A3 gene. GS is traditionally considered a benign disease that is usually not diagnosed until late childhood or adulthood. The average age at onset and diagnosis of GS is 20.0 ± 8.4 years and 23.6 ± 10.4 years, respectively[5]. In this study, the index patient and her third eldest sister were diagnosed with GS earlier than the average age. The two eldest sisters presented without obvious symptoms and were not diagnosed with GS until genetic analysis was performed. Therefore, the prevalence of GS in young children may be underestimated.
GS requires aggressive correction of the associated electrolyte imbalance to avoid the development of chronic kidney disease, abnormal glucose metabolism, or even life-threatening complications such as ventricular arrhythmia. With the advancement of genetic testing technology, it is possible to effectively and quickly diagnose inherited renal tubular disorders, such as GS, in children.
We thank our patients for contributing to the clinical data in this report.
Provenance and peer review: Unsolicited article; Externally peer-reviewed.
Peer-review model: Single-blind
Specialty type: Pediatrics
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Mogahed EA, Navarrete Arellano M, Rodrigues AT, Wierzbicka A S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Cummings BM, Macklin EA, Yager PH, Sharma A, Noviski N. Potassium abnormalities in a pediatric intensive care unit: frequency and severity. J Intensive Care Med. 2014;29:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Molla AM, Rahman M, Sarker SA, Sack DA, Molla A. Stool electrolyte content and purging rates in diarrhea caused by rotavirus, enterotoxigenic E. coli, and V. cholerae in children. J Pediatr. 1981;98:835-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 67] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Kurtz I. Molecular pathogenesis of Bartter's and Gitelman's syndromes. Kidney Int. 1998;54:1396-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Chen H, Ma R, Du H, Liu J, Jin L. Early onset children's Gitelman syndrome with severe hypokalaemia: a case report. BMC Pediatr. 2020;20:366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Tseng MH, Yang SS, Hsu YJ, Fang YW, Wu CJ, Tsai JD, Hwang DY, Lin SH. Genotype, phenotype, and follow-up in Taiwanese patients with salt-losing tubulopathy associated with SLC12A3 mutation. J Clin Endocrinol Metab. 2012;97:E1478-E1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Fujimura J, Nozu K, Yamamura T, Minamikawa S, Nakanishi K, Horinouchi T, Nagano C, Sakakibara N, Shima Y, Miyako K, Nozu Y, Morisada N, Nagase H, Ninchoji T, Kaito H, Iijima K. Clinical and Genetic Characteristics in Patients With Gitelman Syndrome. Kidney Int Rep. 2019;4:119-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Pachulski RT, Lopez F, Sharaf R. Gitelman's not-so-benign syndrome. N Engl J Med. 2005;353:850-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Huang YC, Tsai MH, Fang YW, Tu ML. Normotensive hypokalemic primary hyperaldosteronism mimicking clinical features of anorexia nervosa in a young patient: A case report. Medicine (Baltimore). 2020;99:e20826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Tu ML, Fang YW, Leu JG, Tsai MH. An atypical presentation of high potassium renal secretion rate in a patient with thyrotoxic periodic paralysis: a case report. BMC Nephrol. 2018;19:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Blanchard A, Bockenhauer D, Bolignano D, Calò LA, Cosyns E, Devuyst O, Ellison DH, Karet Frankl FE, Knoers NV, Konrad M, Lin SH, Vargas-Poussou R. Gitelman syndrome: consensus and guidance from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2017;91:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 219] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 11. | Hsu YJ, Yang SS, Chu NF, Sytwu HK, Cheng CJ, Lin SH. Heterozygous mutations of the sodium chloride cotransporter in Chinese children: prevalence and association with blood pressure. Nephrol Dial Transplant. 2009;24:1170-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Tammaro F, Bettinelli A, Cattarelli D, Cavazza A, Colombo C, Syrén ML, Tedeschi S, Bianchetti MG. Early appearance of hypokalemia in Gitelman syndrome. Pediatr Nephrol. 2010;25:2179-2182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Bettinelli A, Bianchetti MG, Girardin E, Caringella A, Cecconi M, Appiani AC, Pavanello L, Gastaldi R, Isimbaldi C, Lama G. Use of calcium excretion values to distinguish two forms of primary renal tubular hypokalemic alkalosis: Bartter and Gitelman syndromes. J Pediatr. 1992;120:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 199] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Al Shibli A, Narchi H. Bartter and Gitelman syndromes: Spectrum of clinical manifestations caused by different mutations. World J Methodol. 2015;5:55-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |