Published online Mar 26, 2022. doi: 10.12998/wjcc.v10.i9.2836
Peer-review started: August 16, 2021
First decision: November 6, 2021
Revised: November 17, 2021
Accepted: February 16, 2022
Article in press: February 16, 2022
Published online: March 26, 2022
Processing time: 218 Days and 1.5 Hours
The emergence of secondary drug resistance when treating epidermal growth factor receptor (EGFR) mutated non-small cell lung cancer (NSCLC) using EGFR-tyrosine kinase inhibitors (EGFR-TKIs), seriously affects the therapeutic efficacy and survival of patients. Here, we report a case of advanced NSCLC focusing on the application of multiple biopsy modalities to reveal the development of multiple resistance mechanisms during targeted therapies.
A 54-year-old male patient presented with EGFR 19Del-mutated advanced lung adenocarcinoma, and exhibited the development of a T790M mutation during initial TKI treatment. Following 3 mo of Osimertinib treatment, a mixed response was observed. Tissue biopsy of the progressive lesion showed transformation to small cell lung cancer (SCLC) harboring RB1 and TP53 mutations, with loss of the original T790M mutation. A standard chemotherapy regimen with Anlotinib for SCLC was administered. Repeat biopsy revealed adenocarcinoma combined with SCLC after tumor progression. The patient’s overall survival was 24 mo.
Multiple biopsy modalities can reveal the development of multiple resistance mechanisms which help with treatment decision-making. Comprehensive treatment regimens according to the drug resistance mechanism significantly improved the prognosis of such patients.
Core Tip: The emergence of secondary drug resistance seriously affects the efficacy of tyrosine kinase inhibitor (TKI) treatment and prognosis of patients with epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer. EGFR-T790M mutation and tumor phenotype transformation are common drug resistance mechanisms in the first-generation TKI treatment process. Thus, it is necessary to determine the potential mechanisms using multiple biopsy methods with the aim of selecting appropriate and effective therapeutic drugs and extending the survival of patients with lung cancer.
- Citation: Hong E, Chen XE, Mao J, Zhou JJ, Chen L, Xu JY, Tao W. Sequential occurrence of T790M mutation and small cell lung cancer transformation in EGFR-positive lung adenocarcinoma: A case report. World J Clin Cases 2022; 10(9): 2836-2843
- URL: https://www.wjgnet.com/2307-8960/full/v10/i9/2836.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i9.2836
Lung carcinoma remains a principal reason for cancer-associated mortality worldwide. However, the treatment strategies for lung cancer, particularly those for non-small cell lung cancer (NSCLC) have undergone revolutionary advancement alongside the emergence of epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs). Within the kinase domain of EGFR, these activating mutations, particularly the deletion of exon 19 as well as the mutation in exon 21 (L858R), occur in 10%-15% of Caucasian patients with lung adenocarcinoma and in up to 50% of patients of Asian origin[1]. Gefitinib and Erlotinib, which are first-generation EGFR-TKIs or Dacomitinib and Afatinib, which are second-generation EGFR-TKIs, have been recommended as the first-line treatment strategy for EGFR mutation-positive NSCLC. Although their effectiveness, as well as safety, have been continuously proven, unfortunately, acquired resistance inevitably emerges, which typically occurs after a median period of 9.2-14.7 mo of TKIs therapy[2]. Three mechanistic categories of acquired resistance have been identified: Bypass signaling pathway activation, target gene mutation and histological transformation[3]. For first-generation EGFR-TKIs, a mutation in EGFR-T790M is the primary mechanism regulating acquired resistance, and is observed in nearly 50%-60% of cases, while 10% of tumors exhibiting EGFR-TKIs resistance transform to small cell lung cancer (SCLC) morphology[2].
Herein, we report a case of NSCLC with EGFR-T790M mutation and conversion to SCLC while treated with EGFR-TKIs. The patient underwent comprehensive treatment, including targeted therapy, platinum-containing chemotherapy, antiangiogenic therapy and immunotherapy, with an overall survival of 24 mo.
A 54-year-old male patient presented to the hospital with dyspnea and productive cough on November 5, 2016, without fever, hemoptysis and pleuritic pain.
The patient had continuously reported symptoms which had persisted for more than 5 mo and were gradually aggravated.
The patient had a history of pulmonary tuberculosis with no history of smoking or malignant tumors.
The patient denied any relevant personal and family history.
Physical examination showed that the patient's body temperature was 36.6ºC, pulse rate was 84 bpm, blood pressure was 126/77 mmHg, respiratory rate was 18 breaths/min, and performance status was 0.
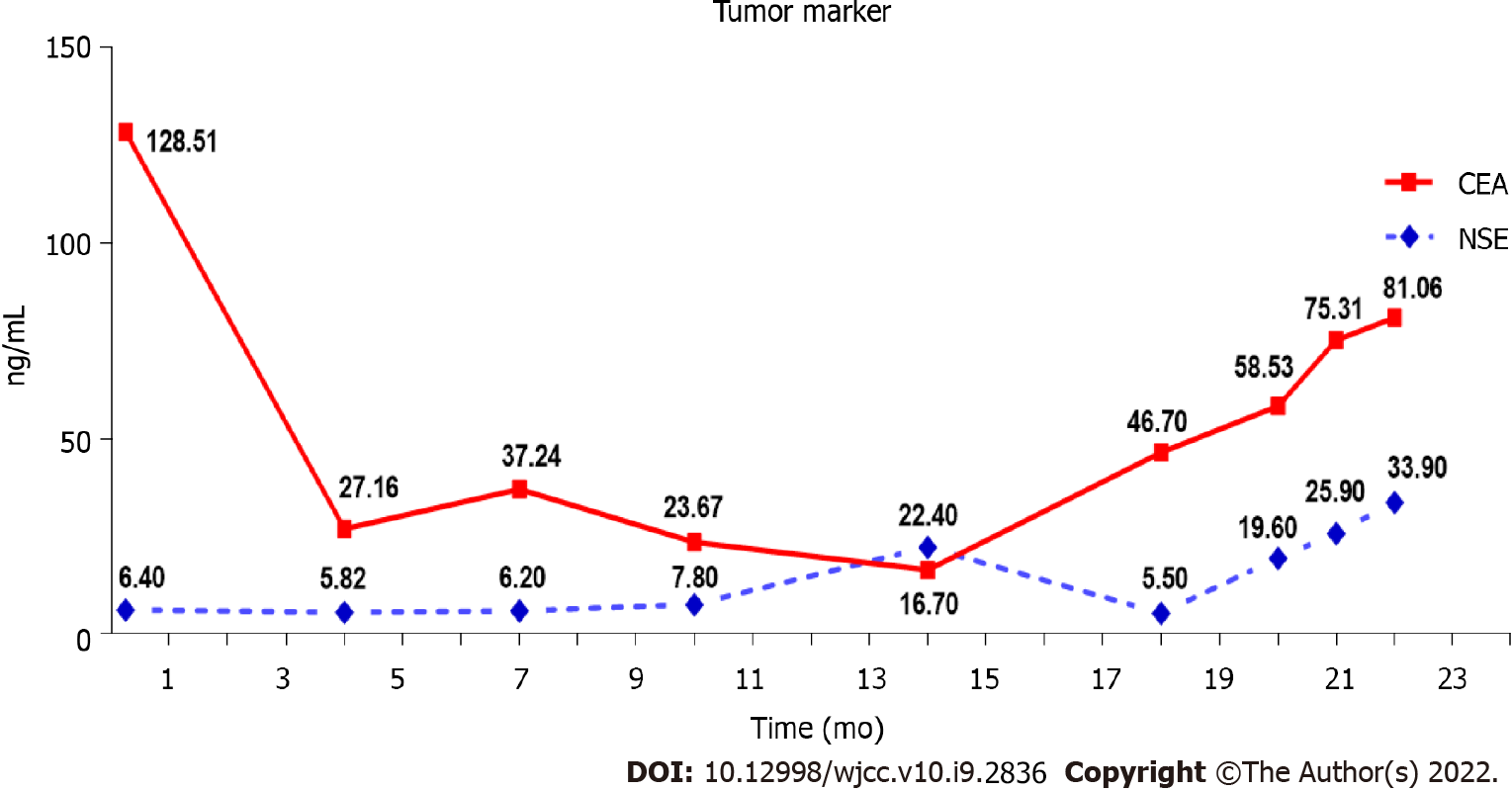
The laboratory results were normal except that the carcinoembryonic antigen (CEA) level was 128.51 ng/mL (the normal range: 0-5.0 ng/mL).
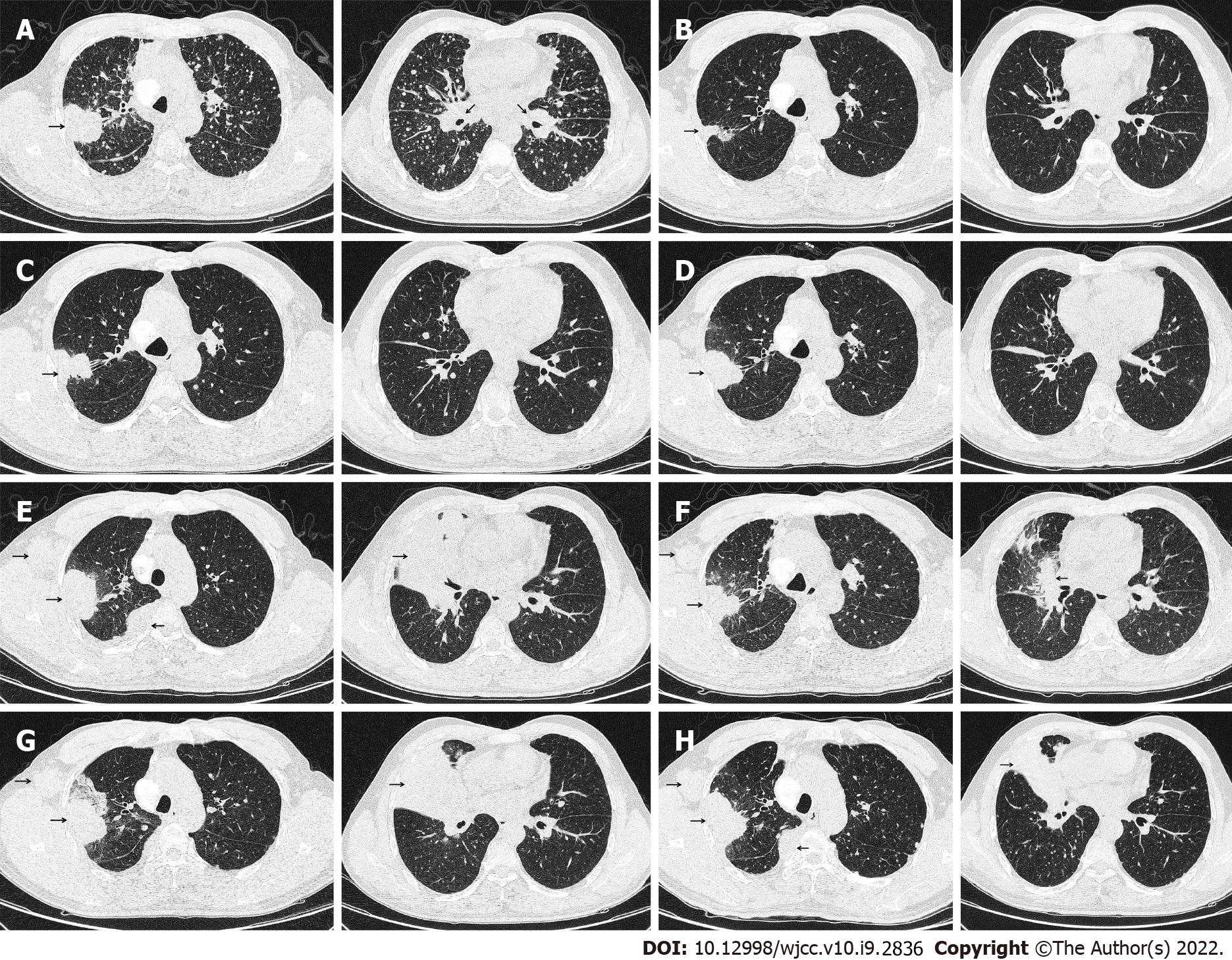
Computed tomography (CT) on November 5, 2016 revealed a 38 mm × 48 mm tumor in the upper right lobe, with multiple pleural and bilateral pulmonary metastases, mediastinal, and hilar lymphadenopathy (Figure 1A). Cranial magnetic resonance imaging, abdominal ultrasound, and bone emission CT were carried out to confirm the absence of distant metastases.
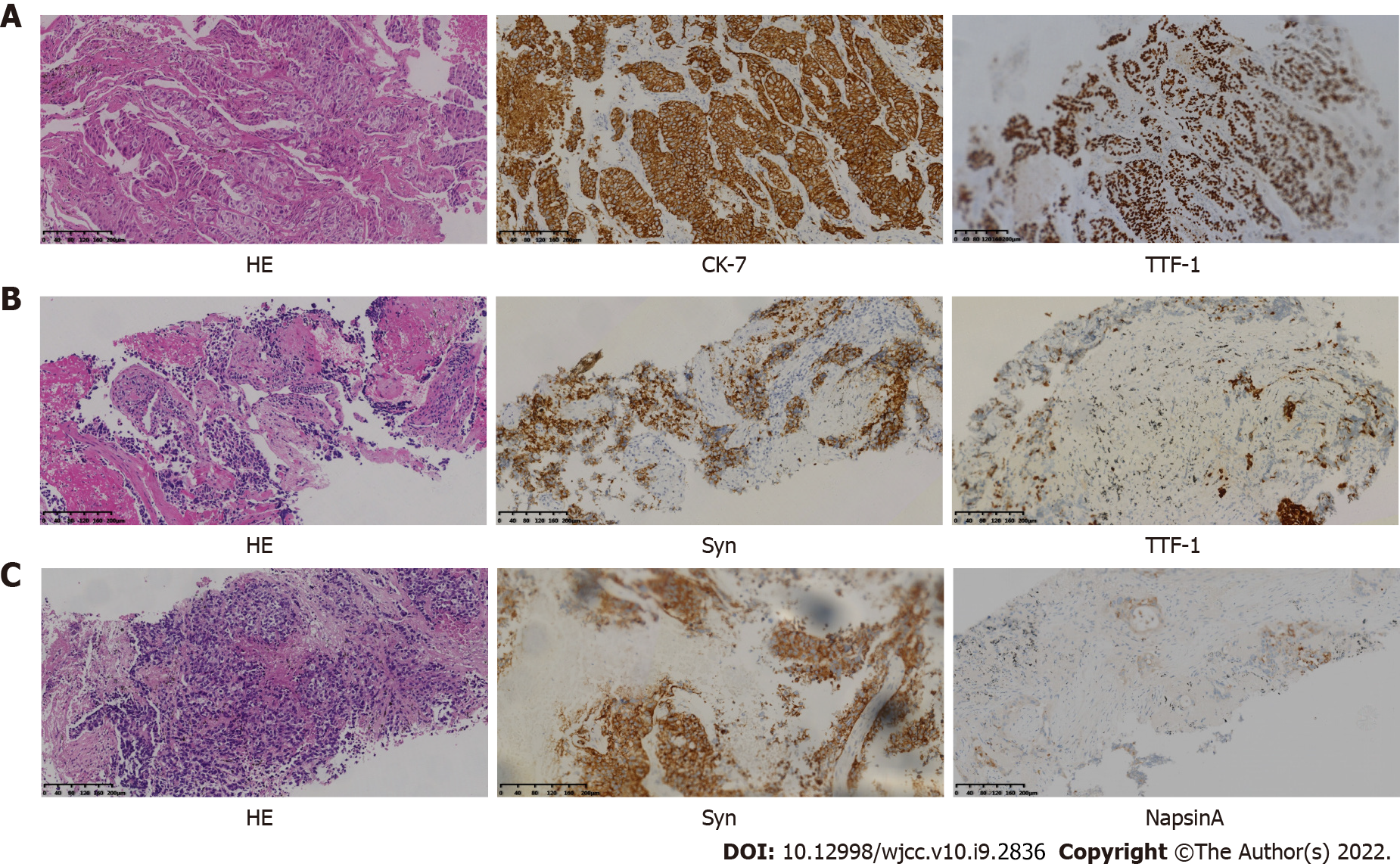
Trans-bronchial biopsy confirmed lung adenocarcinoma on November 10, 2016. Immunohistochemical analysis showed positive immunostaining for cytokeratin 7 (CK7) and thyroid transcription factor-1 (TTF-1) and negative immunostaining for the tumor protein 63 (P63), cytokeratin 5/6 (CK5/6) and synaptophysin (Syn) (Figure 2A). The amplification refractory mutation system technique identified EGFR exon 19 deletion.
The patient was diagnosed with stage IV right lung adenocarcinoma bearing a mutation caused by EGFR exon 19 deletion (cT2bN3M1a in accordance with version 8 of TNM staging).
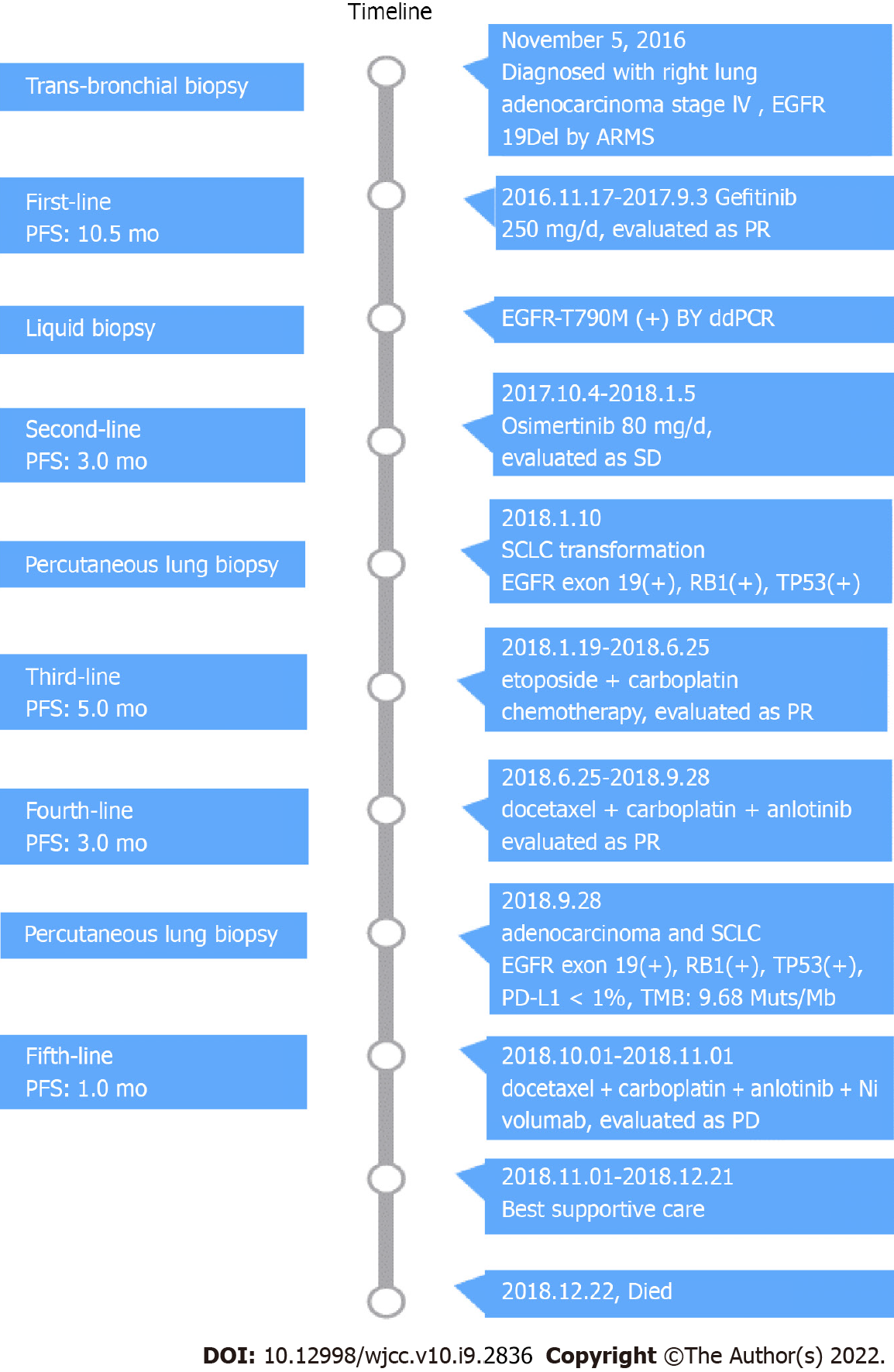
As a first-line treatment, 250 mg of oral Gefitinib was administered once a day starting on November 17, 2016. As per the solid tumor version 1.1 (RECIST 1.1) response evaluation criteria, the best efficacy evaluation was a partial response (PR) after 4 mo (Figure 1B). Serum CEA level significantly decreased to 27.16 ng/mL on March 6, 2017 (Figure 3). After 10.5 mo of progression-free survival (PFS), disease progression was confirmed by CT of the chest on September 3, 2017, which demonstrated an enlarged right upper lobe mass and increased metastatic nodules (Figure 1C). Droplet digital PCR (ddPCR) assay was employed to verify the distinct plasma EGFR-T790M. The therapeutic drug AZD9291 (80 mg daily), also known as Osimertinib, was used as a second-line treatment on October 4, 2017. Unexpectedly, a mixed response was observed after 1 mo, with a growing right upper lobe lesion but the near disappearance of pulmonary metastases (Figure 1D).
Following 3 mo of Osimertinib treatment, chest CT showed a further enlargement of the right upper lobe lesion (58 mm × 36 mm), a sizable mass in the right lung situated in the middle lobe, a local pleural mass with rib destruction, and an enlarged right axillary lymph node (34 mm × 36 mm) on January 5, 2018 (Figure 1E), following a 3.0 mo PFS. Blood tests showed an increase in the serum level of neuron-specific enolase (NSE) to 22.4 ng/mL and persistent decreasing levels of CEA to 16.70 ng/mL (Figure 3). Biopsy of the progressive right upper lobe mass was repeated and showed SCLC transformation according to immunohistochemistry (IHC) and revealed the following: positive Syn and TTF-1 expression, and NapsinA-negative (Figure 2B). Tissues were obtained for next-generation sequencing (NGS) using a panel consisting of 80 genes (3D Medicines, Shanghai, China), which revealed EGFR Exon19 (mutant abundance 58.00%), RB1 (mutant abundance 43.60%), TP53 (mutant abundance 82.60%) mutation and disappearance of EGFR-T790M. Intravenous etoposide 100 mg/m2 from the 1st d to the 3rd d, in combination with carboplatin (EC) (with an area of 5 mg/mL/min under the concentration-time curve, administered intravenously every 21 d on day 1) was used as a third-line treatment. Following the four chemotherapy cycles, the serum NSE level markedly decreased to 5.5 ng/mL (Figure 3), and efficacy evaluation was a partial response (Figure 1F). Unfortunately, the disease progressed after six cycles of EC on June 25, 2018 (Figure 1G). The patient attained a PFS of 5 mo. Oral Anlotinib (12 mg/d, from day 1 to day 14, 21 d) plus 75 mg/m2 docetaxel (injected intravenously on day 1) and intravenous carboplatin (5 mg/mL/min area under the concentration-time curve every 21 d on day 1) were administered as a fourth-line treatment. Lung lesions were stable for more than 3 mo (Figure 1H).
A CT-guided needle puncture of the lesion that had enlarged in size in the upper lobe of the right lung was obtained as the fourth biopsy on September 28, 2018 to monitor progression. Interestingly, the morphology of the lung mass showed poorly differentiated mixed histological subtypes of adenocarcinoma and SCLC (Figure 2C). The IHC staining data revealed the following: partially Syn-positive, partially CgA-positive, partially CD56-positive, partially NapsinA-positive, and PD-L1 < 1% (clone 22C3, DAKO, Agilent, Santa Clara, CA, United States). Tissue samples were detected via NGS containing a panel consisting of 420 genes (3D Medicines, Shanghai, China), which revealed EGFR Exon19 (mutant abundance: 38.28%), RB1 (mutant abundance: 68.48%), TP53 (mutant abundance: 76.97%) and tumor mutation burden (TMB): 9.68 Muts/Mb. Nivolumab was added to the previous therapeutic options as a fifth-line treatment on November 1, 2018 (180 mg intravenously every 2 wk). The entire treatment process is illustrated in Figure 4.
Unfortunately, although the patient underwent the best supportive care due to continuous deterioration in his general condition after 2 cycles of a previous combination treatment (performance status score > 2), he died on December 22, 2018.
The inevitable emergence of acquired drug resistance seriously affects the prognosis of patients with NSCLC and mutations having EGFR-sensitivity. The median time to disease progression after initial TKI treatment is 10.2 mo and the most common symptoms are cough and dyspnea[4]. Various mechanisms of acquired resistance can be categorized such as the activation of alternative signaling pathways, secondary mutations in EGFR, and phenotypic or histologic transformation[5-7]. EGFR-T790M mutation is the most common mechanism of acquired resistance, and amounts to 50-60% of resistance acquired during primary EGFR-TKIs therapy[8]. Evaluation of EGFR T790M status is imperative for the establishment of first- or second-generation TKI-acquired drug resistance, which is required for the selection of subsequent therapies and later proceeding to clinical trials. Recently, liquid biopsy genotyping of circulating tumor DNA (ctDNA) has become an attractive substitute to re-biopsy of tissues, due to its many potential advantages in comparison to tissue biopsy, such as improved safety of patients, reduced costs, shortened turnaround time, and counteracting the heterogeneity of different metastatic lesions[9]. A prospective study revealed that T790M detection based on ctDNA-using ddPCR was up to 77.1% sensitive, with a specificity of 63.2% and a positive predictive value of 79.0%, which indicated an acceptable correlation between tissue re-biopsy and ctDNA[10,11]. Due to the presence of tumor heterogeneity, the entire mechanism for resistance cannot be represented by a single tumor biopsy technique.
The scientific community has increasingly acknowledged that EGFR-mutant NSCLC can undergo SCLC transformation[12]. The conversion of tumor phenotype has been demonstrated to be a target-independent drug resistance mechanism. For patients with unsuccessful EGFR-TKIs treatment, up to 10% of tumors have transformed into SCLC[8,13]. These transformed tumor cells show similar molecular characteristics to SCLC, including the disappearance of TP53 and RB activity[14,15]. Interestingly, these transformed tumors still possess the original EGFR mutation, with a marked reduction in the expression of EGFR, which may suggest that the EGFR signaling pathway is no longer the driving force for the origination of these tumors[14].
In our case, the patient who had advanced lung adenocarcinoma with EGFR 19Del was treated with Gefitinib for a period of 10.0 mo and developed secondary drug resistance. Osimertinib (third-generation TKI) was administered as the second-line TKI treatment at the time of detection of plasma T790M. A dramatic clinical response was observed during treatment with Osimertinib. The main lesion in the right upper lung continued to progress but the metastatic lesions in both lungs significantly regressed. Pathology of the upper right lung lesion biopsy indicated SCLC transformation. The EGFR Exon19 mutation was persistent, while T790M mutation had disappeared as shown by NGS, accompanied by the concurrence of RB1 and TP53 mutations. The above findings suggest that tumor cells tend to successively develop target-dependent and target-independent drug resistance mechanisms due to tumor heterogeneity and TKI selection pressure.
Marcoux et al[12] recently reported the largest retrospective investigation comprising 58 patients bearing EGFR-mutant NSCLC that exhibited transformation into SCLC following one or more types of EGFR-TKIs treatments. The median overall survival from the time of diagnosis was found to be 31.5 mo (24.8 to 41.3 mo, 95%CI), whereas the median survival time from the initiation of SCLC transformation was 10.9 mo (8.0 to 13.7 mo, 95%CI). Following transformation, clinical behavior was identical to classical SCLC in many aspects, with responses to taxanes and platinum-etoposide except for immune checkpoint inhibitors. There is still insufficient evidence for a standard therapy for relapsed SCLC. The unique TKI, Anlotinib has a highly selective inhibitory impact on multiple targets, particularly on fibroblast growth factor receptor, stem cell growth factor receptor (c-Kit), vascular endothelial growth factor receptor, and platelet-derived growth factor receptors, and can inhibit tumor angiogenesis and tumor growth. The outcomes of the ALTER1202 study showed that compared to the placebo arm (4.1 mo vs 0.7 mo; HR = 0.19; 0.12-0.32; P < 0.0001, 95%CI), the median PFS in the Anlotinib arm was considerably prolonged, which should be considered as further-line treatment[16,17]. In our case, the response duration for this patient with PR was 5.0 mo, who underwent six cycles of etoposide plus carboplatin after SCLC transformation. The response to treatment for relapsed SCLC with Anlotinib in combination with docetaxel and carboplatin chemotherapy was more than 3.0 mo. Unfortunately, his disease responded less well to Nivolumab. The length of time from the initial diagnosis to transformation was almost 14.0 mo and the patient's overall survival duration was 24.0 mo.
We report a patient with sequential occurrence of multiple drug resistance after treatment with gefitinib. Comprehensive treatment regimens according to the drug resistance mechanism significantly improved the prognosis of this patient, including the EGFR-TKIs (Gefitinib, Osimertinib), chemotherapy, antiangiogenic drugs and immunotherapy. In summary, for the majority of patients with TKI-resistance, the resistance mechanisms are largely unknown. Repeated biopsies and application of the dynamic technique for multiple biopsies could disclose more comprehensive resistance mechanisms. Therefore, optimal therapy may be determined accordingly by increasing treatment efficacy and prolonging the duration of survival.
We thank the patient and his family for giving permission for his inclusion in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Haddadi S S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wu YXJ
| 1. | Chan BA, Hughes BG. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res. 2015;4:36-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 328] [Reference Citation Analysis (0)] |
| 2. | Westover D, Zugazagoitia J, Cho BC, Lovly CM, Paz-Ares L. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. 2018;29:i10-i19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 510] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 3. | Gao J, Li HR, Jin C, Jiang JH, Ding JY. Strategies to overcome acquired resistance to EGFR TKI in the treatment of non-small cell lung cancer. Clin Transl Oncol. 2019;21:1287-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 4. | Kim HR, Lee JC, Kim YC, Kim KS, Oh IJ, Lee SY, Jang TW, Lee MK, Shin KC, Lee GH, Ryu JS, Jang SH, Son JW, Lee JE, Kim SY, Kim HJ, Lee KY. Clinical characteristics of non-small cell lung cancer patients who experienced acquired resistance during gefitinib treatment. Lung Cancer. 2014;83:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Tan CS, Gilligan D, Pacey S. Treatment approaches for EGFR-inhibitor-resistant patients with non-small-cell lung cancer. Lancet Oncol. 2015;16:e447-e459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 311] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 6. | Stewart EL, Tan SZ, Liu G, Tsao MS. Known and putative mechanisms of resistance to EGFR targeted therapies in NSCLC patients with EGFR mutations-a review. Transl Lung Cancer Res. 2015;4:67-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 157] [Reference Citation Analysis (0)] |
| 7. | Morgillo F, Della Corte CM, Fasano M, Ciardiello F. Mechanisms of resistance to EGFR-targeted drugs: lung cancer. ESMO Open. 2016;1:e000060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 322] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 8. | Yu HA, Arcila ME, Rekhtman N, SimaCS, ZakowskiMF, PaoW, KrisMG, MillerVA, LadanyiM, RielyGJ. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19:2240-2247. [RCA] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 452] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 9. | Haber DA, Velculescu VE. Blood-based analyses of cancer: circulating tumor cells and circulating tumor DNA. Cancer Discov. 2014;4:650-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 555] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 10. | Sacher AG, Paweletz C, Dahlberg SE, Alden RS, O'Connell A, Feeney N, Mach SL, Jänne PA, Oxnard GR. Prospective Validation of Rapid Plasma Genotyping for the Detection of EGFR and KRAS Mutations in Advanced Lung Cancer. JAMA Oncol. 2016;2:1014-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 454] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 11. | Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald NQ, Butler A, Jones D, Raine K, Latimer C, Santos CR, Nohadani M, Eklund AC, Spencer-Dene B, Clark G, Pickering L, Stamp G, Gore M, Szallasi Z, Downward J, Futreal PA, Swanton C. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6102] [Cited by in RCA: 5940] [Article Influence: 456.9] [Reference Citation Analysis (0)] |
| 12. | Marcoux N, Gettinger SN, O'Kane G, Arbour KC, Neal JW, Husain H, Evans TL, Brahmer JR, Muzikansky A, Bonomi PD, Del Prete S, Wurtz A, Farago AF, Dias-Santagata D, Mino-Kenudson M, Reckamp KL, Yu HA, Wakelee HA, Shepherd FA, Piotrowska Z, Sequist LV. EGFR-Mutant Adenocarcinomas That Transform to Small-Cell Lung Cancer and Other Neuroendocrine Carcinomas: Clinical Outcomes. J Clin Oncol. 2019;37:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 344] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 13. | Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger S, Cosper AK, Akhavanfard S, Heist RS, Temel J, Christensen JG, Wain JC, Lynch TJ, Vernovsky K, Mark EJ, Lanuti M, Iafrate AJ, Mino-Kenudson M, Engelman JA. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2619] [Cited by in RCA: 2725] [Article Influence: 194.6] [Reference Citation Analysis (0)] |
| 14. | Niederst MJ, Sequist LV, Poirier JT, Mermel CH, Lockerman EL, Garcia AR, Katayama R, Costa C, Ross KN, Moran T, Howe E, Fulton LE, Mulvey HE, Bernardo LA, Mohamoud F, Miyoshi N, VanderLaan PA, Costa DB, Jänne PA, Borger DR, Ramaswamy S, Shioda T, Iafrate AJ, Getz G, Rudin CM, Mino-Kenudson M, Engelman JA. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun. 2015;6:6377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 376] [Cited by in RCA: 501] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 15. | Lee JK, Lee J, Kim S, Youk J, Park S, An Y, Keam B, Kim DW, Heo DS, Kim YT, Kim JS, Kim SH, Lee JS, Lee SH, Park K, Ku JL, Jeon YK, Chung DH, Park PJ, Kim J, Kim TM, Ju YS. Clonal History and Genetic Predictors of Transformation Into Small-Cell Carcinomas From Lung Adenocarcinomas. J Clin Oncol. 2017;35:3065-3074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 354] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 16. | Cheng Y, Wang Q, Li K, ShiJ, WuL, HanB, ChenG, HeJ, WangJ, QinH, LiX. Anlotinib as Third-Line or Further-Line Treatment in Relapsed SCLC: A Multicentre, Randomized, DoubleBlind Phase 2 Trial. J Thorac Oncol. 2018;13:S351-S352. [RCA] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Gao Y, Liu P, Shi R. Anlotinib as a molecular targeted therapy for tumors. Oncol Lett. 2020;20:1001-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |