Published online Mar 26, 2022. doi: 10.12998/wjcc.v10.i9.2811
Peer-review started: July 27, 2021
First decision: October 22, 2021
Revised: November 2, 2021
Accepted: February 19, 2022
Article in press: February 19, 2022
Published online: March 26, 2022
Processing time: 238 Days and 0.5 Hours
Mutations in the aggrecan (ACAN) gene are identified in patients with: spondyloepiphyseal dysplasia, Kimberley type; short stature with advanced bone age (BA); in the presence or absence of heterozygous ACAN mutation-induced early-onset osteoarthritis and/or osteochondritis dissecans; and spondyloepimetaphyseal dysplasia, ACAN type. Heterozygous mutations contribute to spondyloepiphyseal dysplasia, Kimberley type (MIM#608361), which is a milder skeletal dysplasia. In contrast, homozygous mutations cause a critical skeletal dysplasia, which is called spondyloepimetaphyseal dysplasia, ACAN type (MIM#612813). Lately, investigations on exome and genome sequencing have shown that ACAN mutations can also lead to idiopathic short stature with or without an advanced BA, in the presence or absence of early-onset osteoarthritis and/or osteochondritis dissecans (MIM#165800). We herein reported a heterozygous defect of ACAN in a family with autosomal dominant short stature, BA acceleration, and premature growth cessation.
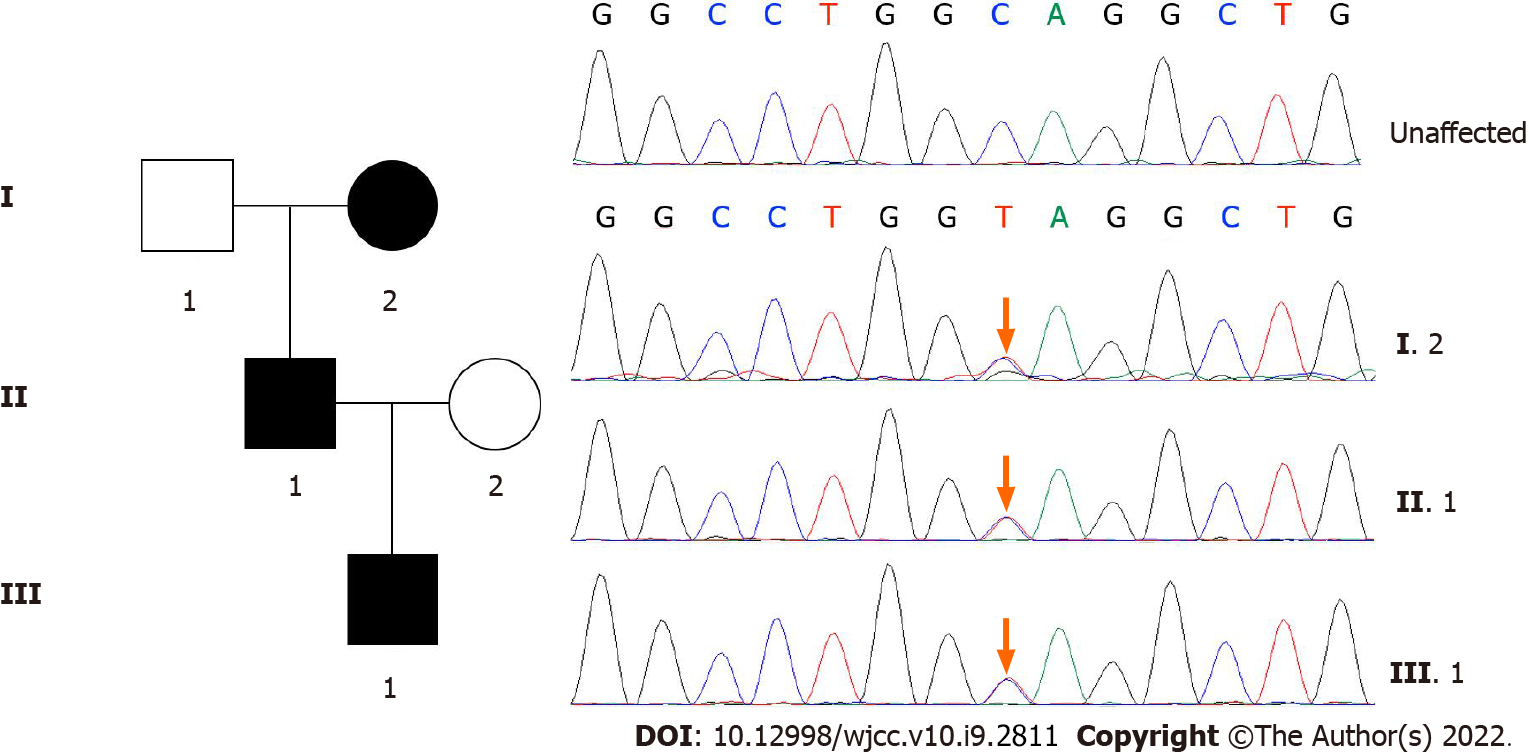
A 2-year-old male patient visited us due to growth retardation. The patient presented symmetrical short stature (height 79 cm, < -2 SD) without facial features and other congenital abnormalities. Whole-exome sequencing revealed a heterozygous pathogenic variant c. 871C>T (p. Gln291*) of ACAN, which was not yet reported in cases of short stature. This mutation was also detected in his father and paternal grandmother. According to the Human Gene Mutation Database, 67 ACAN mutations are registered. Most of these mutations are genetically inheritable, and very few children with short stature are associated with ACAN mutations. To date, heterozygous ACAN mutations have been reported in approximately 40 families worldwide, including a few individuals with a decelerated BA.
Heterozygous c. 871C>T (p. Gln291*) variation of the ACAN gene was the disease-causing variant in this family. Collectively, our newly discovered mutation expanded the spectrum of ACAN gene mutations.
Core Tip: Because of the diversity of clinical manifestations, phenotype overlap, and high genetic heterogeneity of short stature, the etiology of dwarfism cannot be determined by merely inquiring about the medical history, clinical performances, and routine laboratory examination. Gene detection can provide clear clinical diagnostic evidence, decrease the medical error and missed diagnosis of the disorder, instruct genetic counseling, and supply a trustworthy principle for fetal diagnosis. This case expanded the spectrum of aggrecan gene mutations.
- Citation: Yin LP, Zheng HX, Zhu H. Short stature associated with a novel mutation in the aggrecan gene: A case report and literature review. World J Clin Cases 2022; 10(9): 2811-2817
- URL: https://www.wjgnet.com/2307-8960/full/v10/i9/2811.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i9.2811
Children often visit pediatric endocrinologists because of their short stature. However, the clinical definition and therapeutic regimen of pediatric growth disorders have been significantly changed by recent advances in genetic methodology. Idiopathic short stature and advanced bone age (BA), in the presence or absence of heterozygous aggrecan (ACAN) mutation-induced early-onset osteoarthritis and/or osteochondritis dissecans exemplify these changes well. Herein, we presented the case and his affected members with symmetrical short stature, and a heterozygous variant of the ACAN gene was the disease-causing variant in this family.
A 2-year-old boy with growth retardation for over 1 year.
This boy was born at 40 wk of gestation following a common pregnancy and parturition. At birth, his weight was 2.75 kg, while there was no specific body length measurement. At 2 years of age, the patient visited us due to his short stature. His height was 79 cm (-2.7 SD), with a bodyweight of 10 kg, and his occipitofrontal circumference was 49 cm. No dysmorphic features were detected. His mental and motor development were normal.
The patient was not associated with any previous illness.
The height of his father and paternal grandmother was 152 cm (< -3.0 SD) and 138 cm (< -3.0 SD), respectively.
The physical test showed retarded growth (height, 79 cm; weight, 10 kg). He presented symmetrical short stature without facial features and other congenital abnormalities.
The peripheral blood was collected from the patient and his family members, followed by DNA extraction. Whole-exome sequencing was performed using an xGen Exam research panel v1.0 (IDT) on a HiSeq 4000 (Illumina). Any known disease associations were determined using the Online Mendelian Inheritance of Man (http://www.omim.org) database. A heterozygous mutation in ACAN (NM_013227.3) was identified in all affected individuals. This ACAN mutation was predicted to cause the resultant termination at codon 291 (c.871C>T; p.Gln291*) (Figure 1).
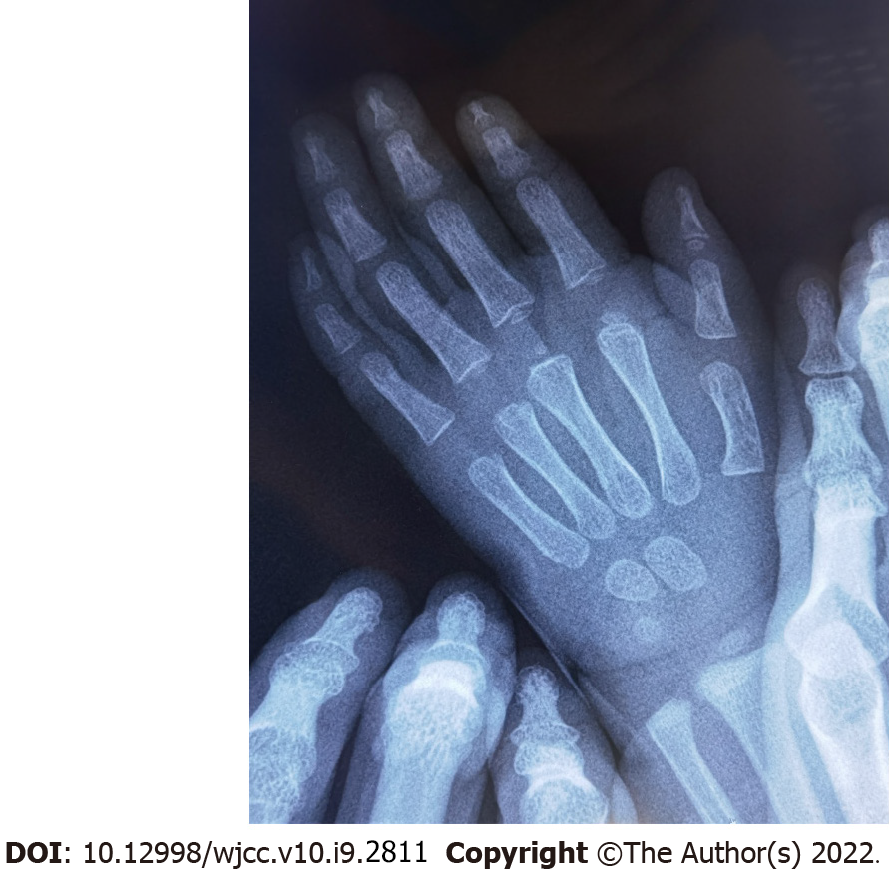
His BA was evaluated as 3 years and 6 mo to 4 years (Figure 2).
Short stature caused by ACAN mutation.
The patient received growth hormone (GH) treatment.
The height of the patient was increased by about 1 cm per month after GH treatment. During the treatment, no adverse events were recorded.
The ACAN gene encoding aggrecan is usually localized on chromosome 15q26[1,2]. Its full-length clone has been obtained by Doege et al[3]. The kernel protein of ACAN is composed of three disulfide-bonded globular domains (G1, G2, and G3) and intervening extended domains[4]. The interglobular domain is a protruding site for breaking proteins into smaller polypeptides or amino acids, and many proteinases can cleave between the G1 and G2 domains[5,6]. An extended GAG-attachment region separates the G2 and G3 domains, which is differentiated into three parts. The keratan sulfate (KS)-rich domain lies adjacent to the G2 domain. The KS-rich domain is likened to the chondroitin sulfate (CS)-rich domain, which is differentiated into two subdomains (CS1 and CS2), and the amino acid sequences of these two subdomains are different. The CS2 domain is connected to the G3 domain, which is located at the carboxy terminus of the core protein. The G3 region consists of two epidermal growth factor-like domains, one C-type lectin-like domain, and one complement regulatory protein-like domain[7]. The G3 region plays a fundamental role in the normal trafficking of ACAN within the chondrocytes, and such a region is also involved in the release of ACAN into the extracellular matrix[8]. In the extracellular matrix, the G3 domain is not detected in some ACAN molecules[9,10], which can probably be attributed to proteolytic cleavage.
There are 19 exons in the human ACAN gene[11]. The G1 region, interglobular domain, and G2 region are encoded by exons 3-6, exon 7, and exons 8-10, respectively. The GAG attachment region is encoded by exons 11 and 12, in which exon 11 encodes the first part of the KS-rich domain, and the large exon 12 encodes the remainder of the KS-rich domain as well as the CS1 and CS2 domains. The exons 13-19 encode the G3 region, exons 13 and 14 each encode an epidermal growth factor-like domain, exons 15-17 encode the lectin-like domain, and exon 18 encodes the complement regulatory protein-like domain.
ACAN is the main proteoglycan of the extracellular matrix of the growth plate cartilage[12]. Mutations in ACAN are associated with growth defects[13]. The research of Gleghorn et al[14] first reported an ACAN mutation that causes human disease. They have identified the heterozygosity for a 1-bp insertion in the ACAN gene with spondyloepiphyseal dysplasia, Kimberley type-affected members. This mutation can forecast the synthesis of a truncated protein that is about 60% of the normal size. The truncated protein lacks half of the CS1 domain, the complete CS2 domain, and the G3 domain, while it includes a novel sequence of 212 aa.
Watanabe et al[15] showed that heterozygotes have two obvious phenotypes: slight dwarfism and age-related hyperlordosis, the anterior concavity in the curvature of the spine. In the families analyzed by some studies[16-18], heterozygotes are detected in all affected members, who exhibit the clinical features of short stature and advanced BA. These data indicate that various pathogenic heterozygous ACAN variants (Table 1) affect the chondrogenesis of the growth plate in a similar pattern. Therefore, the growth plate chondrogenesis is impaired by functional haploinsufficiency of ACAN rather than various mutation-specific mechanisms. However, a dysfunctional C-type lectin domain in the ACAN protein leads to a more severe phenotype, impairing the functions of the growth plate and articular cartilage. All these studies provide a reasonable explanation for why those families have short stature but no evidence of early-onset osteoarthritis. The proband and affected members of our case also presented with autosomal dominant short stature and no indications of chondrodysplasia.
| cDNA | Variant |
| c.223T>C | p.Trp75Arg |
| c.273del | p.Arg93fs |
| c.1172del | p.Gly391fs |
| c.1227del | p.Ser410fs |
| c.1425del | p.Val478fs |
| c.1745del | p.Phe582fs |
| c.2026+1G>A | - |
| c.2541del | p.Val848fs |
| c.3758dup | p.Gly1254fs |
| c.4138G>T | p.Val1380Phe |
| c.4186del | p.Ser1396fs |
| c.4657G>T | p.Glu1553Ter |
| c.5061T>A | p.Ser1687Arg |
| c.5337del | p.Phe1780fs |
| c.5391del | p.Gln1798fs |
| c.5491_5500del | p.Phe1831fs |
| c.6534del | p.Thr2179fs |
| c.7178T>C | p.Leu2393Pro |
| c.7204C>T | p.Gln2402Ter |
| c.7255G>A | p.Asp2419Asn |
| c.7317G>A | p.Trp2439Ter |
| c.7363G>A | p.Val2455Met |
The combination of short stature and advanced BA is rare. Most known causative mutations either impair proteoglycan synthesis[19-21] or reduce signaling through the cAMP-protein kinase A signaling pathway[22-24].
In the clinical studies of ACAN patients, the length in the lower part of most heterozygous carriers of ACAN variants show a normal range at birth, while some of them are born short for gestational age[25]. Some researchers have suggested that individuals treated with GH have an improvement in adult height. In addition to GH treatment, some patients also simultaneously receive treatment with gonadotropin-releasing hormone analogue. This treatment can be given to patients after the administration of an aromatase inhibitor, which can successfully postpone bone maturation, and such therapy can benefit those carrying confirmed ACAN mutations[26-31].
In the present study, a heterozygous mutation in the ACAN gene was identified in a Chinese family with short stature. We hypothesized that this mutation could induce early truncation of the ACAN protein. Genetic testing is important for diagnosis and treatment.
We gratefully acknowledge the kind cooperation of the patient, the family members, and the staff from the unit for their assistance in conducting this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Genetics and heredity
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Scicchitano P S-Editor: Liu JH L-Editor: Filipodia P-Editor: Liu JH
| 1. | Korenberg JR, Chen XN, Doege K, Grover J, Roughley PJ. Assignment of the human aggrecan gene (AGC1) to 15q26 using fluorescence in situ hybridization analysis. Genomics. 1993;16:546-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Just W, Klett C, Vetter U, Vogel W. Assignment of the human aggrecan gene AGC1 to 15q25-->q26.2 by in situ hybridization. Hum Genet. 1993;92:516-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Doege KJ, Sasaki M, Kimura T, Yamada Y. Complete coding sequence and deduced primary structure of the human cartilage large aggregating proteoglycan, aggrecan. Human-specific repeats, and additional alternatively spliced forms. J Biol Chem. 1991;266:894-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 290] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Kiani C, Chen L, Wu YJ, Yee AJ, Yang BB. Structure and function of aggrecan. Cell Res. 2002;12:19-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 502] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 5. | Fosang AJ, Hardingham TE. Isolation of the N-terminal globular protein domains from cartilage proteoglycans. Identification of G2 domain and its lack of interaction with hyaluronate and link protein. Biochem J. 1989;261:801-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 84] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Paulsson M, Yurchenco PD, Ruben GC, Engel J, Timpl R. Structure of low density heparan sulfate proteoglycan isolated from a mouse tumor basement membrane. J Mol Biol. 1987;197:297-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 124] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Day JM, Olin AI, Murdoch AD, Canfield A, Sasaki T, Timpl R, Hardingham TE, Aspberg A. Alternative splicing in the aggrecan G3 domain influences binding interactions with tenascin-C and other extracellular matrix proteins. J Biol Chem. 2004;279:12511-12518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Aspberg A. The different roles of aggrecan interaction domains. J Histochem Cytochem. 2012;60:987-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Dennis JE, Carrino DA, Schwartz NB, Caplan AI. Ultrastructural characterization of embryonic chick cartilage proteoglycan core protein and the mapping of a monoclonal antibody epitope. J Biol Chem. 1990;265:12098-12103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Wiedemann H, Paulsson M, Timpl R, Engel J, Heinegård D. Domain structure of cartilage proteoglycans revealed by rotary shadowing of intact and fragmented molecules. Biochem J. 1984;224:331-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Valhmu WB, Palmer GD, Rivers PA, Ebara S, Cheng JF, Fischer S, Ratcliffe A. Structure of the human aggrecan gene: exon-intron organization and association with the protein domains. Biochem J. 1995;309 ( Pt 2):535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Lauing KL, Cortes M, Domowicz MS, Henry JG, Baria AT, Schwartz NB. Aggrecan is required for growth plate cytoarchitecture and differentiation. Dev Biol. 2014;396:224-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Gibson BG, Briggs MD. The aggrecanopathies; an evolving phenotypic spectrum of human genetic skeletal diseases. Orphanet J Rare Dis. 2016;11:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Gleghorn L, Ramesar R, Beighton P, Wallis G. A mutation in the variable repeat region of the aggrecan gene (AGC1) causes a form of spondyloepiphyseal dysplasia associated with severe, premature osteoarthritis. Am J Hum Genet. 2005;77:484-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Watanabe H, Nakata K, Kimata K, Nakanishi I, Yamada Y. Dwarfism and age-associated spinal degeneration of heterozygote cmd mice defective in aggrecan. Proc Natl Acad Sci U S A. 1997;94:6943-6947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 111] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Stattin EL, Wiklund F, Lindblom K, Onnerfjord P, Jonsson BA, Tegner Y, Sasaki T, Struglics A, Lohmander S, Dahl N, Heinegård D, Aspberg A. A missense mutation in the aggrecan C-type lectin domain disrupts extracellular matrix interactions and causes dominant familial osteochondritis dissecans. Am J Hum Genet. 2010;86:126-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Nilsson O, Guo MH, Dunbar N, Popovic J, Flynn D, Jacobsen C, Lui JC, Hirschhorn JN, Baron J, Dauber A. Short stature, accelerated bone maturation, and early growth cessation due to heterozygous aggrecan mutations. J Clin Endocrinol Metab. 2014;99:E1510-E1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 18. | Quintos JB, Guo MH, Dauber A. Idiopathic short stature due to novel heterozygous mutation of the aggrecan gene. J Pediatr Endocrinol Metab. 2015;28:927-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Kim OH, Nishimura G, Song HR, Matsui Y, Sakazume S, Yamada M, Narumi Y, Alanay Y, Unger S, Cho TJ, Park SS, Ikegawa S, Meinecke P, Superti-Furga A. A variant of Desbuquois dysplasia characterized by advanced carpal bone age, short metacarpals, and elongated phalanges: report of seven cases. Am J Med Genet A. 2010;152A:875-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Nizon M, Huber C, De Leonardis F, Merrina R, Forlino A, Fradin M, Tuysuz B, Abu-Libdeh BY, Alanay Y, Albrecht B, Al-Gazali L, Basaran SY, Clayton-Smith J, Désir J, Gill H, Greally MT, Koparir E, van Maarle MC, MacKay S, Mortier G, Morton J, Sillence D, Vilain C, Young I, Zerres K, Le Merrer M, Munnich A, Le Goff C, Rossi A, Cormier-Daire V. Further delineation of CANT1 phenotypic spectrum and demonstration of its role in proteoglycan synthesis. Hum Mutat. 2012;33:1261-1266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Bui C, Huber C, Tuysuz B, Alanay Y, Bole-Feysot C, Leroy JG, Mortier G, Nitschke P, Munnich A, Cormier-Daire V. XYLT1 mutations in Desbuquois dysplasia type 2. Am J Hum Genet. 2014;94:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 22. | Mantovani G, Ferrante E, Giavoli C, Linglart A, Cappa M, Cisternino M, Maghnie M, Ghizzoni L, de Sanctis L, Lania AG, Beck-Peccoz P, Spada A. Recombinant human GH replacement therapy in children with pseudohypoparathyroidism type Ia: first study on the effect on growth. J Clin Endocrinol Metab. 2010;95:5011-5017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Linglart A, Menguy C, Couvineau A, Auzan C, Gunes Y, Cancel M, Motte E, Pinto G, Chanson P, Bougnères P, Clauser E, Silve C. Recurrent PRKAR1A mutation in acrodysostosis with hormone resistance. N Engl J Med. 2011;364:2218-2226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 24. | Lindstrand A, Grigelioniene G, Nilsson D, Pettersson M, Hofmeister W, Anderlid BM, Kant SG, Ruivenkamp CA, Gustavsson P, Valta H, Geiberger S, Topa A, Lagerstedt-Robinson K, Taylan F, Wincent J, Laurell T, Pekkinen M, Nordenskjöld M, Mäkitie O, Nordgren A. Different mutations in PDE4D associated with developmental disorders with mirror phenotypes. J Med Genet. 2014;51:45-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Hauer NN, Sticht H, Boppudi S, Büttner C, Kraus C, Trautmann U, Zenker M, Zweier C, Wiesener A, Jamra RA, Wieczorek D, Kelkel J, Jung AM, Uebe S, Ekici AB, Rohrer T, Reis A, Dörr HG, Thiel CT. Genetic screening confirms heterozygous mutations in ACAN as a major cause of idiopathic short stature. Sci Rep. 2017;7:12225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Hu X, Gui B, Su J, Li H, Li N, Yu T, Zhang Q, Xu Y, Li G, Chen Y, Qing Y; Chinese Genetic Short Stature Consortium, Li C, Luo J, Fan X, Ding Y, Li J, Wang J, Wang X, Chen S, Shen Y. Novel pathogenic ACAN variants in non-syndromic short stature patients. Clin Chim Acta. 2017;469:126-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | van der Steen M, Pfundt R, Maas SJWH, Bakker-van Waarde WM, Odink RJ, Hokken-Koelega ACS. ACAN Gene Mutations in Short Children Born SGA and Response to Growth Hormone Treatment. J Clin Endocrinol Metab. 2017;102:1458-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 28. | Crippa M, Giangiobbe S, Villa R, Bestetti I, De Filippis T, Fatti L, Taurino J, Larizza L, Persani L, Bellini F, Finelli P, Bonati MT. A balanced reciprocal translocation t(10;15)(q22.3;q26.1) interrupting ACAN gene in a family with proportionate short stature. J Endocrinol Invest. 2018;41:929-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Gkourogianni A, Andrew M, Tyzinski L, Crocker M, Douglas J, Dunbar N, Fairchild J, Funari MF, Heath KE, Jorge AA, Kurtzman T, LaFranchi S, Lalani S, Lebl J, Lin Y, Los E, Newbern D, Nowak C, Olson M, Popovic J, Pruhová Š, Elblova L, Quintos JB, Segerlund E, Sentchordi L, Shinawi M, Stattin EL, Swartz J, Angel AG, Cuéllar SD, Hosono H, Sanchez-Lara PA, Hwa V, Baron J, Nilsson O, Dauber A. Clinical Characterization of Patients With Autosomal Dominant Short Stature due to Aggrecan Mutations. J Clin Endocrinol Metab. 2017;102:460-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 30. | Stavber L, Hovnik T, Kotnik P, Lovrečić L, Kovač J, Tesovnik T, Bertok S, Dovč K, Debeljak M, Battelino T, Avbelj Stefanija M. High frequency of pathogenic ACAN variants including an intragenic deletion in selected individuals with short stature. Eur J Endocrinol. 2020;182:243-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Dateki S, Nakatomi A, Watanabe S, Shimizu H, Inoue Y, Baba H, Yoshiura KI, Moriuchi H. Identification of a novel heterozygous mutation of the Aggrecan gene in a family with idiopathic short stature and multiple intervertebral disc herniation. J Hum Genet. 2017;62:717-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |