Published online Mar 26, 2022. doi: 10.12998/wjcc.v10.i9.2773
Peer-review started: October 19, 2021
First decision: December 10, 2021
Revised: December 24, 2021
Accepted: January 29, 2022
Article in press: January 29, 2022
Published online: March 26, 2022
Processing time: 154 Days and 8.5 Hours
Sedation during endoscopic ultrasonography (EUS) poses many challenges and moderate-to-deep sedation are often required. The conventional method to preform moderate-to-deep sedation is generally intravenous benzodiazepine alone or in combination with opioids. However, this combination has some limitations. Intranasal medication delivery may be an alternative to this sedation regimen.
To determine, by continual reassessment method (CRM), the minimal effective dose of intranasal sufentanil (SUF) when combined with intranasal dexmede
Thirty patients aged 18-65 and scheduled for EUS were recruited in this study. Subjects received intranasal DEX and SUF for sedation. The dose of DEX (1 μg/kg) was fixed, while the dose of SUF was assigned sequentially to the subjects using CRM to determine ED95. The sedation status was assessed by modified observer’s assessment of alertness/sedation (MOAA/S) score. The adverse events and the satisfaction scores of patients and endoscopists were recorded.
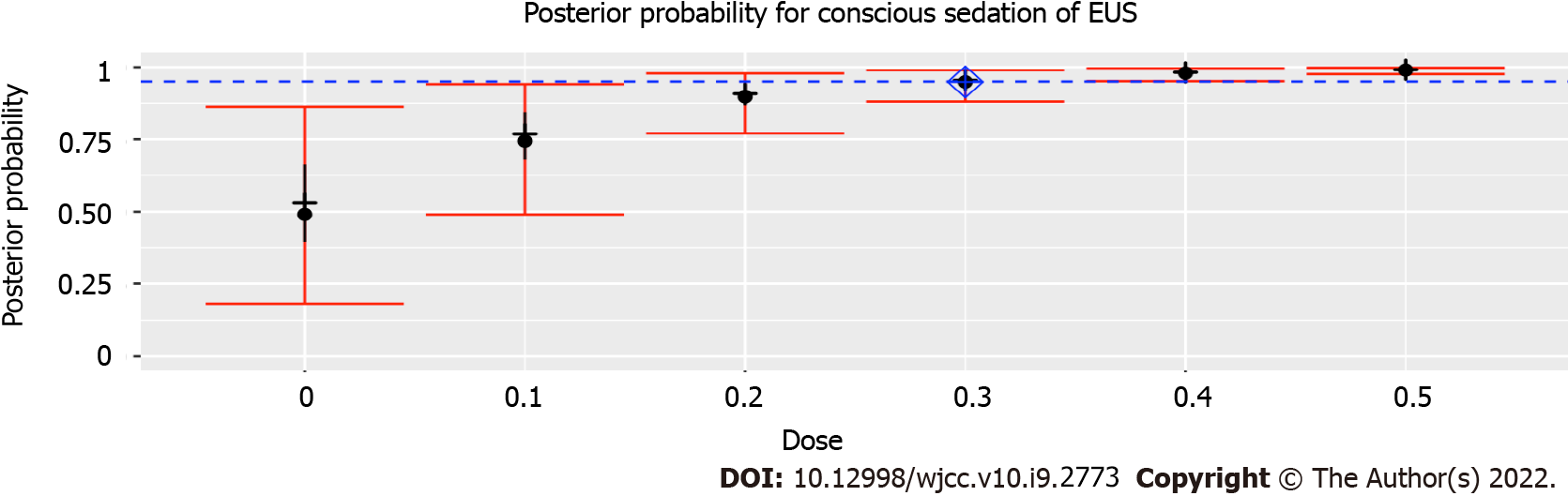
The ED95 was intranasal 0.3 μg/kg SUF when combined with intranasal 1 μg/kg DEX, with an estimated probability of successful moderate sedation for EUS of 94.9% (95% confidence interval: 88.1%-98.9%). When combined with intranasal 1 μg/kg DEX, probabilities of successful moderate sedation at each dose level of intranasal SUF were as follows: 0 μg/kg SUF, 52.8%; 0.1 μg/kg SUF, 75.4%; 0.2 μg/kg SUF, 89.9%; 0.3 μg/kg SUF, 94.9%; 0.4 μg/kg SUF, 98.0%; 0.5 μg/kg SUF, 99.0%.
The ED95 needed for moderate sedation for EUS is intranasal 0.3 μg/kg SUF when combined with intranasal 1 μg/kg DEX, based on CRM.
Core Tip: The conventional method to preform moderate-to-deep sedation is generally intravenous benzodiazepine alone or in combination with opioids. There are many limitations to this method, including an inability for patients to tolerate it due to the possible longevity of endoscopic ultrasonography (EUS) procedures. This study aimed to provide an effective method for moderate-to-deep sedation in EUS via an intranasal approach. We believe that our study makes a significant contribution to the literature because it provides an alternative to intravenous administration by administering intranasal 1 μg/kg DEX in combination with 0.3 μg/kg sufentanil to patients scheduled for EUS.
- Citation: Zou Y, Li N, Shao LJZ, Liu FK, Xue FS, Tao X. Determination of the ED95 of intranasal sufentanil combined with intranasal dexmedetomidine for moderate sedation during endoscopic ultrasonography. World J Clin Cases 2022; 10(9): 2773-2782
- URL: https://www.wjgnet.com/2307-8960/full/v10/i9/2773.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i9.2773
Sedation during endoscopic ultrasonography (EUS) poses many challenges. First, EUS is an advanced endoscopic procedure that generally requires moderate-to-deep sedation due to the large size of the echoendoscope. Second, EUS is a time-consuming procedure that takes longer time than routine endoscopy. Third, because of the properties of ultrasound, a disposable latex balloon or injection of 200-500 of water is needed to provide a liquid interface between the ultrasound probe and the tissue. The injection of a large amount of water increases the risk of pulmonary aspiration. Thus, the best sedation regimen for this procedure is still under debate.
Moderate-to-deep sedation using the conventional method with intravenous benzodiazepine alone or in combination with opioids is usually performed for EUS. However, this regimen cannot be tolerated well by patients due to potentially long duration of the EUS procedure. Intranasal medication delivery is an innovative approach with a more gradual onset and fewer side effects than intravenous administration. Intranasal administration of sufentanil (SUF) offers several potential advantages, including high levels of acceptability, rapid onset, and good bioavailability[1]. Therefore, it is widely used in many fields. Midazolam is a sedative agent that is used commonly, but induces a burning unpleasant sensation and mucosal irritation when applied intranasally[2,3]. Intranasal dexmedetomidine (DEX) is an effective and safe alternative to midazolam as it provides a consistent level of sedation for long procedures.
Therefore, to provide an innovative and effective approach to identify the optimal sedation for EUS, this study used a continual reassessment method (CRM) to determine the minimal effective dose of intranasal SUF when combined with intranasal DEX for moderate sedation in at least 95% of patients (ED95).
Prior to participant enrolment, the study was approved by the Bioethics Committee of Beijing Friendship Hospital, Capital Medical University (Ethics Committee number: 2018-P2-164-02), and registered with the Chinese Clinical Trial Registry (registration number: ChiCTR1800019273). Written informed consent was obtained from each subject prior to enrolment.
Male and female patients, aged 18 to 65 years, with an American Society of Anesthesiologists (ASA) physical status of I to III, body mass index (BMI) of 18 to 25 kg/m2, and scheduled for EUS were enrolled. Patients with nasal diseases (such as status of post nasal surgery, rhinitis, nasal polyposis), heart rate (HR) < 50 beat per minute (bpm), third-degree heart block, history of allergy to DEX and SUF, blood pressure (BP) > 180/110 mmHg, acute myocardial infarction in the past 12 wk, unstable angina, history of digestive tract surgery, digestive malformation, respiratory rate (RR) ≤ 10 /min, Mallampati classification of 3 or 4, or pre-existing hypoxia (oxygen saturation < 90%) were excluded.
Heart rate, peripheral oxygen saturation (SpO2), and BP were noninvasively monitored throughout the study by an anesthesiologist. An oxygen flow of 2 L/min was continuously delivered through a nasal cannula. A total dose of 1 μg/kg DEX (undiluted parenteral solution, 100 μg/mL, Yangtze River Pharmaceutical Co., Ltd, China) was administered, divided equally over both nostrils using a mucosal atomization device (LMA MAD NasalTM, Teleflex Medical Europe Ltd., Dublin, Ireland) 45 min before the EUS. After a 25 min interval, SUF (undiluted parenteral solution, 50 μg/mL, Yichang Humanwell Pharmaceutical CO., Ltd., China) was administrated, divided equally over both nostrils. The tip of an MAD NasalTM device was placed against the nostril with an administration angle > 60° and slight head tilt, and the syringe plunger (1 mL LS 25 GA 5/8 IN 0.5 mm × 16 mm, Becton Dickinson Medical Pte. Ltd., Singapore) was briskly compressed to deliver SUF and DEX into both nostrils[2]. All intranasal drug administrations were performed by an anesthesiologist. Forty-five min after the administration of intranasal DEX, the EUS procedure was performed by a single endoscopist. After the procedure, patients were transferred to the post-anesthesia care unit (PACU) until they met the discharge criteria. Each patient received a venous cannulation for safety before the application of DEX and SUF. In case of oversedation, intravenous naloxone was used to reverse SUF oversedation. As all recruited patients were originally assigned for EUS without sedation, no rescue dose was administered if adequate sedation was not achieved.
Sedation status was assessed from the application of DEX until discharge by an independent observer every 5 min with Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) score. The MOAA/S score[4] was developed to assist in determining the level of sedation. The sedation scoring was as follows: 5 points, conscious; 4 points, minimal sedation; 2-3 points, moderate (conscious) sedation; 1 point, deep sedation; 0 point, general anesthesia[5]. Responsiveness scores of MOAA/S are shown in Table 1[4,6]. Successful moderate sedation was defined as a score ≤ 3 on the MOAA/S scale, as assessed by the observer, even when there were fluctuations in sedation levels caused by repeated stimulations or elimination of stimulations.
| Responsiveness | Score |
| Responds readily to name spoken in normal tone | 5 |
| Lethargic response to name spoken in normal tone | 4 |
| Responds only after name is called loudly and/or repeatedly | 3 |
| Responds only after mild prodding or shaking | 2 |
| Responds only after painful trapezius squeeze | 1 |
| Does not respond to painful trapezius squeeze | 0 |
Satisfaction was evaluated according to a numerical rating scale (NRS), with scores ranging from 1 (extremely dissatisfied) to 10 (extremely satisfied). The endoscopist’s satisfaction was measured at the end of the procedure, and patient satisfaction was assessed before discharge. Mucosal irritation, clinically significant respiratory depression (defined as a respiratory rate of < 8 or an oxygen saturation of < 90%[7,8]), hypotension, nausea and vomiting, and any other adverse events were recorded by the observer.
All drugs were prepared by a nurse who was not involved in the drug administration or observation. The observers, the endoscopist, and patients were blinded to the treatment (dose).
CRM was used to estimate the minimal effective dose of intranasal SUF when combined with intranasal DEX needed for moderate sedation in at least 95% of patients (ED95) scheduled for EUS. Similar to other studies[9,10] performed in the absence of previous dose-finding reports, the prior probability of moderate sedation for intranasal DEX and SUF was assessed at six doses of 0.50, 0.75, 0.90, 0.95, 0.98, and 0.99 for SUF and 0, 0.1, 0.2, 0.3, 0.4, and 0.5 μg/kg for DEX, based on previous experience and previous reports (Table 2)[2,11]. Three patients were recruited per cohort at any given dose. The DEX dose (1 μg/kg) was then fixed. The starting dose of SUF was 0.3 μg/kg, corresponding to the closest to 0.95 prior probability. In the present study, intranasal SUF combined with intranasal DEX dose response was defined as a binary outcome (successful moderate sedation = 1, unsuccessful moderate sedation = 0). Response was considered successful if MOAA/S score was ≤ 3. The probabilities were re-evaluated based on the responses from all previous cohorts. Subsequent doses were those with an updated posterior probability of response closest to the target rate of 95%. The CRM continued until the planned total sample size of 30 patients was reached, when the posterior response probability was either too low or too high for all doses, or when a reliable estimation of the ED95 was obtained[12].
| Dose level, μg/kg | Response probability |
| 0 | 0.5 |
| 0.1 | 0.75 |
| 0.2 | 0.9 |
| 0.3 | 0.95 |
| 0.4 | 0.98 |
| 0.5 | 0.99 |
The dose-finding allocation was performed using the dose-finding allocation using the bcrm package (http://www.jstatsoft.org/v54/i13/) in R software version 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria). We used a one-parameter working model to create a dose-response relationship. Trials can be conducted interactively, or operating characteristics can be obtained using the built-in simulation code[13]. Continuous values are presented as mean (standard deviation, SD) or median (range). Data were analyzed using SPSS 26.0 (IBM, Chicago, IL, United States), and the threshold of significance was set at P < 0.05.
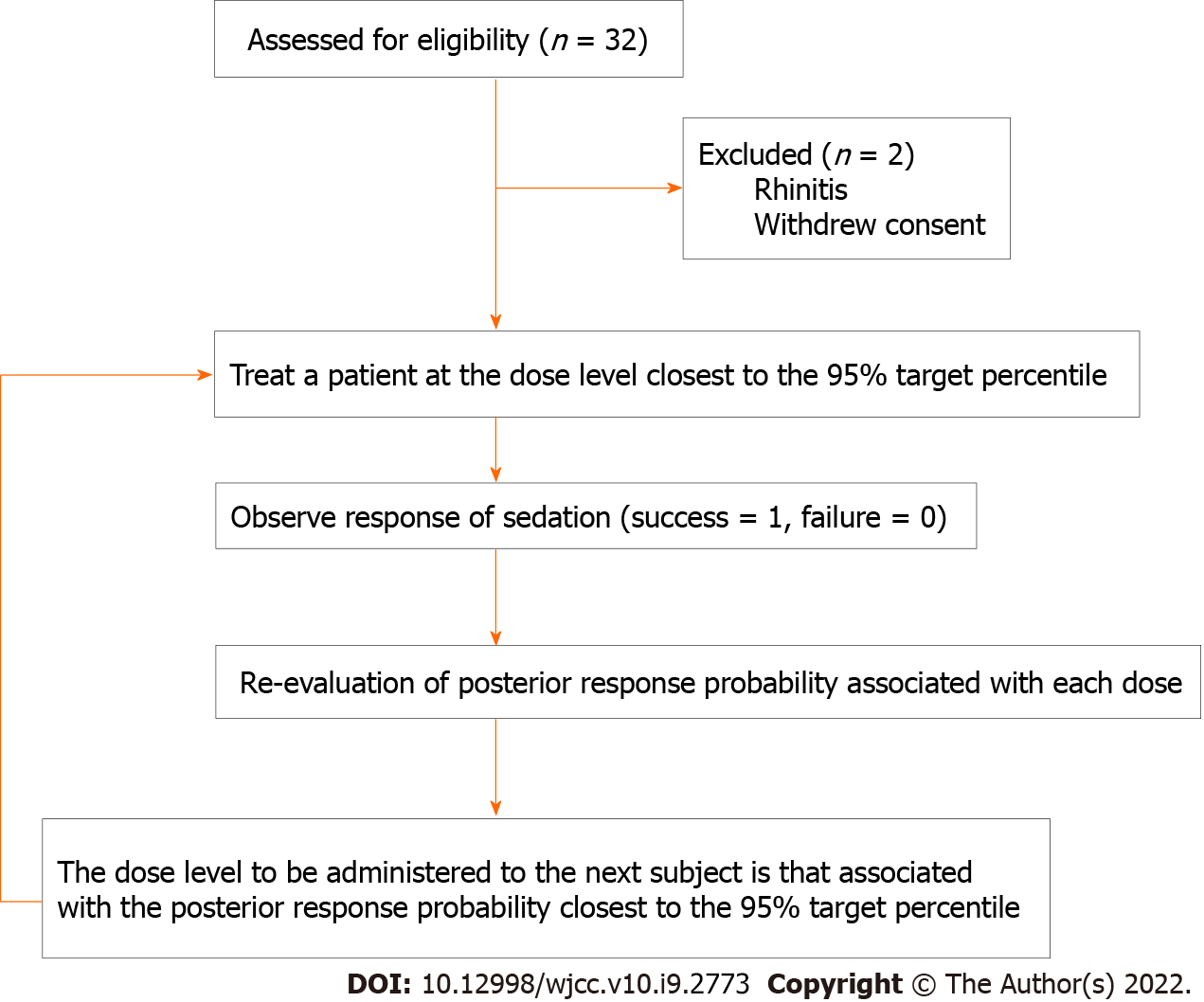
Patients were enrolled from September 1 to December 2, 2020. We screened 32 patients for inclusion in this study. A total of 30 patients were recruited, and no patients were excluded after dose allocation. Patient characteristics and EUS durations are shown in Table 3. A schematic representation of the CRM applied and subjects’ flow through the trial is shown in Figure 1.
| Parameter | Value |
| Age (yr) | 42 (26-59) |
| Height (cm) | 167.77 (7.58) |
| Weight (kg) | 66.00 (9.64) |
| BMI (kg/m2) | 23.46 (2.99) |
| Gender (F/M) | 15/15 |
| ASA-I/II | 21/9 |
| Baseline heart rate (per min) | 75.80 (12.10) |
| Baseline SpO2 (%) | 99 (96-100) |
| Procedure duration (min) | 29.80 (3.63) |
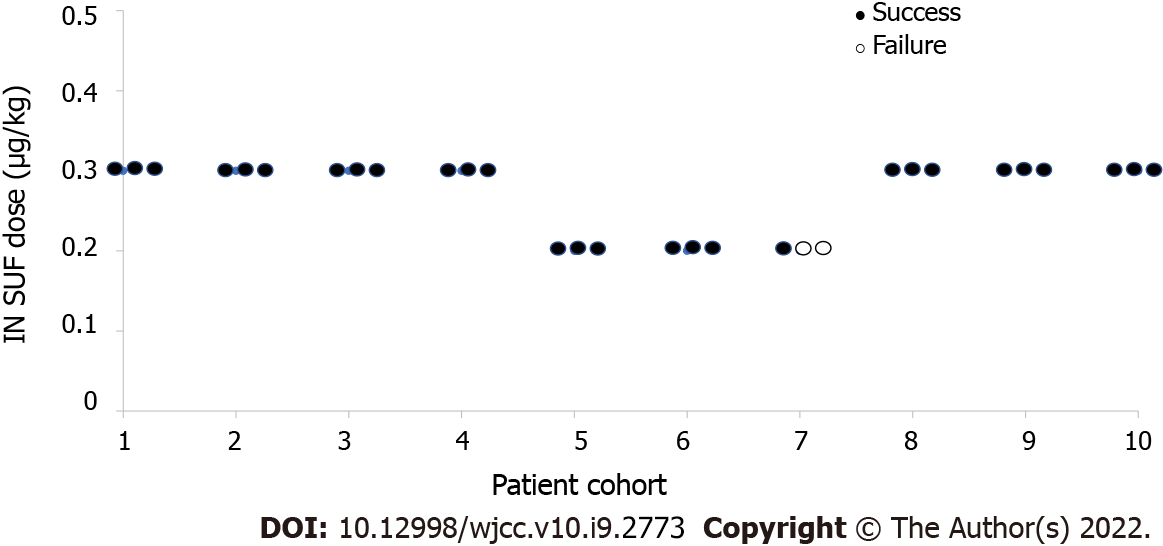
The prior probabilities of dose levels (Table 2) suggested a starting dose of 0.3 μg/kg SUF. This study yielded 28 of 30 successful sedatives. The dose level changed a total of two times throughout this trial (Figure 2). There was no failure of moderate sedation in the dose level of 0.3 μg/kg SUF. Due to the high rate of successful sedation with the 0.3 μg/kg SUF dose, the CRM never recommended higher doses. Thus, the 0.4 and 0.5 μg/kg SUF doses were never tested. Two unsuccessful sedations occurred at dose level of 0.2 μg/kg SUF. As a result of two failures encountered with the 0.2 μg/kg SUF dose, lower doses were not recommended. The dose closest to the ED95 was 0.3 μg/kg SUF, with an estimated success probability of 94.9% (95% credibility interval: 88.1%-98.9%; Figure 3). When combined with intranasal dose of 1 μg/kg DEX, the probability of successful moderate sedation at each dose level was as follows: 0 μg/kg SUF, 52.8%; 0.1 μg/kg SUF, 75.4%; 0.2 μg/kg SUF, 89.9%; 0.3 μg/kg SUF, 94.9%; 0.4 μg/kg SUF, 98.0%; 0.5 μg/kg SUF, 99.0% (Table 4).
| Patients | Allocated SUF dose, μg/kg | Clinical response(Success 1, Failure 0) | SUF dose, μg/kg | |||||
| 0 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | |||
| Prior estimated probability for each dose (%) | ||||||||
| 50 | 75 | 90 | 95 | 98 | 99 | |||
| Posterior probability for each dose (%) | ||||||||
| 1-3 | 0.3 | 1,1,1 | 62.5 | 80.0 | 91.6 | 95.7 | 98.3 | 99.1 |
| 4-6 | 0.3 | 1,1,1 | 65.4 | 82.0 | 92.5 | 96.2 | 98.5 | 99.2 |
| 7-9 | 0.3 | 1,1,1 | 67.8 | 83.6 | 93.3 | 96.6 | 98.6 | 99.3 |
| 10-12 | 0.3 | 1,1,1 | 70.0 | 84.9 | 93.9 | 96.9 | 98.8 | 99.4 |
| 13-15 | 0.2 | 1,1,1 | 73.6 | 87.0 | 94.8 | 97.4 | 99.0 | 99.5 |
| 16-18 | 0.2 | 1,1,1 | 76.4 | 88.7 | 95.5 | 97.8 | 99.1 | 99.6 |
| 19-21 | 0.2 | 1,0,0 | 47.4 | 71.7 | 88.2 | 94.0 | 97.6 | 98.8 |
| 22-24 | 0.3 | 1,1,1 | 49.3 | 73.1 | 88.8 | 94.3 | 97.7 | 98.9 |
| 25-27 | 0.3 | 1,1,1 | 51.1 | 74.3 | 89.4 | 94.6 | 97.8 | 98.9 |
| 28-30 | 0.3 | 1,1,1 | 52.8 | 75.4 | 89.9 | 94.9 | 98.0 | 99.0 |
In general, the patients and endoscopist were satisfied with used sedation regimen. The patients’ mean satisfaction ratings were 9.0 ± 1.1 and 8.1 ± 1.0 with doses of 0.2 μg/kg and 0.3 μg/kg SUF (P = 0.052), respectively, and over 90% of the ratings were greater than 7. Endoscopist’s satisfaction scores were 8.7 ± 0.9 and 8.5 ± 1.3 with doses of 0.2 μg/kg and 0.3 μg/kg SUF (P = 0.654), respectively (Table 5).
| SUF dose, μg/kg | P value | ||
| 0.2 (n = 9) | 0.3 (n = 21) | ||
| Hypotension, n | 0 | 1 | |
| Nausea and vomiting, n | 1 | 2 | |
| Local mucosal irritation, n | 1 | 1 | |
| Endoscopist’s satisfaction, mean (SD) | 8.7 (0.9) | 8.5 (1.3) | 0.654 |
| Patients’ satisfactions, mean (SD) | 9.0 (1.1) | 8.1 (1.0) | 0.052 |
The numbers of patients who experienced hypotension, mucosal irritation, and nausea and vomiting are shown in Table 5. Hypotension occurred in one patient after she was transferred to the PACU, and a single 6-mg dose of ephedrine had to be administrated for management. No further hypotension or other adverse events were observed during the study period. Nausea and vomiting occurred in three patients with motion sickness or a history of postoperative nausea and vomiting (PONV). Two patients experienced mucosal irritation. However, it was tolerable. No patient experienced oversedation in this study. No bradycardia or conduction abnormalities on electrocardiographic monitoring or hypoxemia were observed in this trial. No EUS was interrupted due to side effects of this sedation regimen.
This study has used CRM to determine the ED95 of intranasal SUF + DEX for achieving moderate sedation during EUS. Obtained results have showed an ED95 of 0.3 μg/kg SUF + 1 μg/kg DEX. In this study, moderate sedation was chosen as the patient maintained ventilatory function and was able to elicit purposeful responses to tactile or verbal stimulation[14]. Intranasal administration is emerging as a promising method for delivering medications. The intranasal route has been associated with stable hemodynamics compared to the intravenous route due to a slower and more gradual onset[15]. Intranasal DEX is associated with a bioavailability of 40.6%-65%, an onset of 45-60 min, and a peak sedation effect occurring at 90-105 min[15,16]. In addition, the duration of sedation is similar for intravenous and intranasal DEX at 1 μg/kg[15], which allowed completion of EUS procedures that lasted 29.8 min on average in our study. Intravenous infusion of DEX requires an initial use of a loading dose, which may be associated with a delay in recovery and blood pressure or heart rate issues. A dose of 1 μg/kg DEX administered intranasally produced a modest decrease in blood pressure and heart rate[17].
However, intranasal DEX alone may not induce sufficient sedation and analgesia for some advanced endoscopic procedures, such as EUS. It was demonstrated that 30-45 min after intranasal administration of 1 μg/kg DEX alone, the mean MOAA/S score was 3.526[17]. DEX in combination with a potent intranasal opioid offers the potential for superior efficacy of sedation with only one agent increasing the risk of respiratory depression. SUF, a potent opioid analgesic, can be easily absorbed from the nasal mucosa with a rapid onset of 20 min, excellent bioavailability of 78%, and stable hemodynamics and arterial oxygen tension[1,18].
In our study, we chose DEX at a fixed dose of 1 μg/kg and the dose of SUF was determined by CRM. CRM is a model-based design with potential advantages over traditional rule-based designs such as the 3 + 3 design. The advantages of CRM include a small number of required subjects, possible double-blind evaluation, no placebo group necessary, and allowing the best candidate dose to be administered to each subsequent patient[19,20].
DEX was administrated approximately 45 min prior to the commencement of the procedure, as the onset of action of 1 μg/kg intranasal DEX was 47.5 min (95%CI: 25-135 min)[15]. Since the onset of intranasal SUF was 20 min, SUF was administrated approximately 25 min after the delivery of DEX[1]. The ideal volume for absorption into the nasal cavity was 0.5 mL or less in each nare[21]. In our study, the maximum volume of DEX or SUF was less than 1 mL, and the interval between the two drugs was 25 min, which ensured the absorption. None of the patients complained of an unpleasant taste with intranasal DEX and SUF, which may indicate that no drug excess got into the oral cavity.
Three patients in our study experienced nausea and vomiting, although no patients had nausea in other trials[11,17]. This difference is likely due to several reasons. First, one of the three patients who experienced nausea and vomiting had a history of motion sickness, and the other two patients had experienced PONV. Motion sickness and PONV are both predisposing factors for nausea and vomiting[22]. Second, while no such adverse events have been reported in adults with intranasal 1 μg/kg DEX alone[17], our study combined intranasal 1 μg/kg DEX with intranasal SUF. The use of opioids is a well-known risk factor for PONV[23], and nausea and vomiting have been reported in patients with intranasal SUF[24,25]. Third, the populations studied in this and previous studies were different. In a study of pediatric patients aged 3-7 years, nausea was not associated with the co-administration of intranasal DEX and SUF[11]. Further studies are needed to investigate the incidence of nausea and vomiting among different populations.
This study had some limitations. First, according to our trial results, intranasal 0.3 μg/kg SUF when combined with intranasal 1 μg/kg DEX is effective in achieving an MOAA/S score ≤ 3 in 95% of patients undergoing EUS. However, the duration of effective sedation to maintain a score ≤ 3 remains unclear. Similar to other sedation regimens aimed at inducing moderate sedation, there were fluctuations in sedation levels throughout the study period. Repeated stimulation of endoscopic performance slightly reduced the level of sedation, whereas elimination of the stimulations resulted in slightly increased sedation levels. However, there was no oversedation in this study, and satisfaction scores were high even when there were fluctuations. Although no patient required rescue sedation during the procedure, this did not indicate that the regimen was able to provide ideal sedation for EUS. Further studies are needed to confirm the efficacy of this regimen and explore the stability of sedation levels of different sedation regimens. Second, the incidence of respiratory depression was significantly higher after intravenous administration of SUF than after intranasal administration of SUF[1]. This was consistent with our findings as no patient reported hypoxemia. However, large-scale trials are needed to assess the influence of this sedation regimen on respiratory depression and to determine the safety of intranasal DEX and SUF. Third, although the satisfaction scores of patients and endoscopist were high, the sample size was small. Further prospective randomized controlled trials are required to compare intranasal DEX and SUF with other procedural sedation regimens, such as target-controlled infusion using short-acting drugs. Fourth, we only selected patients with normal BMI in this study. Due to minimal respiratory depression, DEX is especially suitable for obese patients or patients with obstructive sleep apnea (OSA)[26,27]. The different effects of intranasal DEX and SUF in patients with OSA would be an interesting area for further research. Finally, the sedation preparation before the EUS took 45 min in this study (25 min for DEX and then 20 min for SUF action). This can limit the role of the intranasal DEX and SUF combination for a procedure of short duration especially in high volume centers, because it can waste the endoscopy suit time and increase the procedure cost unless the preparation is done in a separate induction room (with full monitoring) to save the endoscopy suit time.
Intranasal 0.3 μg/kg SUF combined with intranasal 1 μg/kg DEX is effective in providing moderate sedation in 95% of patients scheduled for EUS. It offers a noninvasive, well tolerated alternative to intravenous administration. Further research is needed to compare the effectiveness of sedation and the incidence of side effects with this new intranasal sedation combination with a regularly used sedation regimen.
Endoscopic ultrasonography (EUS) progressively developed from a primary diagnostic modality to an interventional procedure in the past two decades. Moderate or deep sedation is considered for diagnostic and some interventional EUS procedures because of the large size of the echoendoscope and potentially prolonged procedure.
The conventional method to preform moderate-to-deep sedation is generally intravenous benzodiazepine alone or in combination with opioids. However, there are many limitations to the conventional method, including an inability for patients to tolerate it due to the possible longevity of EUS procedures. Intranasal medication delivery is an innovative approach with a more gradual onset and fewer side effects than intravenous administration.
The purpose of this study was to determine the minimal effective dose of intranasal sufentanil (SUF) when combined with intranasal dexmedetomidine (DEX) for moderate sedation of EUS in at least 95% of patients (ED95).
This study has used continual reassessment method (CRM) to determine the minimal effective dose of intranasal SUF when combined with intranasal DEX for moderate sedation of EUS in at least 95% of patients (ED95). The sedation status was assessed by Modified Observer’s Assessment of Alertness/ Sedation (MOAA/S) score. The adverse events and the satisfaction scores of patients and endoscopists were recorded.
The ED95 was intranasal 0.3 μg/kg SUF when combined with intranasal 1 μg/kg DEX, with an estimated probability of successful moderate sedation for EUS of 94.9% (95% confidence interval: 88.1-98.9%).
The ED95 needed for moderate sedation for EUS is intranasal 0.3 μg/kg SUF when combined with intranasal 1 μg/kg DEX, based on CRM.
This study provides an alternative to intravenous administration for sedation that both patients and endoscopists were satisfied with. We believe that our study makes a significant contribution to the literature because it provides an alternative to intravenous administration by administering intranasal 1 μg/kg DEX in combination with 0.3 μg/kg SUF to patients scheduled for EUS.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anesthesiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arslan M, Shetabi H, Solanki SL S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Helmers JH, Noorduin H, Van Peer A, Van Leeuwen L, Zuurmond WW. Comparison of intravenous and intranasal sufentanil absorption and sedation. Can J Anaesth. 1989;36:494-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 59] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Zou Y, Shao L, Tian M, Zhang Y, Liu F. Determination of the maximum tolerated dose of intranasal sufentanil and midazolam in Chinese: a pilot study. Acta Anaesthesiol Scand. 2018;62:773-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Kogan A, Katz J, Efrat R, Eidelman LA. Premedication with midazolam in young children: a comparison of four routes of administration. Paediatr Anaesth. 2002;12:685-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Chernik DA, Gillings D, Laine H, Hendler J, Silver JM, Davidson AB, Schwam EM, Siegel JL. Validity and reliability of the Observer's Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990;10:244-251. [PubMed] |
| 5. | American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1313] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 6. | Kurita T, Doi M, Katoh T, Sano H, Sato S, Mantzaridis H, Kenny GN. Auditory evoked potential index predicts the depth of sedation and movement in response to skin incision during sevoflurane anesthesia. Anesthesiology. 2001;95:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Candiotti KA, Bergese SD, Bokesch PM, Feldman MA, Wisemandle W, Bekker AY; MAC Study Group. Monitored anesthesia care with dexmedetomidine: a prospective, randomized, double-blind, multicenter trial. Anesth Analg. 2010;110:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 169] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 8. | Brzezinski M, Hammer GB, Candiotti KA, Bergese SD, Pan PH, Bourne MH, Michalsky C, Wase L, Demitrack MA, Habib AS. Low Incidence of Opioid-Induced Respiratory Depression Observed with Oliceridine Regardless of Age or Body Mass Index: Exploratory Analysis from a Phase 3 Open-Label Trial in Postsurgical Pain. Pain Ther. 2021;10:457-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Liu H, Huang Y, Diao M, Li H, Ma Y, Lin X, Zhou J. Determination of the 90% effective dose (ED90) of phenylephrine for hypotension during elective cesarean delivery using a continual reassessment method. Eur J Obstet Gynecol Reprod Biol. 2015;194:136-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Jæger P, Jenstrup MT, Lund J, Siersma V, Brøndum V, Hilsted KL, Dahl JB. Optimal volume of local anaesthetic for adductor canal block: using the continual reassessment method to estimate ED95. Br J Anaesth. 2015;115:920-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Hitt JM, Corcoran T, Michienzi K, Creighton P, Heard C. An evaluation of intranasal sufentanil and dexmedetomidine for pediatric dental sedation. Pharmaceutics. 2014;6:175-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Zohar S, Chevret S. The continual reassessment method: comparison of Bayesian stopping rules for dose-ranging studies. Stat Med. 2001;20:2827-2843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Liu S, Pan H, Xia J, Huang Q, Yuan Y. Bridging continual reassessment method for phase I clinical trials in different ethnic populations. Stat Med. 2015;34:1681-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | ASGE Standards of Practice Committee; Early DS, Lightdale JR, Vargo JJ 2nd, Acosta RD, Chandrasekhara V, Chathadi KV, Evans JA, Fisher DA, Fonkalsrud L, Hwang JH, Khashab MA, Muthusamy VR, Pasha SF, Saltzman JR, Shergill AK, Cash BD, DeWitt JM. Guidelines for sedation and anesthesia in GI endoscopy. Gastrointest Endosc. 2018;87:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 363] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 15. | Li A, Yuen VM, Goulay-Dufaÿ S, Sheng Y, Standing JF, Kwok PCL, Leung MKM, Leung AS, Wong ICK, Irwin MG. Pharmacokinetic and pharmacodynamic study of intranasal and intravenous dexmedetomidine. Br J Anaesth. 2018;120:960-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 16. | Iirola T, Vilo S, Manner T, Aantaa R, Lahtinen M, Scheinin M, Olkkola KT. Bioavailability of dexmedetomidine after intranasal administration. Eur J Clin Pharmacol. 2011;67:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 17. | Zhang X, Bai X, Zhang Q, Wang X, Lu L. The safety and efficacy of intranasal dexmedetomidine during electrochemotherapy for facial vascular malformation: a double-blind, randomized clinical trial. J Oral Maxillofac Surg. 2013;71:1835-1842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Wolfe TR, Braude DA. Intranasal medication delivery for children: a brief review and update. Pediatrics. 2010;126:532-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | O'Quigley J, Pepe M, Fisher L. Continual reassessment method: a practical design for phase 1 clinical trials in cancer. Biometrics. 1990;46:33-48. [PubMed] |
| 20. | Houle TT, Turner DP. Bayesian statistical inference in anesthesiology. Anesthesiology. 2013;119:4-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Dale O, Hjortkjaer R, Kharasch ED. Nasal administration of opioids for pain management in adults. Acta Anaesthesiol Scand. 2002;46:759-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 154] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Gan TJ. Risk factors for postoperative nausea and vomiting. Anesth Analg. 2006;102:1884-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 358] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 23. | Cohen MM, Duncan PG, DeBoer DP, Tweed WA. The postoperative interview: assessing risk factors for nausea and vomiting. Anesth Analg. 1994;78:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 311] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 24. | Good P, Jackson K, Brumley D, Ashby M. Intranasal sufentanil for cancer-associated breakthrough pain. Palliat Med. 2009;23:54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Steenblik J, Goodman M, Davis V, Gee C, Hopkins CL, Stephen R, Madsen T. Intranasal sufentanil for the treatment of acute pain in a winter resort clinic. Am J Emerg Med. 2012;30:1817-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: a review of clinical applications. Curr Opin Anaesthesiol. 2008;21:457-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 265] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 27. | Bamgbade OA, Alfa JA. Dexmedetomidine anaesthesia for patients with obstructive sleep apnoea undergoing bariatric surgery. Eur J Anaesthesiol. 2009;26:176-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |