Published online Mar 26, 2022. doi: 10.12998/wjcc.v10.i9.2700
Peer-review started: July 15, 2021
First decision: October 18, 2021
Revised: October 29, 2021
Accepted: February 19, 2022
Article in press: February 19, 2022
Published online: March 26, 2022
Processing time: 250 Days and 4.4 Hours
Pulmonary embolism (PE) is a fatal clinical syndrome that is generally caused by an embolus from unstable deep venous thrombosis (DVT). However, clinical and biochemical factors that are related to the stability of DVT are not fully understood.
To evaluate the relationships between plasma antigen levels of factor XII (FXII:Ag) and factor XI (FXI:Ag) with the stability of DVT.
Patients with DVT and no PE, DVT and PE, and controls with no DVT or PE that matched for age, gender, and comorbidities were included in this study. FXII:Ag and FXI:Ag in peripheral venous blood were measured using enzyme-linked immunosorbent assays.
Using the 95th percentile of FXI:Ag in patients with DVT and PE as the cut-off, a higher FXI:Ag was associated with a higher risk of unstable DVT (odds ratio: 3.15, 95% confidence interval: 1.18-8.43, P = 0.019). Stratified analyses showed consistent results in patients ≤ 60 years (P = 0.020), but not in those > 60 years (P = 0.346).
Higher plasma FXI:Ag might be a marker for unstable DVT, which might be associated with PE in these patients.
Core Tip: In this case-control study, we found that plasma level of factor XI (FXI:Ag) was significantly higher in patients with deep venous thrombosis (DVT), and pulmonary embolism (PE) compared with those with only DVT. In addition, using the 95th percentile of FXI:Ag as cut-off values, a higher plasma level of FXI:Ag was associated with unstable DVT, as shown by the prevalence of PE in patients with DVT. Since the majority of PE is caused by thrombus that falls out from unstable DVT, these findings might suggest that a higher plasma level of FXI:Ag could be a marker for unstable DVT.
- Citation: Meng Y, Li Y, Ye YJ, Ma Q, Zhang JB, Qin H, Deng YY, Tian HY. Associations between coagulation factor XII, coagulation factor XI, and stability of venous thromboembolism: A case-control study. World J Clin Cases 2022; 10(9): 2700-2709
- URL: https://www.wjgnet.com/2307-8960/full/v10/i9/2700.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i9.2700
Venous thromboembolism (VTE), which typically includes deep venous thrombosis (DVT) and pulmonary embolism (PE), have been established as a group of key clinical syndromes that contribute significantly to the morbidity and mortality of people worldwide[1-3]. Many factors have been associated with an increased incidence of DVT, such as aging, immobilization, pregnancy, cancer, obesity, and the use of certain medications, such as oral contraceptives[4]. Therefore, the incidence of DVT varied according to the characteristics of the population studied[5,6]. It has been reported that approximately 80% of patients with DVT could be asymptomatic, which means that the early diagnosis of DVT can be difficult[4,5]. In contrast, PE is often characterized by severe symptoms of dyspnea, chest pain, syncope, or sudden death[7]. Previous studies have shown that only 7% of fatal PE cases were diagnosed at the time of death, and the mortality of patients with PE was high[7,8]. Patients that survived PE were vulnerable to severe clinical consequences, such as chronic thromboembolic pulmonary hypertension and right cardiac failure, which led to poor quality of life in these patients[7,8]. Overall, 90% of PE is caused by thrombus that has fallen from unstable DVT. Currently, PE is considered to be a severe but continuous stage with the progression of unstable DVT[9]. For some patients, the risk of PE remains high despite the application of prophylactic anticoagulation[10]. Therefore, the identification of the key processes that play a role in the pathogenesis of unstable DVT might be important for risk stratification and the development of a novel treatment strategy against the incidence of PE in DVT patients.
Recent studies show that factor XII (FXII) and factor XI (FXI), which are two key components of the intrinsic coagulation pathway, might have important functions in the regulation of the stability of thrombosis[11-13]. Conventionally, thrombus formation is mainly initiated by the tissue factor pathway (extrinsic coagulation pathway), but the resulting thrombus from this pathway is nonocclusive and unstable[14-16]. The intrinsic coagulation pathway is then initiated by activated FXII, which participates in the enlargement and expansion of the thrombus[14-16]. Then, the cascade of downstream factors that includes FXI and FIX are activated, which promotes the production of thrombin, enhances the density of the fibrin clot, causes the binding of FXII with fibrin, and the enlargement and expansion of the thrombus[17]. Previous experimental studies in Atherosclerosis-prone apolipoprotein E-deficient (Apoe -/-) mice showed that knock-out of the FXII gene was associated with significantly decreased whole-blood thrombus and fibrin formation on immobilized plaque homogenates in vitro, and the results of localization studies that used confocal microscopy showed that FXII was bound to thrombi and fibrin in luminal-exposed thrombus areas of ruptured atherosclerotic plaques[18]. In addition, a study has shown that treatment with FXI antisense oligonucleotides could prevent thrombus formation on acutely ruptured atherosclerotic plaques in mice[19]. These results demonstrated that FXII and FXI might contribute to the stabilization of thrombus formed during the rupture of atherosclerotic plaques. Therefore, a subsequent study in females < 50 years showed that increased FXI antigen levels (FXI:Ag) were associated with a significantly increased risk of myocardial infarction and a marginally increased risk of ischemic stroke. However, an increased FXII antigen level (FXII:Ag) was not associated with the risks of these diseases[20,21]. In addition, a recent prospective cohort study that included patients after a first mild to moderate ischemic stroke event showed that high activity levels of FXI but not FXII were associated with worse vascular outcomes in the 3 years after the first ischemic stroke. In combination, higher FXI, and possibly FXII, have been related to higher risks of arterial thrombotic events. By taking into consideration their roles in coagulation, it could be hypothesized that these factors might exert their arterial pro-thrombotic efficacy by regulating the stability of the thrombosis[22,23]. However, to the best of the authors’ knowledge, studies that evaluate the relationship between FXII and FXI with the stability of venous thrombosis have not been reported. Therefore, this study aims to perform a pilot study to determine the potential association between plasma FXII:Ag and FXI:Ag and the stability of DVT.
This study was as a case-control study, which included patients with DVT, patients with DVT and PE, and patients without DVT or PE as controls. Patients that were admitted to the Department of Peripheral Vascular Diseases, the First Affiliated Hospital of Xi’an Jiaotong University between 1, March, 2018 and 31, May, 2020 were screened for possible inclusion. All eligible patients provided written informed consent before being enrolled in the study. The protocol of the study was approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University before this study was carried out (number: XJTU1AF2018LSK-010).
Patients aged between 14 and 85 years, with newly diagnosed DVT with or without PE (within 1 mo), without previous antithrombotic therapies, that were able to understand the purpose of the study and that cooperated to complete the follow-up were candidates for inclusion. Diagnosis of DVT was based on the clinical manifestations and formation of central or mixed DVT of lower extremities, such as the iliac vein, femoral vein, and popliteal vein, and confirmed by ultrasound or other imaging examinations, such as computed-tomographic angiography (CTA) or digital subtraction angiography (DSA) of the lower extremity veins. Diagnosis of PE was based on symptoms and evidence from CTA or DSA of the pulmonary arteries. For patients with DVT only, PE was excluded based on negative findings from CTA or DSA of the pulmonary arteries. Patients were excluded from this study if they met either of the following criteria: (1) Diagnosis of DVT, or PE, or both > 30 d before study enrollment; (2) Uncontroll-able mental history or dementia, and unable to complete the informed consent process or with poor compliance judged by the researchers; or (3) Unable or unwilling to cooperate with the clinical evaluation that was requested for this study. In addition, participants without DVT or PE that matched for age, gender, smoking status, and comorbidities of hypertension, diabetes mellitus, and coronary artery disease (CAD) were included as controls. All the included participants underwent routine biochemical and coagulation blood tests on admission using venous blood samples obtained during fasting status.
FXII:Ag and FXI:Ag levels were measured by enzyme-linked immunosorbent assay (ELISA) that used commercially available kits according to the manufacturer’s instructions. Briefly, after admission, 3 mL of peripheral venous blood was collected from each patient and placed into a sodium citrate anticoagulant tube. The blood was centrifuged for at 1500 g for 10 min. The plasma was then separated and placed stored room temperature. The measurements of FXII:Ag and FXI:Ag was performed within 4 h of this procedure according to the manufacturer’s instructions to avoid the degradation of the factors being measured.
Continuous variables are presented as means and standard deviations if they were normally distributed and categorized variables are expressed as numbers and proportions. Comparisons between the participants in the two groups were performed using independent t-test, and comparisons between categorized variables of the groups were performed using a Chi-squared test. For comparisons of continuous variables between participants of multiple groups, ANOVA was performed. The association between FXII:Ag and FXI:Ag with the risk of PE was analyzed within the controls and patients with DVT. Using the 95th percentile of FXII:Ag and FXI:Ag in DVT patients as cut-off values, the association between FXII:Ag and FXI:Ag with the risk of DVT were calculated as odds ratios (ORs) and their 95% confidence intervals (CIs). Similarly, the association between FXII:Ag and FXI:Ag with the stability of DVT was analyzed in patients with DVT and patients with DVT and PE. Using the cut-off values of the 95th percentile of FXII:Ag and FXI:Ag in patients with DVT and PE, the association between FXII:Ag and FXI:Ag with the risk of unstable DVT were calculated as ORs and their corresponding 95%CI. A P value < 0.05 was considered as statistically significant. The statistical analyses were performed using SPSS 19.0 software.
A total of 58 patients with DVT, 53 patients with DVT and PE, and 61 controls without DVT or PE were included. The characteristics of the included patients are listed in Table 1. Briefly, these patients were matched for age, sex, smoking status, and comorbidities that included hypertension, diabetes, and CAD (all P > 0.05). However, compared with the controls, patients with only DVT and those with DVT and PE had significantly higher white blood cell counts, neutrophil proportions, high-sensitivity C-reactive protein, fibrinogen, and fibrinogen degradation products (all P < 0.05), which appeared significantly increased in patients with DVT and PE (Table 1). In addition, the plasma level of N-terminal pro-B-type natriuretic peptide was significantly increased in patients with DVT and PE, compared with those with only DVT or the controls (P < 0.05).
| DVT (n = 58) | DVT + PE (n = 53) | Control (n = 61) | P value1 | |
| Mean age (yr) | 61.6 (14.9) | 56.8 (18.3) | 61.7 (8.34) | 0.57 |
| Male (%) | 44.0 | 58.3 | 45.0 | 0.76 |
| Hypertension (%) | 20.0 | 8.33 | 30.0 | 0.78 |
| Diabetes (%) | 8.00 | 0 | 20.0 | 0.62 |
| CAD (%) | 6.00 | 8.33 | 15.0 | 0.85 |
| Smoking (%) | 28.0 | 16.7 | 30.0 | 0.81 |
| WBC (109/L) | 8.16 (4.30) | 9.95 (3.63) | 5.49 (1.80) | 0.002 |
| Neutrophil, % | 72.0 (12.7) | 77.3 (8.14) | 59.9 (8.32) | 0.002 |
| Platelet (109/L) | 213.6 (62.8) | 203.2 (82.3) | 193.8 (46.4) | 0.57 |
| hs-CRP (mg/L) | 8.19 (3.30) | 9.21 (2.62) | 1.63 (1.50) | < 0.001 |
| hs-TNT (ng/mL) | 0.08 (0.19) | 0.02 (0.02) | 0.01 (0.001) | 0.63 |
| FDP (mg/L) | 25.4 (39.8) | 33.5 (32.5) | 1.62 (2.57) | 0.009 |
| FIB (g/L) | 3.60 (1.38) | 4.08 (1.30) | 2.64 (0.39) | 0.002 |
| NT-proBNP | 203.8 (229.3) | 1038 (1653) | 205.6 (555.0) | 0.015 |
| D-Dimer (mg/L) | 8.64 (14.0) | 12.2 (11.5) | 0.72 (1.08) | 0.001 |
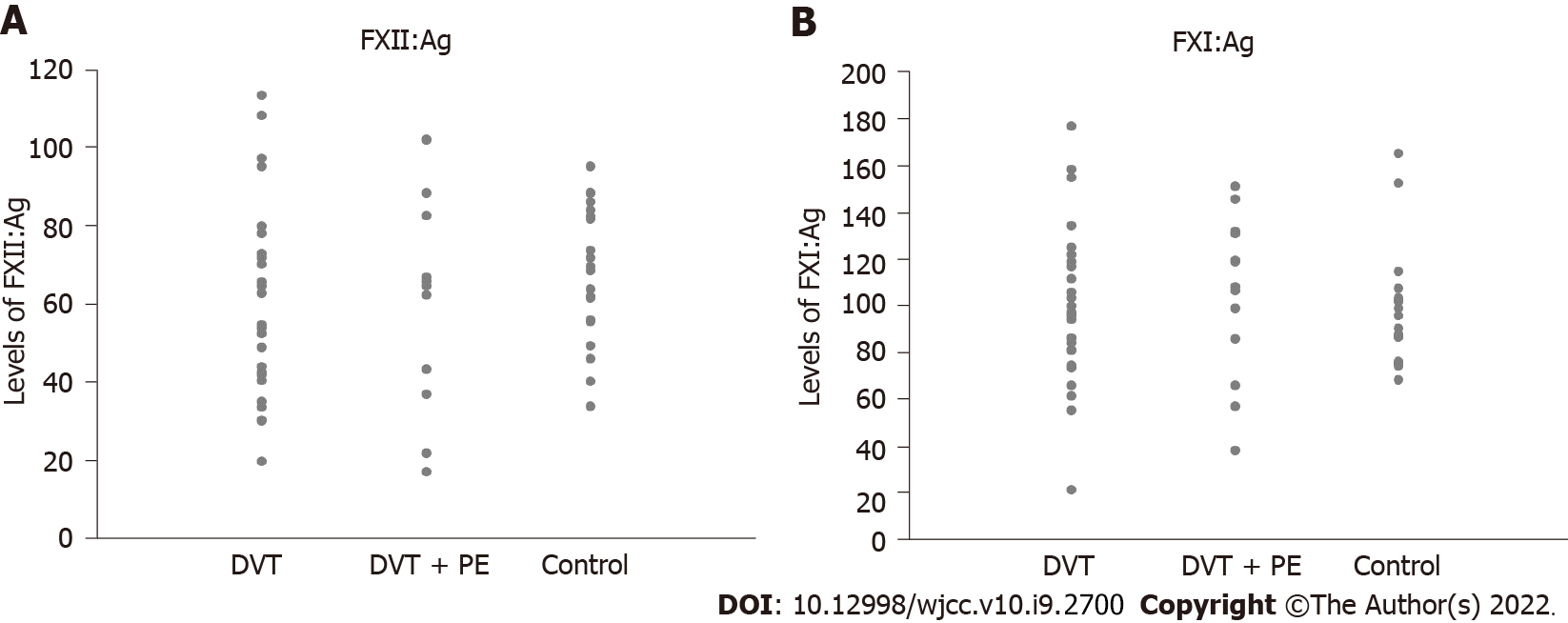
The distributions of FXII:Ag and FXI:Ag levels in the participants from each group are shown in Figure 1 as a scatter graph. The levels of FXII:Ag (58.6 ± 25.2 vs 59.2 ± 22.5, P = 0.968) and FXI:Ag (99.1 ± 33.3 vs 93.0 ± 24.2, P = 0.484) were not statistically different between patients with only DVT and control participants (Table 2). In addition, although the levels of FXII:Ag (58.6 ± 25.2 vs 56.1 ± 27.9, P = 0.603) were not statistically different between patients with only DVT and those with DVT and PE, the levels of FXI:Ag (99.1 ± 33.3 vs 103.2 ± 33.7, P = 0.043) were significantly lower in patients with only DVT compared with patients with DVT and PE (Table 2).
| DVT (n = 58) | DVT + PE (n = 53) | Control (n = 61) | P value (DVT vs control) | P value (DVT vs DVT + PE) | |
| FXII:Ag (%) | 58.6 (25.2) | 56.1 (27.9) | 59.2 (22.5) | 0.968 | 0.603 |
| FXI:Ag (%) | 99.1 (33.3) | 103.2 (33.7) | 93.0 (24.2) | 0.484 | 0.043 |
The potential association between levels of FXII:Ag and FXI:Ag and the risk of DVT were explored in patients with only DVT and the controls. As given in Table 3, using the 95th percentile of FXII:Ag and FXI:Ag in patients with DVT as the cut-off value, higher FXII:Ag (OR: 1.45, 95%CI: 0.68-3.09, P = 0.332) or FXI:Ag (OR: 1.61, 95%CI: 0.73-3.58, P = 0.236) were not associated with a higher risk of DVT.
| Cut-off1 | Control | DVT | χ2 | OR | 95%CI | P value | |
| FXII:Ag | ≤ 63.331 | 42 | 35 | 0.942 | 1.453 | 0.683-3.092 | 0.332 |
| > 63.331 | 19 | 23 | |||||
| FXI:Ag | ≤ 100.905 | 46 | 38 | 1.401 | 1.614 | 0.729-3.576 | 0.236 |
| > 100.905 | 15 | 20 |
The potential association between levels of FXII:Ag and FXI:Ag and the stability of DVT were explored in patients with only DVT and those with DVT and PE. As given in Table 4, using the 95th percentile of FXII:Ag and FXI:Ag in patients with DVT and PE as the cut-off value, higher FXII:Ag (OR: 0.83, 95%CI: 0.37-1.88, P = 0.407) was not associated with a higher risk of unstable DVT (evidenced by DVT and PE). However, higher FXI:Ag (OR: 3.15, 95%CI: 1.18-8.43, P = 0.019) was associated with a higher risk of unstable DVT. Further stratified analyses based on the age of the patients showed that higher FXII:Ag was not associated with a higher risk of unstable DVT in patients aged ≤ 60 years or in patients aged > 60 years (both P > 0.05) (Table 5). Of interest, a higher FXI:Ag level was associated with higher risk of unstable DVT in patients aged ≤ 60 years (OR: 4.18, 95%CI: 1.19-14.75, P = 0.020), but not in patients aged > 60 years (P = 0.346) (Table 5).
| Cut-off1 | DVT | DVT + PE | χ2 | OR | 95%CI | P value | |
| FXII:Ag | ≤ 68.485 | 39 | 37 | 0.197 | 0.832 | 0.369-1.876 | 0.407 |
| > 68.485 | 19 | 15 | |||||
| FXI:Ag | ≤ 130.117 | 51 | 37 | 5.535 | 3.151 | 1.178-8.427 | 0.019 |
| > 130.117 | 7 | 16 |
In this pilot case-control study, we found that although the plasma level of FXII:Ag was not statistically different between patients with only DVT and those with DVT and PE, the plasma level of FXI:Ag was significantly higher in patients with DVT and PE compared with those with only DVT. In addition, using the 95th percentile of FXII:Ag and FXI:Ag for patients with DVT and PE as cut-off values, the results showed that a higher plasma level of FXI:Ag was associated with unstable DVT, as shown by the prevalence of PE in patients with DVT, and FXII:Ag was not associated with unstable DVT. Finally, stratified analyses based on the age of the patients showed that a higher plasma level of FXI:Ag was associated with unstable DVT in aged ≤ 60 years, but not in those aged > 60 years. Since the majority of PE is caused by thrombus that falls out from unstable DVT, these findings might suggest that a higher plasma level of FXI:Ag could be a marker for unstable DVT. These results need to be validated in a large-scale cohort study of DVT patients without PE at baseline. The potential significance of the increased plasma levels of FXI:Ag as a risk factor, or even a preventive target against the incidence of PE in patients with DVT, should be evaluated in future studies.
Previous studies that evaluated the association between FXII and FXI and the thrombotic events mainly focused on the arterial system, rather than the venous system. It has been suggested that higher FXI is associated with a higher risk of ischemic stroke and myocardial infarction in the adult population; however, for FXII this association was not always observed[20,21,24]. Early studies showed that FXII-mediated FXI activation contributed to thrombus formation in rodents and primates, which is particularly important for the enlargement and stabilization of thrombosis[25]. Currently, increasing evidence suggests that the stability of DVT might determine the risk of PE in these patients. A previous study that induced thrombi in the femoral vein of wild-type, heterozygous, and homozygous factor V Leiden (FVL) mice showed that initial DVT development was similar in FVL and noncarriers; however, thrombi in FVL carriers were more stable, and therefore, less vulnerable to PE than in noncarriers[26], which might partly explain the higher DVT but lower PE incidence in humans with FVL variants. A recent study in new PE patients showed that looser fibrin networks that were composed of thicker fibers increased the susceptibility to lysis and characterized patients with central PE, which suggested that fibrin clot phenotype affected the size of thrombi that occluded the pulmonary arteries[27]. Because of the potential role of FXII and FXI in the stabilization of thrombosis, a higher FXII, or FXI, or both might be associated with unstable DVT. The results of this study that compared FXII:Ag and FXI:Ag levels between patients with DVT and patients with DVT and PE, showed that a higher plasma level of FXI:Ag might be associated with unstable DVT, as shown by simultaneous PE in these patients.
As a pilot study, the results of this study might have several implications for future studies. First, the results of this study did not show a significant difference in FXII:Ag or FXI:Ag between patients with DVT and controls without DVT or PE; however, they did indicate a significant difference in FXI:Ag between patients with only DVT and those with DVT and PE. Because PE in DVT is thought to be caused by unstable DVT, these findings might reflect the pathophysiological significance of FXI as a mediator of thrombosis stability[28]. The higher FXI:Ag that was observed in patients with DVT and PE (those with unstable DVT) might be a result of the feedback mechanism of the unstable thrombosis in DVT, which requires more FXI to stabilize the thrombosis in these patients. Therefore, the measurement of FXI:Ag might be a useful marker for unstable DVT patients, which might have a higher risk of recurrent VTE events, including PE. Large-scale prospective cohort studies that include DVT patients without PE are required to validate the results of this study, and to establish a potential sequential relationship between higher FXI:Ag and the risk of PE in patients with DVT. In addition, the optimal measurement methods and cut-off values for FXI:Ag that predict unstable DVT need to be determined in future large-scale cohort studies. In this study, the levels of FXII and FXI were measured using ELISA for the antigens. It is not known whether the levels of FXII:Ag and FXI:Ag were correlated with its activity, which warrants further studies. In addition, because of the important role of FXI in thrombosis formation and stabilization, it was highlighted that FXI might be a novel target for anticoagulation therapy[29]. Future studies should evaluate the preventive efficacy of anticoagulation therapy that targets FXI against PE in patients with unstable DVT, which is characterized by increased FXI:Ag.
This study has some limitations. The interpretation of the results should be cautious when these limitations are considered. This was a small-scale, case-control study that only included Chinese patients from a single center. In addition, only patients with no previous antithrombotic therapies were included. These factors might have led to selection bias, and the results of this study should be validated in large-scale studies in other centers. Although the baseline characteristics included age, gender, smoking status, and comorbidities that might have affected the coagulative status of the patients, due to the limited sample size, only univariate analysis was performed. The potential effect of other study characteristics could not be excluded, such as prophylactic anticoagulants[30] and concurrent medication that was used by each patient, which might affect the results. Because this was a pilot investigation, our study should be considered as hypothesis generating. Large-scale studies that include multivariate analysis and adequate control of the potential confounding factors are required to validate our findings in the future. Second, this was a case-control study based on cross-sectional data, and a longitudinal association between higher FXI:Ag and unstable DVT could not be established, which should be confirmed in future cohort studies. Third, due to the limited sample size of the patients, the results of the age stratified analyses should be interpreted with caution. In total, 38 patients were included in the analyses for the association between FXI:Ag and unstable DVT in patients aged > 60 years, and the insignificant finding of these analyses might be caused by the insufficient statistical power results by the limited sample size. Therefore, large-scale studies are required to determine the potential characteristics of the patients on the outcome, in addition to the age of the patients. In addition, all the included patients were from the Chinese population. It was not determined whether an ethnicity difference existed for the association between FXI:Ag and unstable DVT. The potential ethnicity difference should be considered in future studies, because FXI:Ag[31] and VTE risk[32] could be affected by genetic factors. Finally, a causative association between higher FXI:Ag and unstable DVT was not determined from the results, because it was an observational study.
In summary, this pilot case-control study suggested that higher plasma FXI:Ag might be a marker for unstable DVT, which might be associated with PE in these patients. Large-scale cohort studies are required to confirm these findings, and to evaluate the potential predictive value of higher FXI:Ag for PE in high-risk DVT patients.
Pulmonary embolism (PE) is a fatal clinical syndrome that is generally caused by an embolus from unstable deep venous thrombosis (DVT). However, clinical and biochemical factors that related to the stability of DVT remain not fully understood.
PE is a fatal clinical syndrome that is generally caused by an embolus from unstable DVT. However, clinical and biochemical factors that related to the stability of DVT remain not fully understood.
This study aims to evaluate the relationships between plasma antigen levels of factor XII (FXII:Ag) and factor XI (FXI:Ag) with the stability of DVT.
Patients with DVT and no PE, DVT and PE, and controls with no DVT or PE that matched for age, gender, and comorbidities were included in this study. FXII:Ag and FXI:Ag in peripheral venous blood were measured using enzyme-linked immunosorbent assays.
Using the 95th percentile of FXI:Ag in patients with DVT and PE as the cut-off, a higher FXI:Ag was associated with a higher risk of unstable DVT (odds ratio: 3.15, 95% confidence interval: 1.18-8.43, P = 0.019). Stratified analyses showed consistent results in patients ≤ 60 years (P = 0.020), but not in those > 60 years (P = 0.346).
Higher plasma FXI:Ag might be a marker for unstable DVT, which might be associated with PE in these patients.
Future large-scale studies with multivariate analyses are needed to validate our findings, and to evaluate the potential predictive value of higher FXI:Ag for PE in high-risk DVT patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Oley MH S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Kearon C, Kahn SR. Long-term treatment of venous thromboembolism. Blood. 2020;135:317-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 2. | Weitz JI, Chan NC. Novel antithrombotic strategies for treatment of venous thromboembolism. Blood. 2020;135:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Parakh RS, Sabath DE. Venous Thromboembolism: Role of the Clinical Laboratory in Diagnosis and Management. J Appl Lab Med. 2019;3:870-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Olaf M, Cooney R. Deep Venous Thrombosis. Emerg Med Clin North Am. 2017;35:743-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Nicklas JM, Gordon AE, Henke PK. Resolution of Deep Venous Thrombosis: Proposed Immune Paradigms. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Stone J, Hangge P, Albadawi H, Wallace A, Shamoun F, Knuttien MG, Naidu S, Oklu R. Deep vein thrombosis: pathogenesis, diagnosis, and medical management. Cardiovasc Diagn Ther. 2017;7:S276-S284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 190] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 7. | Giordano NJ, Jansson PS, Young MN, Hagan KA, Kabrhel C. Epidemiology, Pathophysiology, Stratification, and Natural History of Pulmonary Embolism. Tech Vasc Interv Radiol. 2017;20:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 8. | Piazza G. Advanced Management of Intermediate- and High-Risk Pulmonary Embolism: JACC Focus Seminar. J Am Coll Cardiol. 2020;76:2117-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 9. | Serhal M, Barnes GD. Venous thromboembolism: A clinician update. Vasc Med. 2019;24:122-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Tritschler T, Kraaijpoel N, Le Gal G, Wells PS. Venous Thromboembolism: Advances in Diagnosis and Treatment. JAMA. 2018;320:1583-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 217] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 11. | Visser M, Heitmeier S, Ten Cate H, Spronk HMH. Role of Factor XIa and Plasma Kallikrein in Arterial and Venous Thrombosis. Thromb Haemost. 2020;120:883-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | Weitz JI, Fredenburgh JC. Factors XI and XII as Targets for New Anticoagulants. Front Med (Lausanne). 2017;4:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Weitz JI. Factor XI and factor XII as targets for new anticoagulants. Thromb Res. 2016;141 Suppl 2:S40-S45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Grover SP, Mackman N. Intrinsic Pathway of Coagulation and Thrombosis. Arterioscler Thromb Vasc Biol. 2019;39:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 142] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 15. | Kleinschnitz C, Braeuninger S, Pham M, Austinat M, Nölte I, Renné T, Nieswandt B, Bendszus M, Stoll G. Blocking of platelets or intrinsic coagulation pathway-driven thrombosis does not prevent cerebral infarctions induced by photothrombosis. Stroke. 2008;39:1262-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Naudin C, Burillo E, Blankenberg S, Butler L, Renné T. Factor XII Contact Activation. Semin Thromb Hemost. 2017;43:814-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | Key NS. Epidemiologic and clinical data linking factors XI and XII to thrombosis. Hematology Am Soc Hematol Educ Program. 2014;2014:66-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Kuijpers MJ, van der Meijden PE, Feijge MA, Mattheij NJ, May F, Govers-Riemslag J, Meijers JC, Heemskerk JW, Renné T, Cosemans JM. Factor XII regulates the pathological process of thrombus formation on ruptured plaques. Arterioscler Thromb Vasc Biol. 2014;34:1674-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 19. | van Montfoort ML, Kuijpers MJ, Knaup VL, Bhanot S, Monia BP, Roelofs JJ, Heemskerk JW, Meijers JC. Factor XI regulates pathological thrombus formation on acutely ruptured atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2014;34:1668-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Siegerink B, Rosendaal FR, Algra A. Antigen levels of coagulation factor XII, coagulation factor XI and prekallikrein, and the risk of myocardial infarction and ischemic stroke in young women. J Thromb Haemost. 2014;12:606-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Siegerink B, Maino A, Algra A, Rosendaal FR. Hypercoagulability and the risk of myocardial infarction and ischemic stroke in young women. J Thromb Haemost. 2015;13:1568-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Bolton-Maggs P, Goudemand J, Hermans C, Makris M, de Moerloose P. FXI concentrate use and risk of thrombosis. Haemophilia. 2014;20:e349-e351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Tillman B, Gailani D. Inhibition of Factors XI and XII for Prevention of Thrombosis Induced by Artificial Surfaces. Semin Thromb Hemost. 2018;44:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 24. | Rohmann JL, Huo S, Sperber PS, Piper SK, Rosendaal FR, Heuschmann PU, Endres M, Liman TG, Siegerink B. Coagulation factor XII, XI, and VIII activity levels and secondary events after first ischemic stroke. J Thromb Haemost. 2020;18:3316-3324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Cheng Q, Tucker EI, Pine MS, Sisler I, Matafonov A, Sun MF, White-Adams TC, Smith SA, Hanson SR, McCarty OJ, Renné T, Gruber A, Gailani D. A role for factor XIIa-mediated factor XI activation in thrombus formation in vivo. Blood. 2010;116:3981-3989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 26. | Shaya SA, Westrick RJ, Gross PL. Thrombus stability explains the factor V Leiden paradox: a mouse model. Blood Adv. 2019;3:3375-3378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Ząbczyk M, Natorska J, Janion-Sadowska A, Metzgier-Gumiela A, Polak M, Plens K, Janion M, Skonieczny G, Mizia-Stec K, Undas A. Loose Fibrin Clot Structure and Increased Susceptibility to Lysis Characterize Patients with Central Acute Pulmonary Embolism: The Impact of Isolated Embolism. Thromb Haemost. 2021;121:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Mohammed BM, Matafonov A, Ivanov I, Sun MF, Cheng Q, Dickeson SK, Li C, Sun D, Verhamme IM, Emsley J, Gailani D. An update on factor XI structure and function. Thromb Res. 2018;161:94-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 29. | Al-Horani RA, Afosah DK. Recent advances in the discovery and development of factor XI/XIa inhibitors. Med Res Rev. 2018;38:1974-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 30. | Scheres LJJ, Lijfering WM, Middeldorp S, Cheung YW, Barco S, Cannegieter SC, Coppens M. Measurement of coagulation factors during rivaroxaban and apixaban treatment: Results from two crossover trials. Res Pract Thromb Haemost. 2018;2:689-695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | O'Connell NM. Factor XI deficiency--from molecular genetics to clinical management. Blood Coagul Fibrinolysis. 2003;14 Suppl 1:S59-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | White RH, Keenan CR. Effects of race and ethnicity on the incidence of venous thromboembolism. Thromb Res. 2009;123 Suppl 4:S11-S17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 305] [Article Influence: 19.1] [Reference Citation Analysis (0)] |