Published online Mar 6, 2022. doi: 10.12998/wjcc.v10.i7.2341
Peer-review started: October 9, 2021
First decision: November 17, 2021
Revised: November 29, 2021
Accepted: January 19, 2022
Article in press: January 19, 2022
Published online: March 6, 2022
Processing time: 143 Days and 18.2 Hours
Spontaneous coronary artery dissection (SCAD) is a frequent cause of acute coronary syndrome in young to middle-aged women with few or no traditional cardiovascular risk factors. Chest pain is the most frequently described presenting symptom, but syncope is extremely rare. Herein, we report on a 16-year-old girl who presented with an episode of syncope occurring during a race. Despite significantly elevated troponin level, the diagnosis of the left main coronary artery SCAD with cardiogenic shock was delayed.
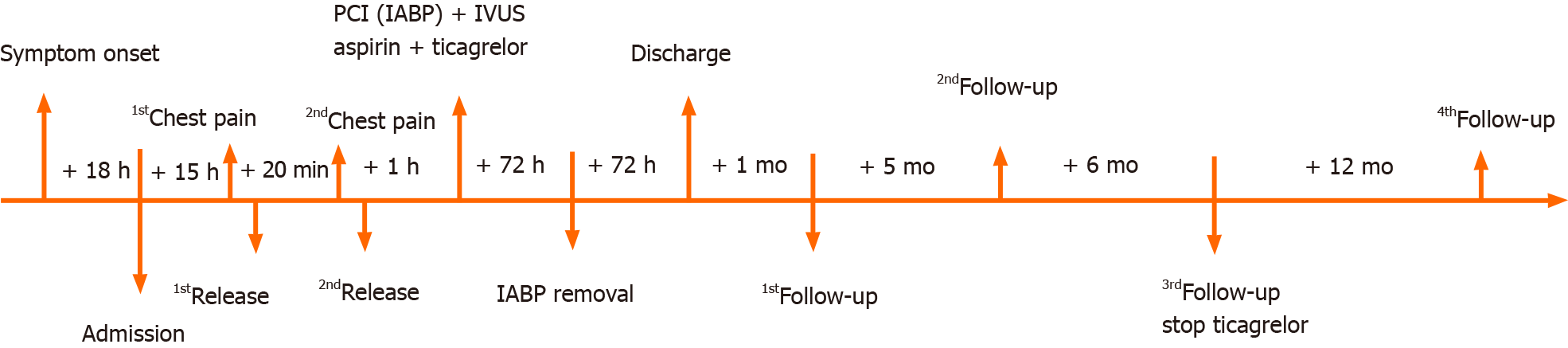
A 16-year-old girl presented with an episode of syncope. Myocardial injury markers were positive. Echocardiography showed a mildly reduced left ventricular ejection fraction (50%). Although initially stable, she later experienced recurrent chest pain accompanying precordial ST segment elevation with dynamic changes and developed cardiogenic shock, necessitating emergent revascularization. Coronary angiography demonstrated almost total occlusion at the ostium and proximal segment of the left main trunk coronary artery (LMT). Intravascular ultrasound confirmed a false lumen with prominent dissection in the LMT. Percutaneous coronary intervention assisted by intra-aortic balloon pump was conducted in the LMT. A 3.5 mm × 24 mm everolimus-eluting stent was deployed to the focal lesions of the LMT. A postprocedural electrocardiogram showed alleviation of the precordial ST-segment elevation. The diagnosis of SCAD was confirmed. Transthoracic echocardiography showed an improved left ventricular ejection fraction (57%). The patient was asymptomatic during the 24-mo. follow-up period.
SCAD should always be considered in the differential diagnosis of acute coronary syndrome presentations in low-risk patients, regardless of age.
Core Tip: Spontaneous coronary artery dissection in adolescents is rare. Few such cases have been reported in the existing peer-reviewed medical literature, highlighting the value of documenting the present case. We report a 16-year-old Chinese female case. We performed percutaneous coronary intervention assisted by intra-aortic balloon pump for the left main trunk coronary artery lesion. A good prognosis was confirmed at the 24-mo. follow-up.
- Citation: Liu SF, Zhao YN, Jia CW, Ma TY, Cai SD, Gao F. Spontaneous dissection of proximal left main coronary artery in a healthy adolescent presenting with syncope: A case report. World J Clin Cases 2022; 10(7): 2341-2350
- URL: https://www.wjgnet.com/2307-8960/full/v10/i7/2341.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i7.2341
Spontaneous coronary artery dissection (SCAD) is rare, accounting for up to 1% to 4% of acute coronary syndrome (ACS) cases overall[1]. Though the pathophysiology of SCAD remains unknown, a link of female sex, pregnancy, fibromuscular dysplasia, physical and emotional stress triggers with SCAD has been established in multiple series[2-4]. Patients with SCAD are at risk of being underdiagnosed, misdiagnosed and mistreated because their relatively young age and absence of atherosclerotic risk factors frequently do not fit the expected phenotype of an atherosclerotic patient with ACS[5]. Accurate and rapid diagnosis of SCAD in the early stages of ACS presentation is paramount because the management and investigation differ vastly from those applied to atherosclerotic forms of coronary artery disease.
Classically, SCAD is diagnosed with coronary angiography. Dedicated intracoronary imaging methods, including intravascular ultrasonography (IVUS) and optical coherence tomography, provide detailed visualization of the arterial wall that aids the diagnosis of SCAD[1]. Medical management is preferred over attempts at revascularization of the SCAD. However, in high-risk patients with ongoing ischemia, left main artery dissection, or hemodynamic instability, urgent intervention with percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) should be considered[6].
Here, we describe an unusual case of SCAD in an otherwise healthy 16-year-old girl. This adolescent group is not well studied and may have a unique clinical feature compared with adult populations. This case emphasizes the importance for cardiologists to be conscious that the quick recognition of SCAD and initiation of treatment in the early stages of ACS presentation may be lifesaving. This recognition is helpful for elucidating this still relatively poorly understood disease and improving the prognosis of patients.
A 16-year-old girl presented to our outpatient department after having a syncopal episode 18 h ago, along with abdominal pain and vomiting.
The patient had been picked up from her high school playground, where she had collapsed from exhaustion during a running race. She had visited the gastroenterology clinic earlier that day, and computed tomography scans of her brain and abdomen revealed no abnormalities. She also reported symptoms of an upper respiratory infection 3 wk before the event.
The patient had a healthy previous medical history. She had no cardiovascular risk factors and was taking no oral medications at the time.
The patient had no family history of inherited diseases or premature coronary heart disease.
On arrival, her vital signs included blood pressure 103/59 mmHg, heart rate 96 beats/min, oxygen saturation 98% on room air, respiratory rate 20 beats/min, and normal physical examination.
On admission, the laboratory findings were as follows: High-sensitivity troponin T was elevated to 1511 ng/L (cut-off > 50 ng/L), N-terminal (NT)-pro hormone BNP (NT-pro BNP) value was 1535 ng/L, alanine transaminase level was high, at 61.3 U/L, as was aspartate transaminase level, at 67 U/L, serum uric acid level was 399.5 µmol/L. Her blood count, blood biochemistry results, C- reactive protein, Creatine Kinase, Creatine Kinase-MB and indicators of blood coagulation function demonstrated no obvious abnormalities (Table 1).
| Complete blood countand coagulation function | Blood biochemistry tests | Cardiac biomarkers and inflammation markers | |||
| WBC (109/L) | 7.25 | ALT (U/L) | 61.3 | hs-CTnT (ng/L) | 1511 |
| NEUT (109/L) | 5 | AST (U/L) | 67 | CK (U/L) | 142.9 |
| NEUT, % | 68.9 | ALP (U/L) | 154 | CK-MB (U/L) | 16.5 |
| LY, % | 25.2 | γ-GTP (U/L) | 127.2 | LDH (U/L) | 440.7 |
| MONO, % | 5.5 | BUN (mmol/L) | 4.35 | NT-proBNP (ng/L) | 1535 |
| EOS, % | 0.3 | Cr (µmol/L) | 49.7 | CRP (mg/L) | 8.42 |
| BASO, % | 0.1 | UA (µmol/L) | 399.5 | ESR (mm/h) | 11.4 |
| RBC (1012/L) | 3.83 | HbA1c, % | 5.5 | RF (IU/mL) | 0 |
| Hb (g/L) | 119 | Glucose (mmol/L) | 7.56 | Complement C3 (g/L) | 1.13 |
| PLT (109/L) | 237 | TC (mmol/L) | 3.7 | Complement C4 (g/L) | 0.27 |
| D-dimmer (mg/L) | 1.5 | TG (mmol/L) | 0.74 | Antinuclear Antibodies | Negative |
| PT (s) | 11.9 | LDL-C (mmol/L) | 2.15 | Antineutrophil cytoplasmic antibodies | Negative |
| APTT (s) | 28.6 | HDL-C (mmol/L) | 1.09 | Aticardiolipin antibody | Negative |
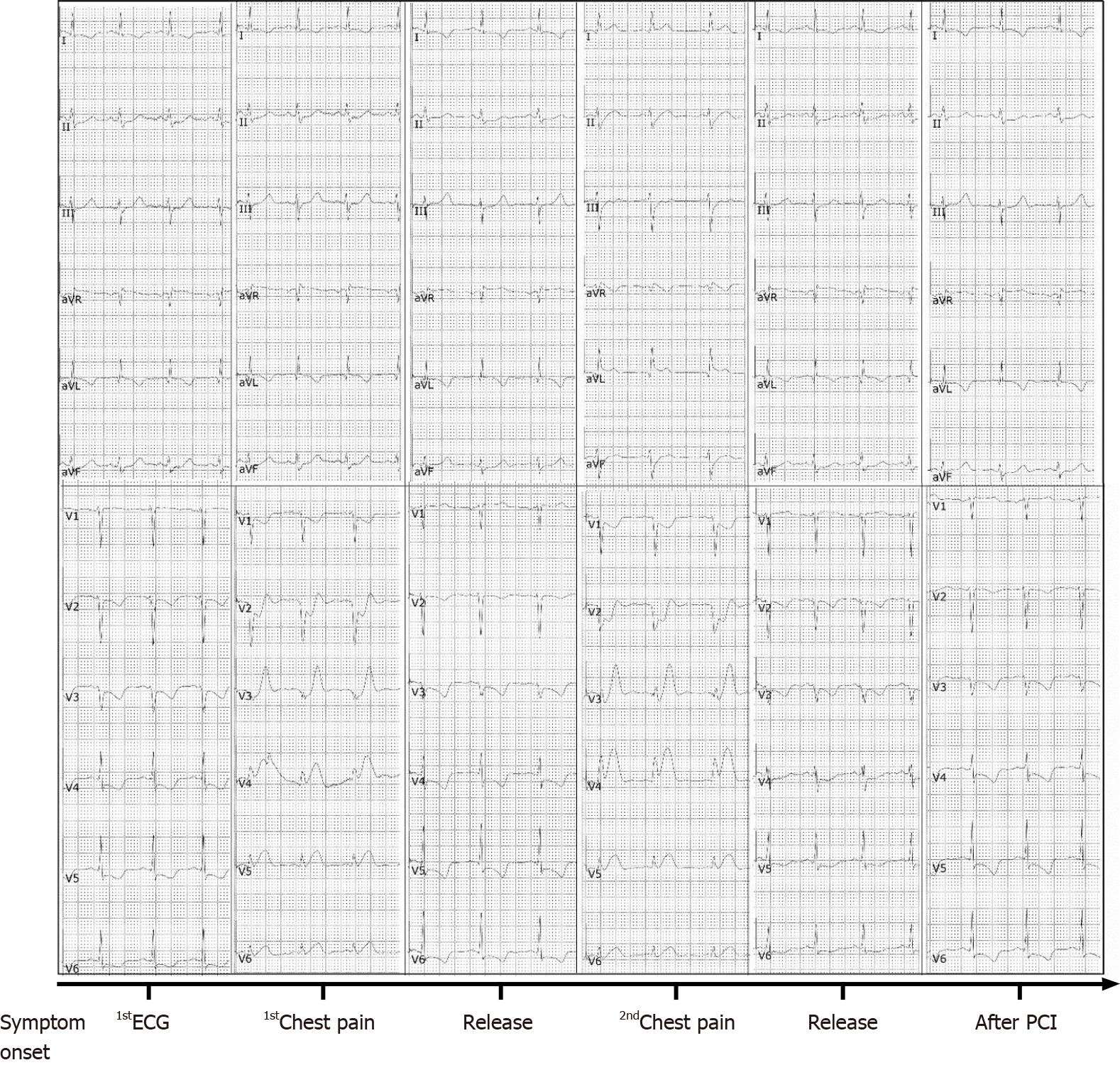
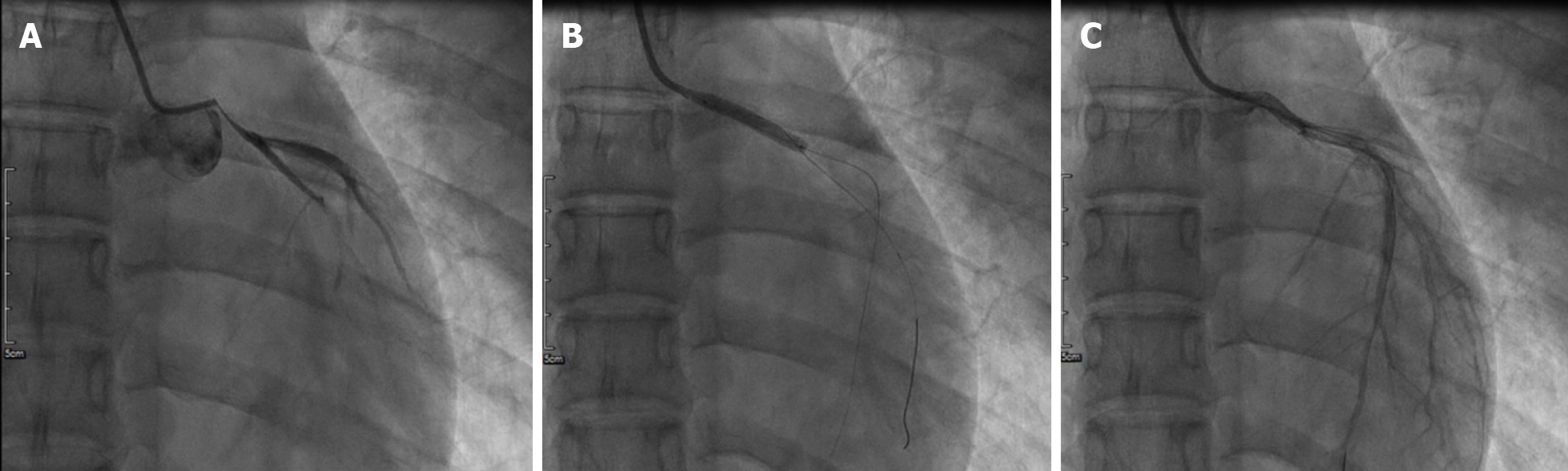
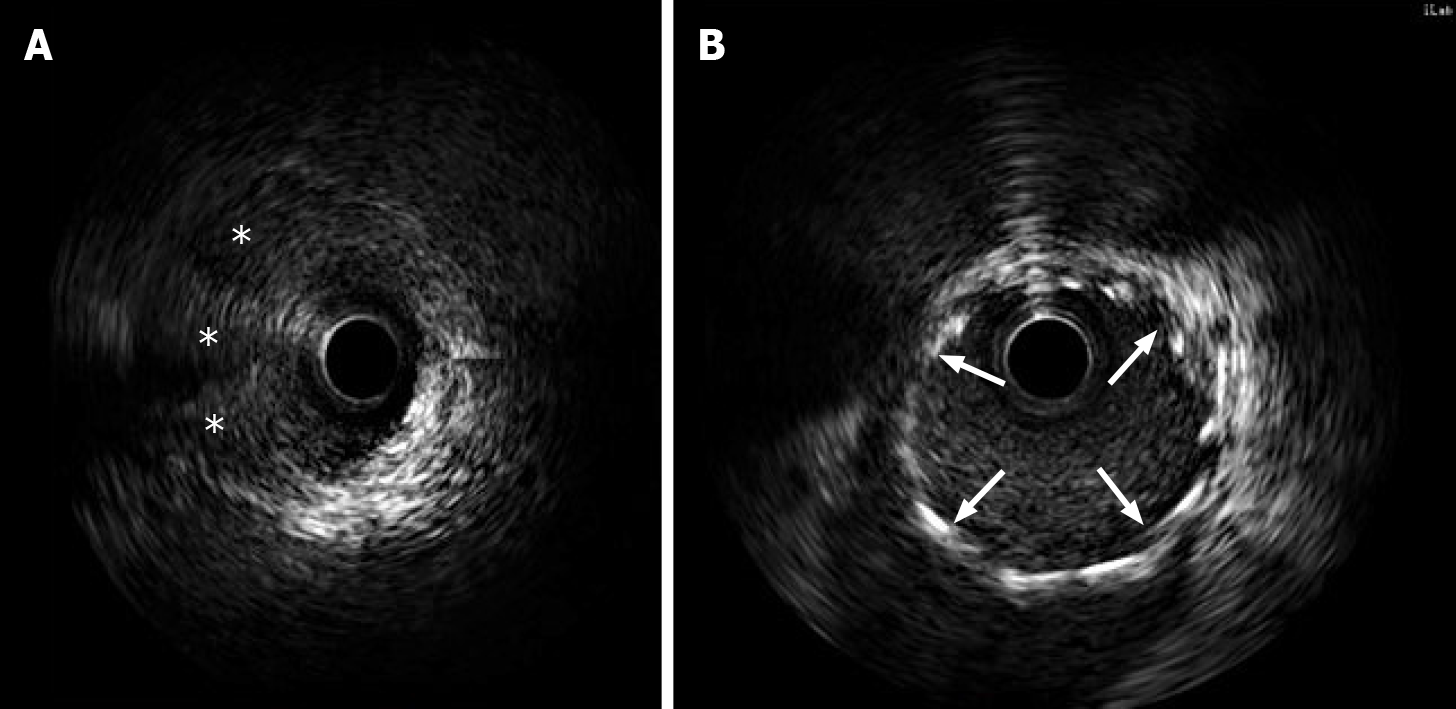
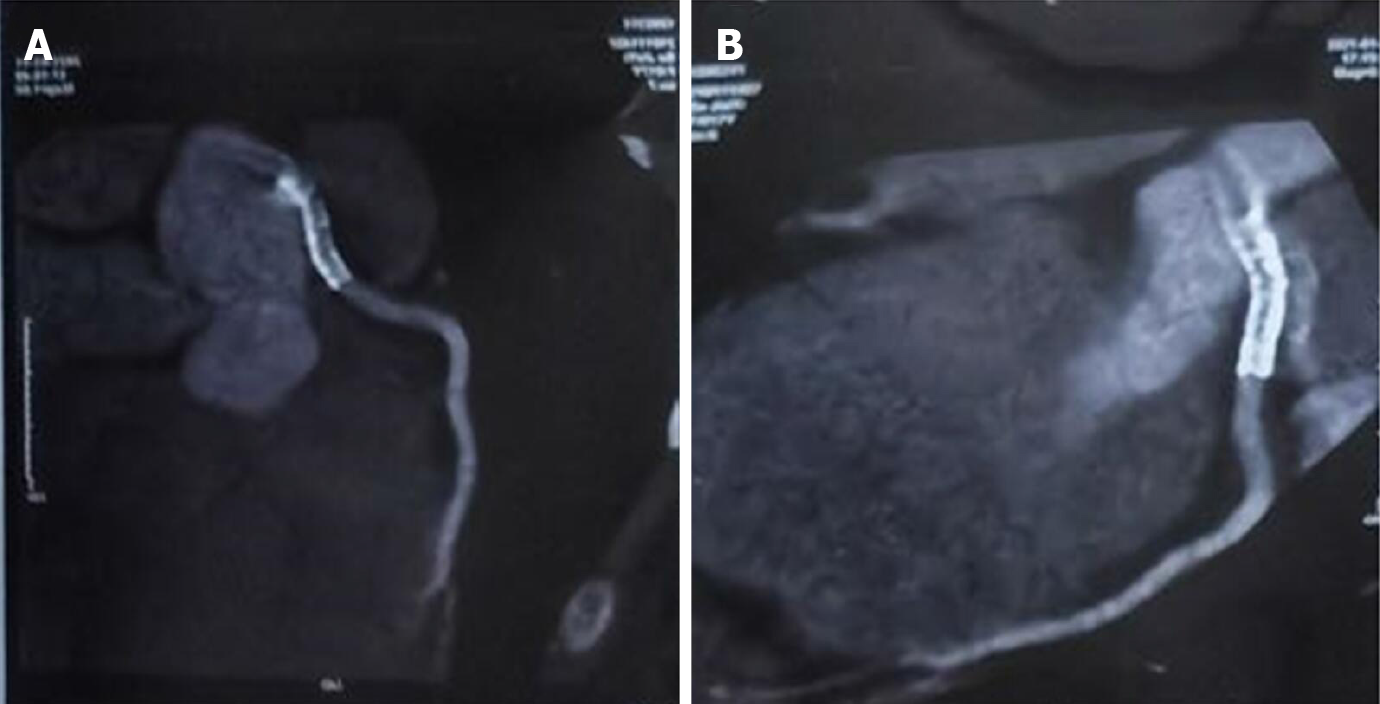
Her initial chest X-ray showed no sign of pulmonary edema. Transthoracic echocardiography revealed left lateral, anterior and posterior ventricular wall hypokinesis; minor mitral insufficiency; and a mildly reduced ejection fraction of 50%. An initial electrocardiogram (ECG) revealed sinus rhythm with poor R wave progression in leads V1 through V3 (Figure 1). Considering the young age, low coronary risk profile and atypical symptoms, the patient was initially diagnosed with suspected acute myocarditis after upper respiratory infection. She had continuous ECG monitoring, while a low dose of β-blocker was used to lower her heart rate. Then she was scheduled for cardiac magnetic resonance imaging (MRI) and coronary computed tomography angiography after 2 d. However, fifteen hours after admission, the patient experienced chest pain and sweating. The ECG indicated greater than 1 mm ST segment elevation in the anterolateral leads and broad ST depression in leads II, III, avF, V1, and V2 (Figure 1). When she was free of pain, the repeated ECG showed that the ST elevations were lesser (Figure 1). Twenty minutes later, she experienced intense chest pain. Repeated ECG indicated ST-segment elevation in leads V3 to V5 (Figure 1). After treatment with nitroglycerin, she did not complain about any discomfort, and the chest pain was substantially relieved. The ECG indicated that the elevation of the ST segment disappeared in multiple leads (Figure 1). Suspecting myocardial infarction, we then performed emergent coronary angiography, which demonstrated almost total occlusion at the ostium and the proximal segment of LMT (Figure 2A). IVUS during angiography identified intramural hematoma severely compressing the true lumen which extended from the LMT to the ostium of the left anterior descending artery (LAD) suggesting SCAD (Figure 3).
The diagnosis of spontaneous coronary artery dissection of the LMT was confirmed.
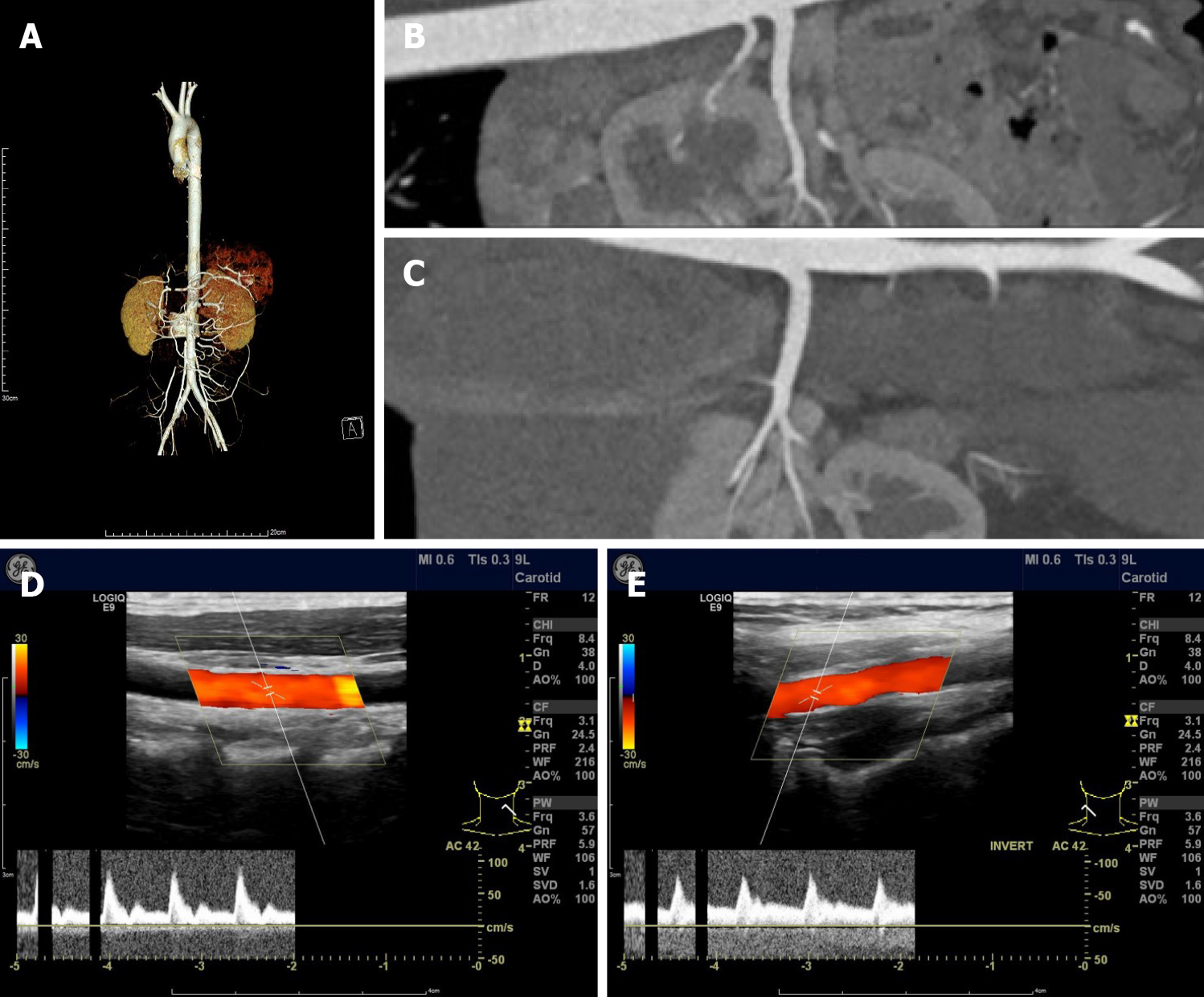
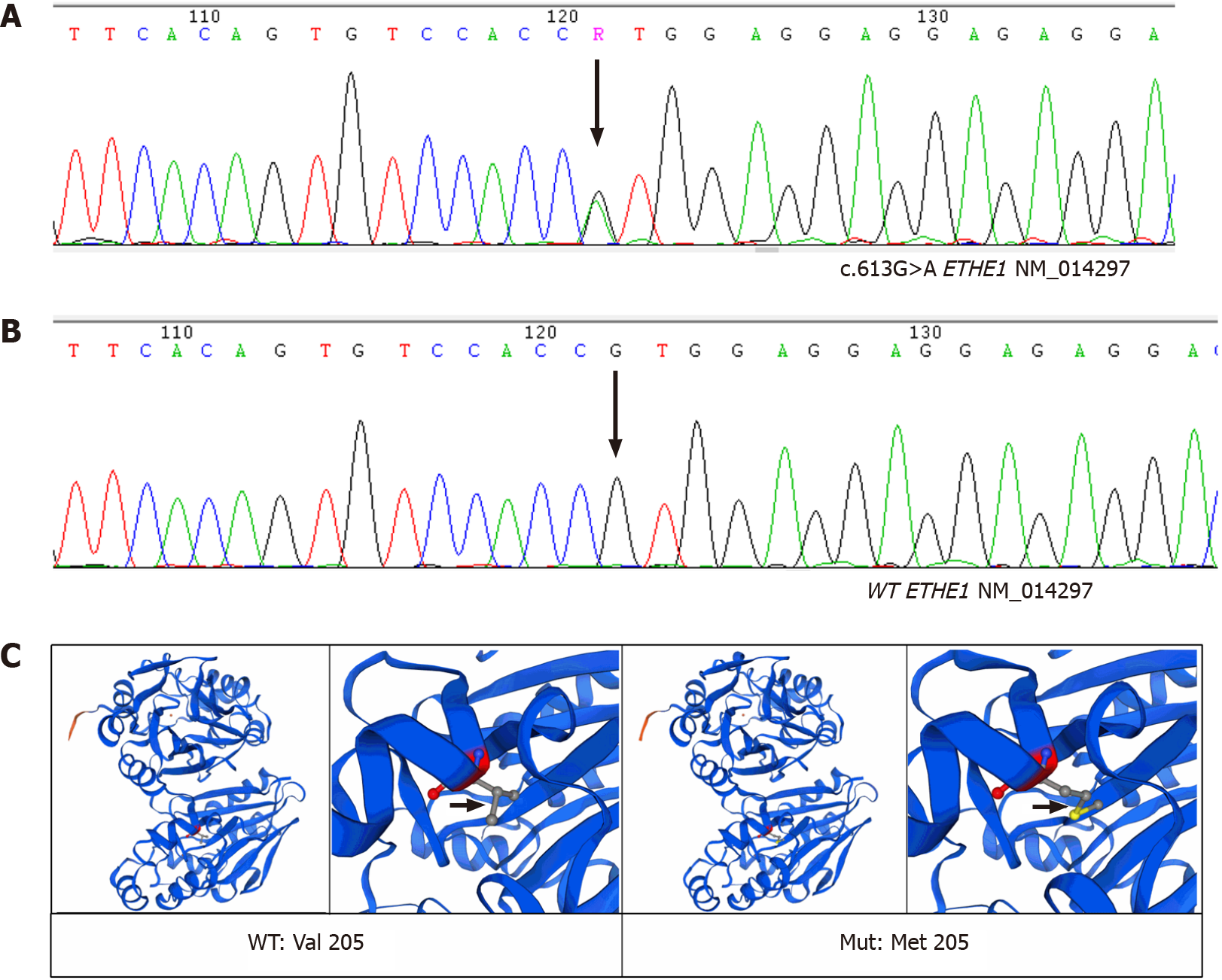
During coronary angiography, the patient suffered from cardiogenic shock and symptoms of congestive heart failure but was stabilized hemodynamically by norepinephrine 0.2 µg/kg/min and an Intra-aortic balloon pump (IABP). Sequentially, an everolimus-eluting stent (3.5 mm × 24 mm) was quickly deployed from the ostium of LMT to the proximal portion of LAD to fully cover the lesion and post-dilated under IVUS guidance using a 4.0-mm noncompliant balloon (Figure 2B and C). During the following week, antiplatelet and antithrombotic therapies were continued. She had no more chest pain, and the IABP was removed 72 h later. The high-sensitivity troponin T progressively decreased to 17.48 pg/mL. Repeat ECG revealed sinus rhythm with poor R wave progression in leads V1 and V2 (Figure 1). Transthoracic echocardiography showed an improved left ventricular ejection fraction of the left ventricle (57%) and normal diastolic function. On further testing, she had no abnormal vascular findings suggestive of fibromuscular dysplasia (Figure 4). She also had no clear evidence of collagen disease according to serum markers, including antinuclear antibodies, antineutrophil cytoplasmic antibodies, and anticardiolipin antibody. The possibly early-onset presentations of SCAD warrant clinical consideration of genetically mediated vascular conditions. To identify potentially causative gene variants, we performed whole-exome sequencing on genomic DNA isolated from the peripheral blood of this patient. No well-known pathological variants (e.g., in FBN1, SMAD3, COL3A1, TSR1, LMX1B, TGFBR1/R2[7-8]) were found, but we identified a novel heterozygous missense variant, c.613G>A (p.V205M), in the ETHE1 gene (Figure 5A). Structural models of the wild-type and mutant proteins were built using SWISSMODEL (https://www.swissmodel.expasy.org) (Figure 5C). Then, the computational algorithms SIFT, PolyPhen2 and MutationTaster were used to predict the pathogenicity of this novel variant. All these prediction tools presented it as a potentially damaging or disease-causing variant. We then advance the screening, no ETHE1 variant was found in her parents or her younger sister (Figure 5B and C). The remainder of the hospital stay was uneventful. The patient was subsequently discharged after 7 d. She was administered 100 mg aspirin, 75 mg ticagrelor bid and 456 mg polyene phosphatidyl choline tid to improve liver function.
The patient was asymptomatic and the Transaminases regressed to normal values at the 1, 6 mo. follow-up visits. 12 mo after this hospitalization, follow-up coronary computed tomography angiography (CCTA) at a local hospital demonstrated a stable condition at the PCI site (Figure 6), and the aspirin monotherapy was continued. At 24-mo. follow-up, the patient remained stable with no symptoms such as chest pain recurred. Bleeding complications were not reported (Figure 7).
SCAD is a frequent cause of ACS in young to middle-aged women with few or no traditional cardiovascular risk factors[9-11]. Most reports are large contemporary series with mean ages ranging from 44 to 53 years[12]. Although SCAD has a wide range of clinical presentations and severities, its presenting symptoms are consistent with atherosclerotic ACS, with chest pain being the most prevalent. As many as 3% to 11% of patients present with ventricular arrhythmias or sudden cardiac death[13]. A minority present with syncope (0.5%)[14]. Although any coronary artery can be affected, the LAD is the most affected (32%-46% of SCAD cases)[15]. In only < 10% of cases are the proximal coronary arteries affected[16].
Delayed diagnosis of SCAD is common because most acute medical and cardiology services are focused on the identification of patients at high risk of obstructive atherosclerotic ACS. In young patients, differential diagnosis during chest pain is not always easy. When ECG findings suggest a cardiac origin of such symptoms, myocarditis is usually the most likely hypothesis. In our patient, despite an invariably increased troponin level, the diagnosis was established hours later after taking into account typical angina symptoms and dynamic changes in the ECG. The young age of the patient and the severity of the condition, as well as the delayed diagnosis of SCAD, which led to a life-threatening condition in an otherwise young healthy girl, highlighting the value of documenting this case.
Data on SCAD in children and adolescents are scarce. Only 7 cases of SCAD in the under 19 population have been identified in the existing peer-reviewed literature (Table 2). In 4 of the 7 published cases, chest pain was the first clinical symptom of SCAD[17-20]. However, one other case of SCAD in an 18-year-old boy was asymptomatic[21]. As in the adult group, the LAD is reportedly the most affected coronary artery in adolescents with SCAD, but not in this case[17-18,21]. However, there were obvious differences between the adolescent group and the adult group with SCAD. First, an intriguing finding is that 5 of the 7 adolescent patients were male, while in adults, SCAD occurred overwhelmingly in females. According to the small sample size, this could be a casual phenomenon. Second, the adolescent group is not well studied and has a unique risk profile with fewer traditional cardiovascular risk factors than the adult group. SCAD has been described in 2 case reports of adolescent patients; one with neurofibromatosis type I[17] and one with systemic lupus erythematosus[22]. Acute triggering events involving the consumption of a caffeine -containing beverage[18], heavy exercise[19] and the use of methylphenidate[20] are suspected causes of SCAD. In one case, details of possible triggers and symptoms were not mentioned[23]. In our patient, the close temporal proximity between heavy exercise and the onset of syncope suggests a causal relationship. When compared to the other two LMT spontaneous dissection cases, our case is unique in that it is the first reported case of SCAD presenting with syncope, a relatively uncommon mani
| Ref. | Age/Sex | Site | Clinical presentation | Treatment | Possible triggers |
| Kothari et al[22], 2007 | 17/Boy | LCX | Unknown | Unknown | SLE |
| Rohit et al[23], 2008 | 14/Boy | LMT | Unknown | Medical treatment | Unknown |
| Uyar et al[17], 2012 | 17/Girl | LAD | Chest pain | CABG | Neurofibromatosis |
| Polat et al[18], 2013 | 13/Boy | LAD | Chest pain | Medical treatment | Caffeinated “energy drinks” |
| Cropp et al[19], 2013 | 14/Girl | LMT | Chest pain | CABG | Heavy exercise |
| Herry et al[21], 2013 | 18/Boy | LAD | Asymptomatic | Medical treatment | Unknown |
| Stammschulte et al[20], 2020 | 6/Boy | RCA | Chest pain | PCI | Methylphenidate use |
With respect to the upper respiratory infection three weeks before the event, further evaluation (serology and inflammation markers) did not yield evidence for an associated myocarditis or a systemic inflammatory response to this infection (Table 1). In addition, an upper respiratory infection has not yet been linked to coronary dissection.
Inherited arteriopathies and connective tissue disorders are infrequently reported as the underlying cause of SCAD (5%-8.2%)[26]. Cardiovascular genetic evaluation seems appropriate for patients with SCAD, especially young ones[27]. In our case, a genetic workup was done, and a mutation (c.613G>A, p.V205M) in the ETHE1 gene was found. ETHE1 is a 30-kDa polypeptide located in the mitochondrial matrix that functions as a homodimeric, Fe-containing sulfur dioxygenase involved in the catabolic oxidation of hydrogen sulfide (H2S) to sulfate[28]. Although H2S acts as a cytoprotective agent in trace amounts, at high concentrations it is a powerful toxic agent that inhibits some important enzymes with antioxidative and energy-producing effects, damaging vascular endothelial cells and contributing to vasculopathy. Our data suggest that patients with ETHE1 mutations may be more likely to experience SCAD. Limited by the one-patient sample, this obviously requires further investigation.
Management of SCAD differs significantly from that of atherosclerotic ACS. Medical management is suggested in SCAD patients who are hemodynamically stable in whom major coronary arteries are not involved. In high-risk patients with ongoing ischemia, left main artery dissection, or hemodynamic instability, it is the consensus that urgent intervention with PCI or CABG should be considered[1,6]. Our patient underwent PCI and received the standard guideline- based antiplatelet therapy after PCI. β-blockers, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers was not used because of her lower blood pressure level. Statin therapy was not adopted either. Transthoracic echocardiogram showed improved LV function, which was 50% on admission and 57% 3 d after PCI. She continues to be symptom-free at the 1, 6, 12, 24 mo. follow-up visits and still active as a college student.
SCAD in adolescents is rare and can easily be undiagnosed or misdiagnosed. Accurate and rapid diagnosis of SCAD in adolescents with suspected coronary ischemia is paramount and may be lifesaving. This rare disease should be known by all cardiologists and should always be actively considered in the differential diagnosis of ACS presentations in low-risk patients, regardless of age. To evaluate the long-term safety and efficacy of PCI for adolescents with LMT SCAD, further research is required.
Provenance and peer review: Unsolicited article; externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and Cardiovascular Systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ito S, Matsuo Y S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Hayes SN, Kim ESH, Saw J, Adlam D, Arslanian-Engoren C, Economy KE, Ganesh SK, Gulati R, Lindsay ME, Mieres JH, Naderi S, Shah S, Thaler DE, Tweet MS, Wood MJ; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous Coronary Artery Dissection: Current State of the Science: A Scientific Statement From the American Heart Association. Circulation. 2018;137:e523-e557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 819] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 2. | Henkin S, Negrotto SM, Tweet MS, Kirmani S, Deyle DR, Gulati R, Olson TM, Hayes SN. Spontaneous coronary artery dissection and its association with heritable connective tissue disorders. Heart. 2016;102:876-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 3. | Nakashima T, Noguchi T, Haruta S, Yamamoto Y, Oshima S, Nakao K, Taniguchi Y, Yamaguchi J, Tsuchihashi K, Seki A, Kawasaki T, Uchida T, Omura N, Kikuchi M, Kimura K, Ogawa H, Miyazaki S, Yasuda S. Prognostic impact of spontaneous coronary artery dissection in young female patients with acute myocardial infarction: A report from the Angina Pectoris-Myocardial Infarction Multicenter Investigators in Japan. Int J Cardiol. 2016;207:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 249] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 4. | Saw J, Aymong E, Sedlak T, Buller CE, Starovoytov A, Ricci D, Robinson S, Vuurmans T, Gao M, Humphries K, Mancini GB. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv. 2014;7:645-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 540] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 5. | Saw J, Mancini GBJ, Humphries KH. Contemporary Review on Spontaneous Coronary Artery Dissection. J Am Coll Cardiol. 2016;68:297-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 390] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 6. | Adlam D, Alfonso F, Maas A, Vrints C; Writing Committee. European Society of Cardiology, acute cardiovascular care association, SCAD study group: a position paper on spontaneous coronary artery dissection. Eur Heart J. 2018;39:3353-3368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 460] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 7. | Sun Y, Chen Y, Li Y, Li Z, Li C, Yu T, Xiao L, Yu B, Zhao H, Tao M, Jiang J, Yan J, Wang Y, Zeng H, Shen X, Zhou Y, Jin L, Song W, Dou K, Wang DW. Association of TSR1 Variants and Spontaneous Coronary Artery Dissection. J Am Coll Cardiol. 2019;74:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Bai B, Zhang M, Zhuang Y, Zhu J, Li W, Ma W, Chen H. The pregnancy-associated spontaneous coronary artery dissection in a young woman with a novel missense mutation in NOTCH1: a case report. BMC Med Genet. 2020;21:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Keepanasseril A, Pfaller B, Metcalfe A, Siu SC, Davis MB, Silversides CK. Cardiovascular Deaths in Pregnancy: Growing Concerns and Preventive Strategies. Can J Cardiol. 2021;37:1969-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Saw J, Mancini GB, Humphries K, Fung A, Boone R, Starovoytov A, Aymong E. Angiographic appearance of spontaneous coronary artery dissection with intramural hematoma proven on intracoronary imaging. Catheter Cardiovasc Interv. 2016;87:E54-E61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 11. | Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, Gersh BJ, Khambatta S, Best PJ, Rihal CS, Gulati R. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012;126:579-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 641] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 12. | Al-Hussaini A, Adlam D. Spontaneous coronary artery dissection. Heart. 2017;103:1043-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 13. | Tweet MS, Eleid MF, Best PJ, Lennon RJ, Lerman A, Rihal CS, Holmes DR Jr, Hayes SN, Gulati R. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv. 2014;7:777-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 447] [Article Influence: 40.6] [Reference Citation Analysis (1)] |
| 14. | Lettieri C, Zavalloni D, Rossini R, Morici N, Ettori F, Leonzi O, Latib A, Ferlini M, Trabattoni D, Colombo P, Galli M, Tarantini G, Napodano M, Piccaluga E, Passamonti E, Sganzerla P, Ielasi A, Coccato M, Martinoni A, Musumeci G, Zanini R, Castiglioni B. Management and Long-Term Prognosis of Spontaneous Coronary Artery Dissection. Am J Cardiol. 2015;116:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 220] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 15. | Rashid HN, Wong DT, Wijesekera H, Gutman SJ, Shanmugam VB, Gulati R, Malaipan Y, Meredith IT, Psaltis PJ. Incidence and characterisation of spontaneous coronary artery dissection as a cause of acute coronary syndrome--A single-centre Australian experience. Int J Cardiol. 2016;202:336-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 148] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 16. | Rogowski S, Maeder MT, Weilenmann D, Haager PK, Ammann P, Rohner F, Joerg L, Rickli H. Spontaneous Coronary Artery Dissection: Angiographic Follow-Up and Long-Term Clinical Outcome in a Predominantly Medically Treated Population. Catheter Cardiovasc Interv. 2017;89:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 188] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 17. | Uyar IS, Uyar B, Okur FF, Akpinar B, Abacilar F, Ates M. A 17-year-old with neurofibromatosis and spontaneous coronary artery dissection. Asian Cardiovasc Thorac Ann. 2012;20:724-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Polat N, Ardıç I, Akkoyun M, Vuruşkan E. Spontaneous coronary artery dissection in a healthy adolescent following consumption of caffeinated "energy drinks". Turk Kardiyol Dern Ars. 2013;41:738-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Cropp EM, Turner JS, Kreutz RP. Spontaneous coronary artery dissection in a 14-year-old. Am J Emerg Med. 2013;31:461.e5-461.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Stammschulte T, Pitzer M, Rascher W, Becker M, Pohlmann U, Ostermayer S, Kerst G. Acute myocardial infarction due to spontaneous coronary artery dissection in a 6-year-old boy with ADHD on the third day of treatment with methylphenidate. Eur Child Adolesc Psychiatry. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Herry Y, Tonang A, Herlambang V, Anggriyani N. Spontaneous coronary artery dissection causing myocardial infarction in an 18-year-old man: A case report. Australas Med J. 2013;6:694-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Kothari D, Ruygrok P, Gentles T, Occleshaw C. Spontaneous coronary artery dissection in an adolescent man with systemic lupus erythematosus. Intern Med J. 2007;37:342-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Rohit MK, Garg PK, Hariram V, Gupta A, Grover A. Idiopathic spontaneous coronary artery dissection presenting as acute myocardial infarction in a young boy. Indian Heart J. 2008;60:346-348. [PubMed] |
| 24. | Macaya F, Salinas P, Gonzalo N, Fernández-Ortiz A, Macaya C, Escaned J. Spontaneous coronary artery dissection: contemporary aspects of diagnosis and patient management. Open Heart. 2018;5:e000884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 25. | Georges A, Albuisson J, Berrandou T, Dupré D, Lorthioir A, D'Escamard V, Di Narzo AF, Kadian-Dodov D, Olin JW, Warchol-Celinska E, Prejbisz A, Januszewicz A, Bruneval P, Baranowska AA, Webb TR, Hamby SE, Samani NJ, Adlam D, Fendrikova-Mahlay N, Hazen S, Wang Y, Yang ML, Hunker K, Combaret N, Motreff P, Chédid A, Fiquet B, Plouin PF, Mousseaux E, Azarine A, Amar L, Azizi M, Gornik HL, Ganesh SK, Kovacic JC, Jeunemaitre X, Bouatia-Naji N. Rare loss-of-function mutations of PTGIR are enriched in fibromuscular dysplasia. Cardiovasc Res. 2021;117:1154-1165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Kaadan MI, MacDonald C, Ponzini F, Duran J, Newell K, Pitler L, Lin A, Weinberg I, Wood MJ, Lindsay ME. Prospective Cardiovascular Genetics Evaluation in Spontaneous Coronary Artery Dissection. Circ Genom Precis Med. 2018;11:e001933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 27. | Di Fusco SA, Rossini R, Zilio F, Pollarolo L, di Uccio FS, Iorio A, Lucà F, Gulizia MM, Gabrielli D, Colivicchi F. Spontaneous coronary artery dissection: Overview of pathophysiology. Trends Cardiovasc Med. 2021;14:S1050-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Goudarzi S, Babicz JT Jr, Kabil O, Banerjee R, Solomon EI. Spectroscopic and Electronic Structure Study of ETHE1: Elucidating the Factors Influencing Sulfur Oxidation and Oxygenation in Mononuclear Nonheme Iron Enzymes. J Am Chem Soc. 2018;140:14887-14902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |