Published online Mar 6, 2022. doi: 10.12998/wjcc.v10.i7.2247
Peer-review started: July 9, 2021
First decision: October 22, 2021
Revised: November 3, 2021
Accepted: January 22, 2022
Article in press: January 22, 2022
Published online: March 6, 2022
Processing time: 235 Days and 20.3 Hours
Complications of vascular closure devices mainly include bleeding, vascular injury, and trapped device that cannot be removed percutaneously. However, arterial stenosis or occlusion induced by vascular injury is rare. This article introduces a rare case with severe acute limb ischemia after using the vascular closure device (StarClose).
A 54-year-old man was admitted because of necrosis of the second toe of the left foot for 2 mo. Ultrasound showed left femoral artery stenosis, and occlusion of the left popliteal, posterior tibial, peroneal, anterior tibial and dorsalis pedis arteries, suggesting arteriosclerosis obliterans of low extremities, gangrene and type 2 diabetes. He underwent an interventional procedure of drug-eluting balloon in the left lower limb via antegrade puncture of the left common femoral artery. He developed acute limb ischemia after 1 h, and severe pain, numbness, pale skin, low skin temperature and weakened sensation in the left foot. Injury of the common femoral artery intima was considered. Exploratory surgery showed occlusion at the puncture point accompanied with bulged vascular lumen and flipped vascular intima caused by StarClose. The flipped intima was removed. The limb blood supply was restored and the limb was saved post-surgery. He recovered well at final follow-up.
Incorrect use of the vascular closure device was the main cause of severe acute limb ischemia in this case.
Core Tip: Clinical reports on the complications of vascular injury caused by vascular closure devices are rare. Here, we report a case of severe acute limb ischemia after using a vascular closure device (StarClose). The case suggests that use of the vascular closure device was the main cause of severe acute limb ischemia in this case. The vascular closure device should be operated carefully.
- Citation: Sun LX, Yang XS, Zhang DW, Zhao B, Li LL, Zhang Q, Hao QZ. Flip-over of blood vessel intima caused by vascular closure device: A case report. World J Clin Cases 2022; 10(7): 2247-2252
- URL: https://www.wjgnet.com/2307-8960/full/v10/i7/2247.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i7.2247
Vascular closure devices overcome the limitations of traditional hemostasis methods, achieve rapid hemostasis and shorten the in-bed time after surgery. However, they also have some complications, with the most common being bleeding and the least common stenosis or occlusion caused by vascular injury[1]. At present, clinical reports on the complications of vascular injury caused by vascular closure devices are rare. In this article, one case of severe acute limb ischemia after using vascular closure device (StarClose) is reported.
The patient was a 54-year-old man who was admitted due to cyanotic necrosis and severe pain in the second toe.
The patient had the necrosis of the second toe of the left foot for 2 mo.
The patient had a history of smoking for 30 years and type 2 diabetes for 1 mo.
The pulse of the femoral artery of the left lower limb was weakened, and those of the popliteal, posterior tibial, and dorsal foot arteries were not palpable.
Blood routine examination showed white blood cell count 9.46 × 109/L, absolute neutrophils 6.43 × 109/L, absolute monocytes 0.78 × 109/L, absolute basophils 0.07 × 109/L, erythrocytes 9.46 × 1012/L, hemoglobin 148 g/L, and platelets 299 × 109/L. Blood biochemical results indicated blood potassium 4.63 mmol/L, alanine aminotransferase 50 U/L, aspartate aminotransferase 22 U/L, total bilirubin 9.2 mol/L, creatinine of 47 mol/L, glomerular filtration rate 120.06 mol/L, homocysteine 11.2 mol/L, glucose 6.48 mmol/L, glycosylated hemoglobin 10.6%, and erythrocyte sedimentation rate 22 mm/h. Blood coagulation test suggested international standardized ratio 1.03, plasma prothrombin time 13.6 s, activated partial thromboplastin time 42.6 s, plasma fibrinogen 5.76 g/L, and thrombin time 16.5 s.
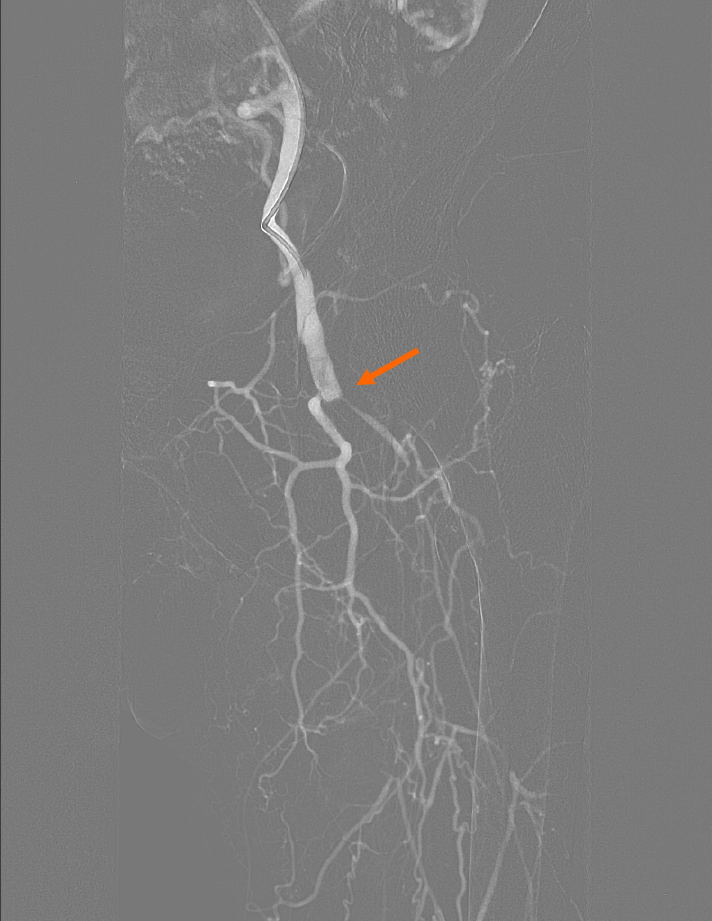
Color Doppler ultrasound showed left femoral artery stenosis, and occlusion of the left popliteal, posterior tibial, peroneal, anterior tibial and dorsalis pedis arteries. The diagnosis of arteriosclerosis obliterans of the lower extremity accompanied with gangrene and type 2 diabetes was made. Under local anesthesia, the interventional procedure of drug-eluting balloon in the left lower limb was performed via antegrade puncture of the left common femoral artery. The operation went smoothly. However, 1 h after the puncture point was closed by the StarClose vascular closure device, the patient developed manifestations of acute ischemia, including severe pain, numbness, pale skin, low skin temperature and weakened sensation in the left foot. The pulse of the left common femoral artery disappeared by palpation. Arterial angiography of the left lower extremity showed occlusion of the middle and distal segment of the common femoral artery, but no thrombus was observed (Figure 1).
The injury of the common femoral artery intima was considered.
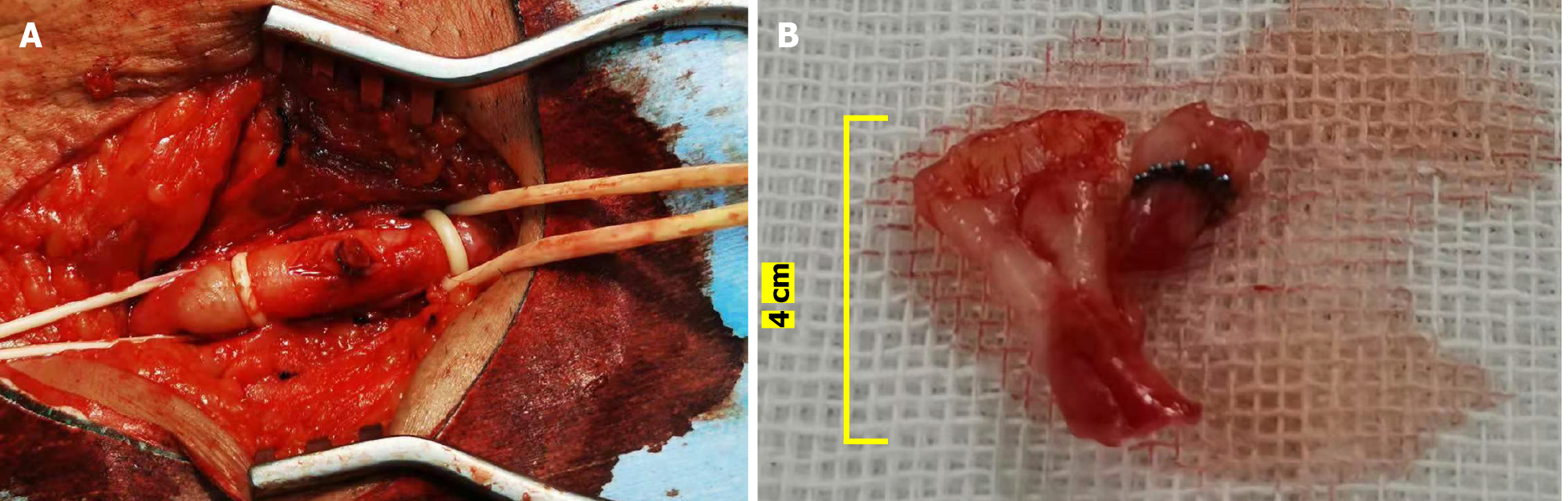
Exploratory surgery was performed on the left common femoral artery. Occlusion at the puncture point was observed with bulged vascular lumen. There was intima tissue in the vascular lumen. An incision of about 1.5 cm was made at the puncture point. We observed that the intima embedded with the nickel–titanium alloy clip of the StarClose vascular closure device flipped over and blocked the lumen (Figure 2A). The flipped intimal tissue of about 4 cm was removed during the operation (Figure 2B). A jet-like blood flow was observed at the distal end of the common femoral artery incision. The common femoral artery incision was sutured and repaired without using the StarClose vascular closure device.
Arterial angiography showed smooth blood flow in the common and superficial femoral artery. Color Doppler ultrasound of the left lower limb artery showed that the blood flow of the left common iliac, common femoral, popliteal, anterior tibial and peroneal arteries was unobstructed. The patient was given antiplatelet, anticoagulation, anti-infection and microcirculatory improvement drugs after surgery. The patient was discharged after hospitalization for 11 d. During follow-up at 6 mo after surgery, the patient recovered well. The patient is under constant follow-up.
For a long time, manual compression of the puncture point was the traditional method of hemostasis after vascular interventional therapy. However, this required no less than 30 min of continuous compression and several or even dozens of hours of immobilization after the operation[2,3]. To overcome the limitations of traditional hemostasis methods, the vascular closure devices have been developed since the 1990s. However, the vascular closure devices have complications during clinical application. Bleeding is the most common one, and the incidence of stenosis or occlusion caused by vascular injury is the lowest[4]. In the present case, the patient had acute limb arterial ischemia after intervention. It is considered that during the placement of StarClose vascular closure device, due to incorrect operation, the nickel–titanium alloy clip of the device clamped the intima of the blood vessel. When it was pulled out violently, the intima of the blood vessel was flipped over, resulting in arterial stenosis and occlusion, and causing ischemic symptoms at the distal end of the limb. Finally, the occluded segment of the intima was removed through femoral artery incision, and the femoral artery was repaired to restore the blood supply of the affected limb. Dzieciuchowicz et al[1] also reported a case of acute limb arterial ischemia caused by the incorrect release of the StarClose vascular closure device in a 31-year-old woman. In our case and the case reported by Dzieciuchowicz et al[1], surgical incision was used to restore the blood supply of the affected limb. Although some scholars have suggested that the stenosis and occlusive complications should be resolved through endovascular treatment[5,6], surgical incision has to be performed in some cases according to the type and location of the lesion as well as the severity of limb ischemia.
According to previous reports, complications occur in 3%-4% of cases intervened with StarClose vascular closure devices[7-9], with bleeding as the most common complication. The inguinal complications are classified into self-limiting hematoma, hematoma requiring blood transfusion, other/minor (pseudoaneurysm and infection) or other/severe (vascular complications) complications. For example, McTaggar et al[10] used StarClose in 281 patients undergoing interventional surgery and found that the incidence of self-limiting hematoma was the highest, and no patients had other/minor complications. Gaba et al[11] observed 83 patients with liver tumors using StarClose vascular closure device as interventional treatment and found that only three (3.6%) developed small inguinal hematomas after the operation. The least common complication of the vascular closure device was acute limb ischemia, vascular stenosis or occlusion. Rodriguez et al[4] reported that among 603 patients receiving intervention with StarClose vascular closure, only two had this complication, including one case of common femoral artery occlusion, and another of common femoral artery stenosis. Another common complication[12] is that the nickel–titanium alloy clip of the vascular closure device is compressed or stuck by the scar tissue or normal tissue at the puncture site and cannot be removed percutaneously. The Medical Device Adverse Events database of the US FDA showed that from July 2009 to October 2010, there were 224 cases with complications of stuck StarClose vascular closure device[13]. The surgeon should pull the handle of the closure device linearly[14] and then remove it smoothly. Studies have shown that the release of the vascular closure device under ultrasound guidance can reduce the number of complications[15-17]. In addition, although the vascular closure device has significant advantages in closing large-sized blood vessels, its operation is complicated[18]. Once the operation fails, it will not only fail to stop bleeding, but also cause some delayed complications (such as secondary thrombosis and pseudoaneurysm)[10,19-21]. The complications secondary to StarClose are summarized in Table 1. Therefore, the proficiency of the surgeons handling the vascular closure device should be improved, and the operation should be cautious and standardized, so as to avoid the occurrence of complications.
| Complications secondary to StarClose | Incidence % (n/N) |
| Failure to stop bleeding immediately[20] | 8.7% (15/171) |
| Small inguinal hematoma[10] | 3.6% (3/83) |
| Pseudoaneurysm[20] | 0.6% (1/156) |
| Retroperitoneal hematoma[21] | 0.43% (461/107710) |
| Acute limb ischemia[11] | 0.33% (2/603) |
| Self-limiting hematoma[10] | 1.4%(4/281) |
| Hematoma requiring blood transfusion[10] | 0.4%(1/281) |
This article reports a rare case of acute limb ischemia after using a vascular closure device. This case suggests that the vascular closure device should be operated carefully and standardized within the scope of the instructions to reduce complications. In addition, strengthening the training and supervision of clinicians is also important.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Peripheral vascular disease
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vunnam SR S-Editor: Ma YJ L-Editor: Kerr C P-Editor: Ma YJ
| 1. | Dzieciuchowicz Ł, Zmysłowski M, Stefaniak K, Oszkinis G. Acute limb ischemia caused by incorrect deployment of a clip-based arterial closure device. Wideochir Inne Tech Maloinwazyjne. 2016;11:111-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Krishnasamy VP, Hagar MJ, Scher DJ, Sanogo ML, Gabriel GE, Sarin SN. Vascular Closure Devices: Technical Tips, Complications, and Management. Tech Vasc Interv Radiol. 2015;18:100-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Kim SH, Behnes M, Baron S, Shchetynska-Marinova T, Tekinsoy M, Mashayekhi K, Hoffmann U, Borggrefe M, Akin I. Differences of bleedings after percutaneous coronary intervention using femoral closure and radial compression devices. Medicine (Baltimore). 2019;98:e15501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Rodriguez A, Katz SG. The use of the StarClose device for obtaining femoral artery hemostasis. Vasc Endovascular Surg. 2011;45:627-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Rekik S, Brunet J, Bayet G, Hager FX, Meille L, Quatre JM, Sainsous J. Percutaneous management of lower limb ischemia after the use of vascular closure devices. Can J Cardiol. 2013;29:1448-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Milnerowicz AI, Milnerowicz AA, Protasiewicz M, Kuliczkowski W. Use of vascular closure devices for endovascular interventions requiring a direct puncture of PETE grafts. Vasa. 2018;47:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Hermiller JB, Simonton C, Hinohara T, Lee D, Cannon L, Mooney M, O'Shaughnessy C, Carlson H, Fortuna R, Zapien M, Fletcher DR, DiDonato K, Chou TM. The StarClose Vascular Closure System: interventional results from the CLIP study. Catheter Cardiovasc Interv. 2006;68:677-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Gonen KA, Hakyemez B, Erdogan C. Analysis of predictive and preventive factors for access complications associated with vascular closure devices in complicated endovascular procedures. Jpn J Radiol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Jaff MR, Hadley G, Hermiller JB, Simonton C, Hinohara T, Cannon L, Reisman M, Braden G, Fletcher DR, Zapien M, Chou TM, DiDonato K. The safety and efficacy of the StarClose Vascular Closure System: the ultrasound substudy of the CLIP study. Catheter Cardiovasc Interv. 2006;68:684-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | McTaggart RA, Raghavan D, Haas RA, Jayaraman MV. StarClose vascular closure device: safety and efficacy of deployment and reaccess in a neurointerventional radiology service. AJNR Am J Neuroradiol. 2010;31:1148-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Gaba RC, Parvinian A, Trinos EM, Padayao SV, Francisco RM, Yap FY, Knuttinen MG, Owens CA, Bui JT. Safety and efficacy of StarClose SE Vascular Closure System in high-risk liver interventional oncology patients. J Vasc Access. 2012;13:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Noori VJ, Eldrup-Jørgensen J. A systematic review of vascular closure devices for femoral artery puncture sites. J Vasc Surg. 68:887-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 13. | US Food and Drug Administration. MAUDE - Manufacturer and user facility device experience. October 20, 2010; updated June 30, 2011. |
| 14. | Durack JC, Thor Johnson D, Fidelman N, Kerlan RK, LaBerge JM. Entrapment of the StarClose Vascular Closure System after attempted common femoral artery deployment. Cardiovasc Intervent Radiol. 2012;35:942-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Lo RC, Fokkema MT, Curran T, Darling J, Hamdan AD, Wyers M, Martin M, Schermerhorn ML. Routine use of ultrasound-guided access reduces access site-related complications after lower extremity percutaneous revascularization. J Vasc Surg. 2015;61:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Kennedy SA, Rajan DK, Bassett P, Tan KT, Jaberi A, Mafeld S. Complication rates associated with antegrade use of vascular closure devices: a systematic review and pooled analysis. J Vasc Surg. 2021;73:722-730.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Choo HJ, Jeong HW, Park JY, Jin SC, Kim ST, Seo JH, Lee SJ, Park YM. Ultrasonographic features of vascular closure devices: initial and 6-month follow-up results. Ultrasonography. 2014;33:283-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Kim SH, Behnes M, Baron S, Shchetynska-Marinova T, Uensal M, Mashayekhi K, Hoffmann U, Borggrefe M, Akin I. Extravascular compared to Intravascular Femoral Closure is Associated with Less Bleeding and Similar MACE after Percutaneous Coronary Intervention. Int J Med Sci. 2019;16:43-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Afify E, Patel R, Elmetwally A, Al-Khaffaf H. Delayed Complication Secondary to Unsuccessful Deployment of the StarClose Device. Ann Vasc Surg. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Burke MN, Hermiller J, Jaff MR. StarClose vascular closure system (VCS) is safe and effective in patients who ambulate early following successful femoral artery access closure--results from the RISE clinical trial. Catheter Cardiovasc Interv. 2012;80:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Resnic FS, Wang TY, Arora N, Vidi V, Dai D, Ou FS, Matheny ME. Quantifying the learning curve in the use of a novel vascular closure device: an analysis of the NCDR (National Cardiovascular Data Registry) CathPCI registry. JACC Cardiovasc Interv. 2012;5:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |