Published online Mar 6, 2022. doi: 10.12998/wjcc.v10.i7.2184
Peer-review started: October 5, 2021
First decision: October 22, 2021
Revised: October 25, 2021
Accepted: January 25, 2022
Article in press: January 25, 2022
Published online: March 6, 2022
Processing time: 148 Days and 3.7 Hours
In recent years, the predictive role of YKL-40 for long-term survival in colorectal cancer patients has been gradually investigated. However, whether it is a reliable and valuable prognostic indicator for patients with colorectal carcinoma has not been verified.
To identify the prognostic value of serum/plasma concentration of YKL-40 or expression status of YKL-40 in tumor cells in colorectal carcinoma patients.
Several electronic databases including the PubMed, EMBASE, Web of Science, CNKI, VIP and WanFang were searched for relevant studies. The hazard ratios (HR) and 95% confidence intervals (CI) were combined and the primary and secondary outcomes were overall survival (OS) and progression-free survival (PFS), respectively. All statistical analysis were conducted by STATA 15.0 software.
A total of nine studies involving 2545 patients were included. The pooled results indicated that YKL-40 was significantly associated with poor OS (HR = 1.80, 95%CI: 1.32-2.45, P < 0.001) and PFS (HR = 1.62, 95%CI: 1.22-2.16, P = 0.001). Subgroup analysis stratified by the treatment, tumor type and source of YKL-40 showed similar results.
Elevated serum/plasma concentration of YKL-40 or positive expression in tumor cells was related with worse prognosis of colorectal carcinoma patients. YKL-40 might serve as a novel and reliable indicator for the evaluation of prognosis in colorectal cancer.
Core Tip: Our study demonstrated that YKL-40 was significantly associated with poor OS (P < 0.001) and PFS (P = 0.001). Subgroup analysis stratified by the treatment, tumor type and source of YKL-40 showed similar results. Elevated serum/plasma concentration of YKL-40 or positive expression in tumor cells was related with worse prognosis of colorectal carcinoma patients. YKL-40 might serve as a novel and reliable indicator for the evaluation of prognosis in colorectal cancer.
- Citation: Wang J, Qi S, Zhu YB, Ding L. Prognostic value of YKL-40 in colorectal carcinoma patients: A meta-analysis. World J Clin Cases 2022; 10(7): 2184-2193
- URL: https://www.wjgnet.com/2307-8960/full/v10/i7/2184.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i7.2184
Colorectal carcinoma is one of the most common cancers worldwide, although a slow decline of the overall risk of colorectal cancer is observed in recent years, especially in elderly population[1-3]. Despite of the great advances in the surgical, neoadjuvant and adjuvant treatments, colorectal cancer patients still suffer from poor prognosis[4]. Moreover, most of colorectal cancer patients received surgical treatment, even those with distant metastasis, but 30%-50% of stage II-III patients and 50% with stage IV patients who receive the surgery will develop a recurrence[5-7].
Up to now, the main predictor of prognosis is still the disease stage at the time of diagnosis. Unfortunately, patients in the same stage of tumor progression might undergo a different course of disease. Thus, it is still an urgent issue to identify more biomarkers to predict posttreatment survival accurately and contribute to the formulation of appropriate treatment strategies, which is crucial to reduce the mortality of colorectal carcinoma patients.
In recent years, a number of studies have indicated that Chitinase-3-like protein 1, also known YKL-40, might be a potential candidate biomarker and therapeutic target in cancers[8-10]. It has been reported that YKL-40 is a highly conserved glycoprotein binding to heparin that is produced by immune cells such as the macrophages, neutrophils, as well as the tumor cells and tumor-associated macrophages[8]. According to previous literatures, the encoding gene of YKL-40 is located on the chromosome 1q32.1 and its increasing expression is usually observed in normal cells with high proliferation, differentiation ability and cellular activity[9,10]. Besides, the overexpression of YKL-40 gene has been reported in several cancers such as the glioblastoma, melanoma, small cell lung cancer and colorectal carcinoma[11,12]. High tissue expression of YKL-40 protein detected by the immunohistochemistry in the glioblastoma is related with poor differentiation, advanced disease stage, poorer radiation response and also worse prognosis[13]. Furthermore, it is also reported that the serum concentration of YKL-40 is elevated in several tumors such as the breast cancer, melanoma, ovarian cancer and renal cell cancer and associated with poor response to therapies, advanced disease stage and poor survival[14-17]. Several investigators explored the clinical role of YKL-40 in colorectal carcinoma patients, especially its prognostic value[18-28]. However, inconsistent results have been reported in their studies.
Thus, the aim of the current meta-analysis was to identify the prognostic value of YKL-40 in colorectal carcinoma, which might help with evaluation of prognosis and formulation of treatment strategy for colorectal cancer patients.
This systematic review and meta-analysis were conducted according to the Preferred reporting items for systematic reviews and meta-analyses guidelines[29].
The PubMed, EMBASE, Web of Science, CNKI, VIP and WanFang databases were searched up to September 27, 2021. The following key words were used: YKL-40, colon, rectum, rectal, colorectal, tumor, cancer, carcinoma, neoplasm, prognosis, survival and prognostic. Meanwhile, the references of included studies were also assessed for availability.
The following inclusion criteria were applied: (1) Patients were pathologically diagnosed with primary colorectal carcinoma; (2) Patients were divided into two groups (elevated vs normal serum/plasma concentration or positive vs negative expression in tumor cells) and the long-term survival of patients were compared between the two groups; (3) HR with corresponding 95%CI for OS or PFS were provided or could be calculated from the Kaplan-Meier survival curves; and (4) High-quality studies with the (Newcastle-Ottawa scale, NOS) score of 6 or higher[30] .
The following exclusion criteria were applied: (1) Duplicated or overlapped data; and (2) Conference abstracts, case reports, letters or reviews.
The following information were collected from included studies: Author, publication year, sample size, gender, age, country, tumor stage, number of colon carcinoma patients, type of treatment (surgery vs non-surgery) and tumor, threshold of YKL-40 and endpoints with corresponding HR with 95%CI.
The quality of included studies was evaluated according to the NOS score[30] and only high-quality studies with a NOS score of 6 or higher were included.
The literature retrieval, selection, data extraction and quality assessment were all conducted by two authors independently (Jian Wang and Yu-Bing Zhu) and any differences were resolved by team discussion.
All statistical analyses were performed by STATA 15.0 software. The HR with corresponding 95%CI were combined to assess the association between YKL-40 and prognosis of colorectal carcinoma patients. If the HRs with 95%CI were not provided in articles directly, then they would be calculated from the Kaplan-Meier survival curves[31]. The heterogeneity among included studies was evaluated by I2 statistics and Q test. When obvious heterogeneity was observed presenting as the I2 > 50% or (and) P < 0.1, the random effect model was used; otherwise, the fix effect model was used[32]. The sensitivity analysis and subgroup analysis based on the treatment (surgery vs no-surgery), tumor type (colorectal carcinoma vs rectal carcinoma vs colon carcinoma) and source of YKL-40 (serum/plasma vs tissue) were performed to detect the source of heterogeneity and evaluated the stability of pooled results. Besides, the Begg’s funnel plot and Egger’s test were conducted to detect publication bias[33]. Significant publication bias was defined as P < 0.05.
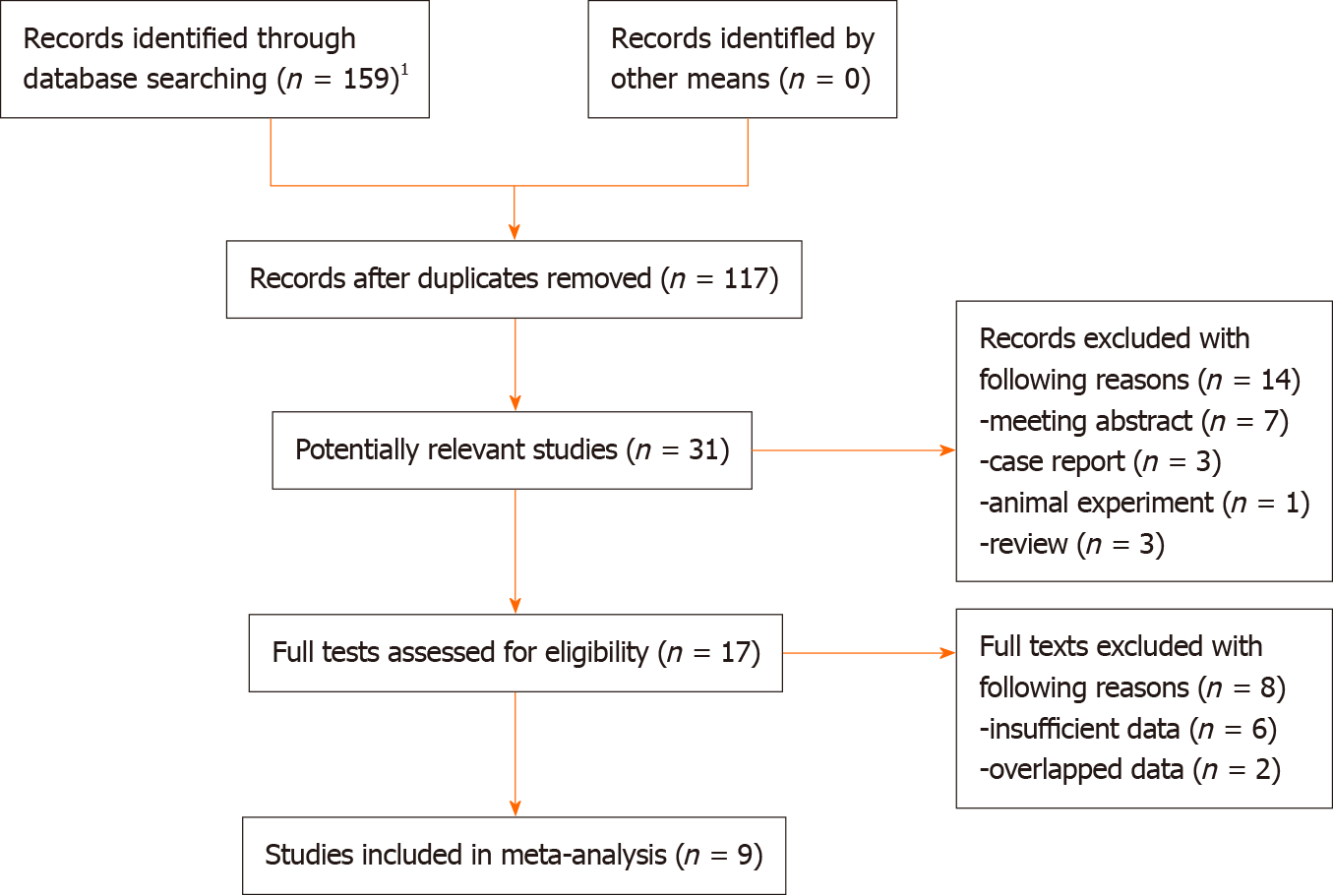
Initially, 159 records from the six electronic databases were identified and then 42 duplicated records were removed. Eight-six irrelevant publications and 14 unavailable records were excluded after reading the titles and abstracts. Then eight studies were excluded because of the insufficient data (n = 6) or overlapping data (n = 2) and nine studies were included into this meta-analysis finally[20-28] (Figure 1).
All included studies were retrospective and a total of 2545 patients were enrolled. Among these 2545 patients, 1480 patients were male. Most of them were from Eastern countries, applied the tumor-node-metastasis (TNM) stage system for tumor staging evaluation, focused on the colorectal carcinoma and detected the serum/plasma concentration of YKL-40. Besides, except for the study conducted by Tarpgaard et al[27], all the other studies only enrolled operated patients. The detailed information was presented in the Table 1.
| Ref. | Sample size | Gender (male) | Age (median, range), years | Country | Stage | Number of colon carcinoma | Treatment | Tumor type | Threshold | Endpoints | NOS |
| Cintin et al[20], 1999 | 603 | 355 | 69 (33-91) | Denmark | Duke A-D | 355 | Surgery | CRC | 247 ug/L (upper 95th percentile) | OS | 7 |
| Cintin et al[21], 2002 | 324 | 192 | 68 (37-90) | Denmark | Duke A-D | 197 | Surgery | CRC | 247 ug/L (upper 95th percentile) | OS, PFS | 7 |
| Liu et al[24], 2014 | 86 | 48 | 60 (38-76) | China | TNMI I-IV | 34 | Surgery | CRC | 216 ng/mL (median) | PFS | 6 |
| Tarpgaard et al[27], 2014 | 510 | 301 | NR | Denmark | TNM IV | 302 | Non-surgery | CRC | 155 ug/L (upper 95th percentile) | OS, PFS | 7 |
| Fuksiewicz et al[22], 2018 | 83 | 59 | 65 (25-82) | Poland | TNM I-III | NR | Surgery | RC | 44.6 pg/mL (upper 95th percentile) | OS, PFS | 6 |
| Hermunen et al[23], 2020 | 147 | 75 | 60 (31-76) | Finland | TNM II-IV | 87 | Surgery | CRC | 70.7 ng/mL (maximum Youden’s index) | OS, PFS | 7 |
| Peltonen et al[26], 2020 | 441 | 260 | 64.9 (33-84) | Denmark | TNM IV | 258 | Surgery (liver resection) | CRC | 34.8 ng/mL (upper 95th percentile) | OS, PFS | |
| Oh et al[25], 2021 | 265 | 134 | NR | Korea | TNM I-IV | NR | Surgery | CRC | Positive in tumor cells | OS, PFS | 7 |
| Yang et al[28], 2021 | 86 | 56 | 60.12 ± 7.32 (mean ± SD) | China | TNMI I-IV | 86 | Surgery | CC | Positive in tumor cells | OS | 7 |
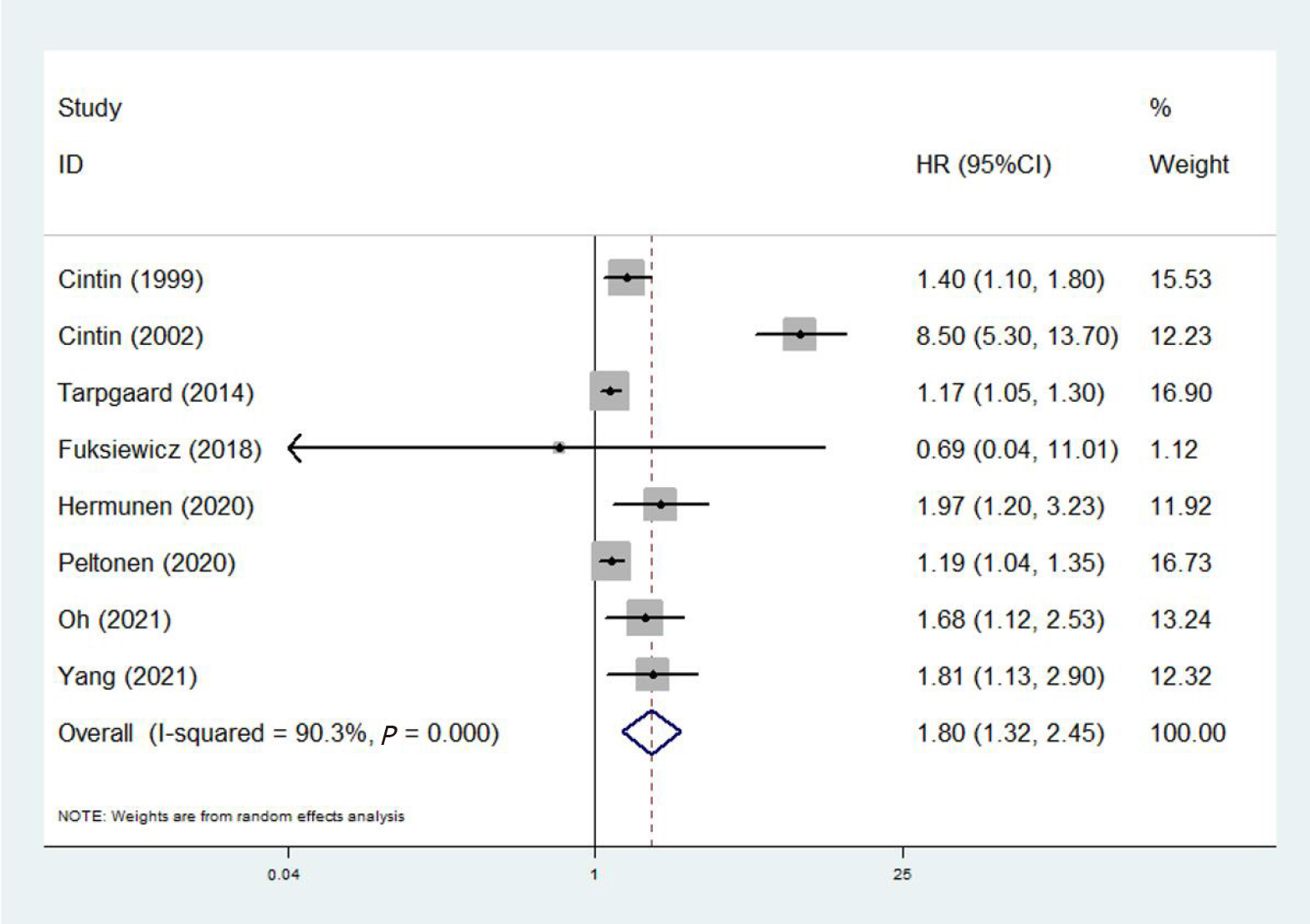
Eight studies involving 2459 patients investigated the predictive role of YKL-40 for OS of colorectal cancer patients[20-23,25-28]. The pooled results demonstrated that YKL-40 was significantly associated with OS of colorectal carcinoma patients (HR = 1.80, 95%CI: 1.32-2.45, P < 0.001; I2% = 90.3%, P < 0.001) (Figure 2). Furthermore, subgroup analysis stratified by the treatment (surgery vs non-surgery), tumor type (colorectal carcinoma vs rectal carcinoma vs colon carcinoma) and source of YKL-40 (serum/plasma vs tissue) were performed. The subgroup analysis showed similar results, except for the unsignificant relationship of YKL-40 with OS in rectal carcinoma patients (HR = 0.69, 95%CI: 0.04-11.5, P = 0.796) (Table 2).
| No. of studies | HR | 95%CI | P value | I2 (%) | P value | |
| Overall survival | 8 | 1.80 | 1.32-2.45 | < 0.001 | 90.3 | < 0.001 |
| Treatment | ||||||
| Surgery | 7 | 1.99 | 1.27-3.12 | 0.003 | 90.8 | < 0.001 |
| Non-surgery | 1 | 1.17 | 1.05-1.30 | 0.004 | - | - |
| Tumor type | ||||||
| Colorectal carcinoma | 6 | 1.83 | 1.30-2.56 | < 0.001 | 92.9 | < 0.001 |
| Rectal carcinoma | 1 | 0.69 | 0.04-11.45 | 0.796 | - | - |
| Colon carcinoma | 1 | 1.81 | 1.13-2.90 | 0.013 | - | - |
| Source of YKL-40 | ||||||
| Serum | 6 | 1.83 | 1.26-2.66 | 0.001 | 92.7 | < 0.001 |
| Tissue | 2 | 1.74 | 1.28-2.36 | < 0.001 | 0.0 | 0.816 |
| Progression-free survival | 7 | 1.62 | 1.22-2.16 | 0.001 | 88.3 | < 0.001 |
| Treatment | ||||||
| Surgery | 6 | 1.93 | 1.21-3.08 | .005 | 87.8 | < 0.001 |
| Non-surgery | 1 | 1.00 | 0.91-1.09 | 1.000 | - | - |
| Tumor type | ||||||
| Colorectal carcinoma | 6 | 1.64 | 1.22-2.20 | 0.001 | 90.2 | < 0.001 |
| Rectal carcinoma | 1 | 1.32 | 0.39-4.46 | 0.655 | - | - |
| Source of YKL-40 | ||||||
| Serum | 6 | 1.54 | 1.15-2.07 | 0.004 | 88.0 | < 0.001 |
| Tissue | 1 | 2.00 | 1.36-2.94 | < 0.001 | - | - |
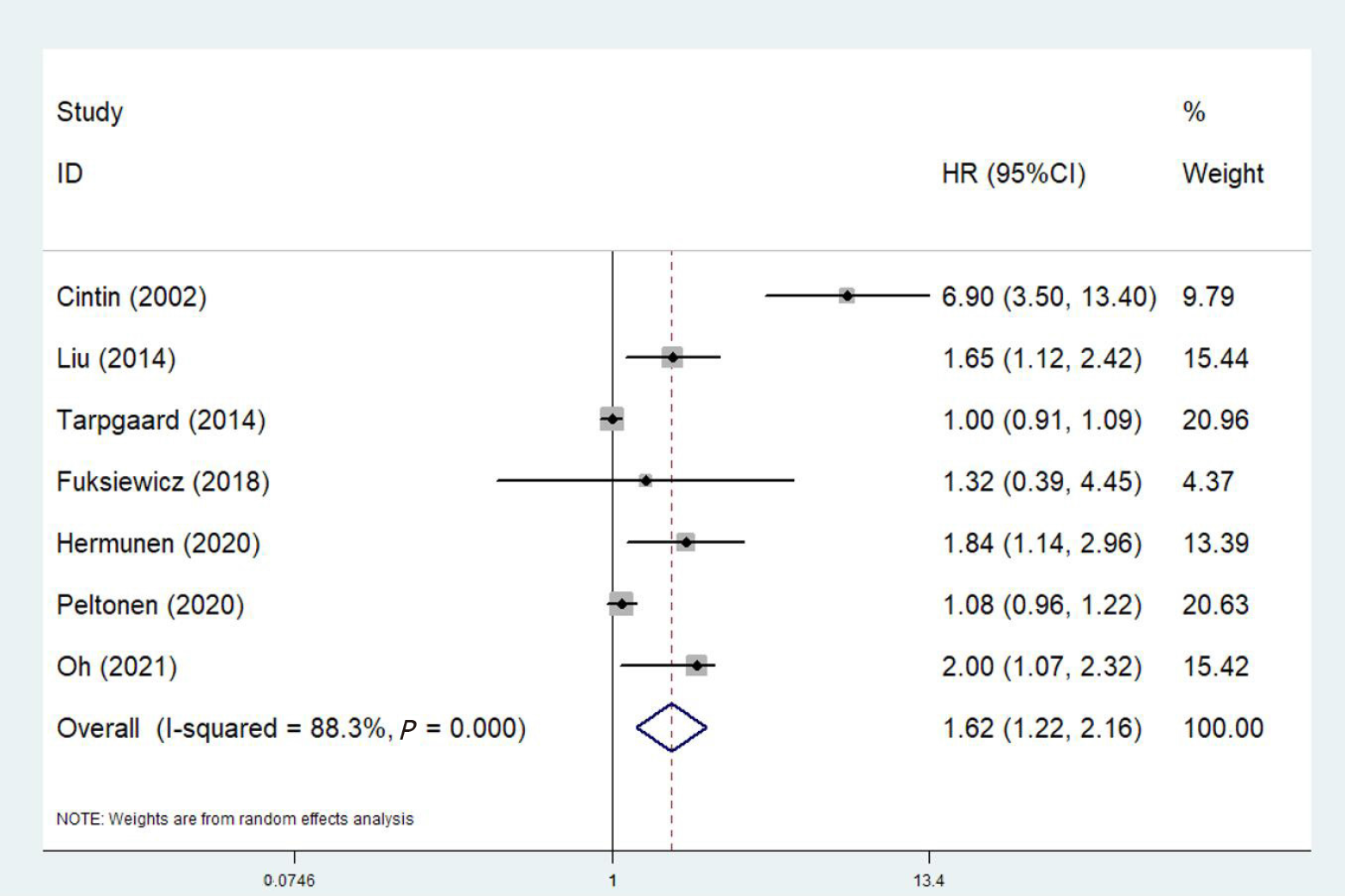
Seven relevant studies involving 1856 participants explored the predictive role of YKL-40 for PFS[21-27]. The pooled results also manifested significant association between YKL-40 and PFS of colorectal carcinoma patients (HR = 1.62, 95%CI: 1.21-2.16, P = 0.001; I2% = 88.3%, P < 0.001) (Figure 3). Then, subgroup analysis based on the treatment (surgery vs non-surgery), tumor type (colorectal carcinoma vs rectal carcinoma) and source of YKL-40 (serum/plasma vs tissue) were further conducted. No significant association of YKL-40 with PFS in patients who did not receive surgery (HR = 1.00, 95%CI: 0.91-1.09, P = 1.00) or with rectal cancer (HR = 1.32, 95%CI: 0.39-4.46, P = 0.655) (Table 2).
In overall, YKL-40 was supposed to be an important prognostic indicator in colorectal carcinoma patients based on above results.
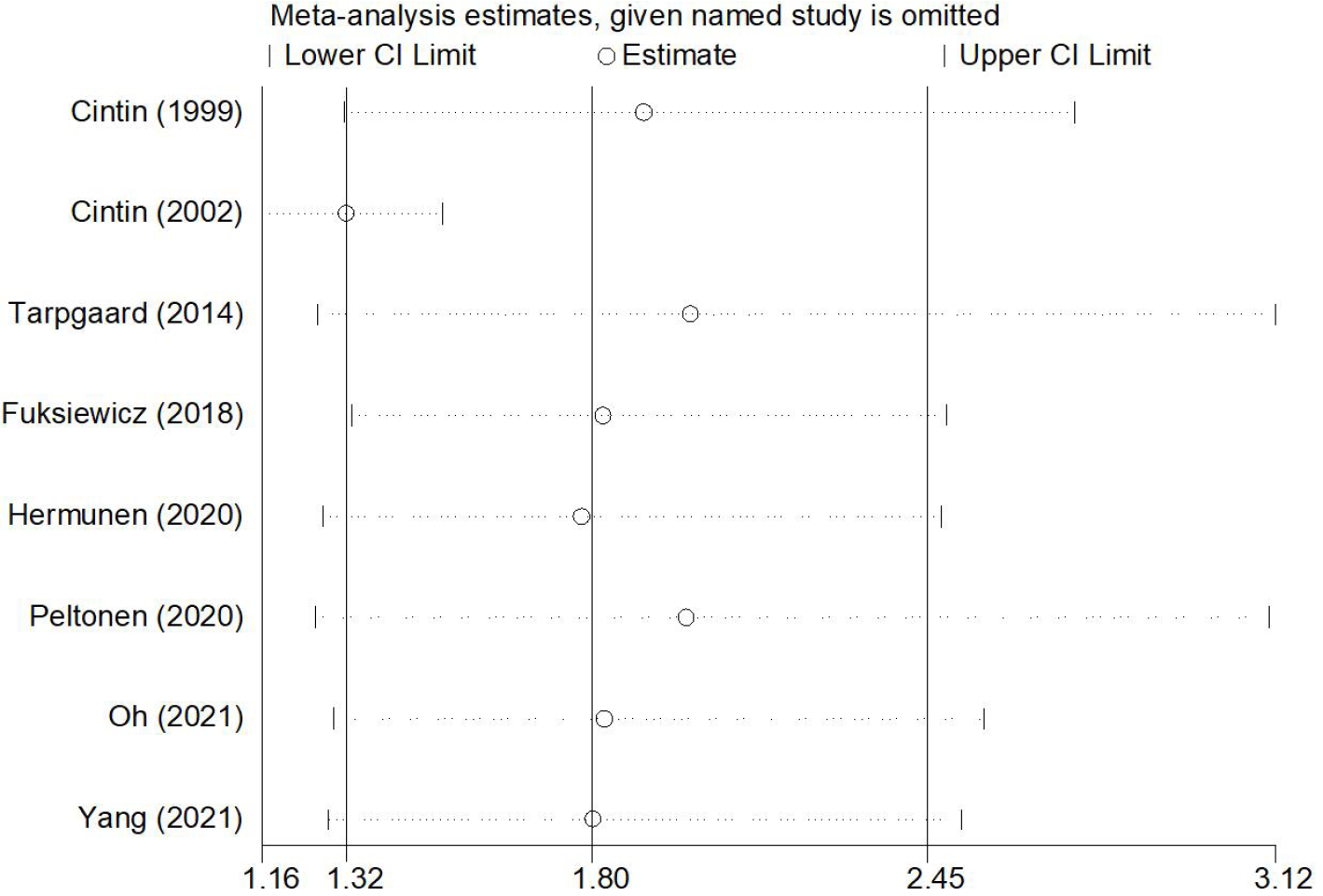
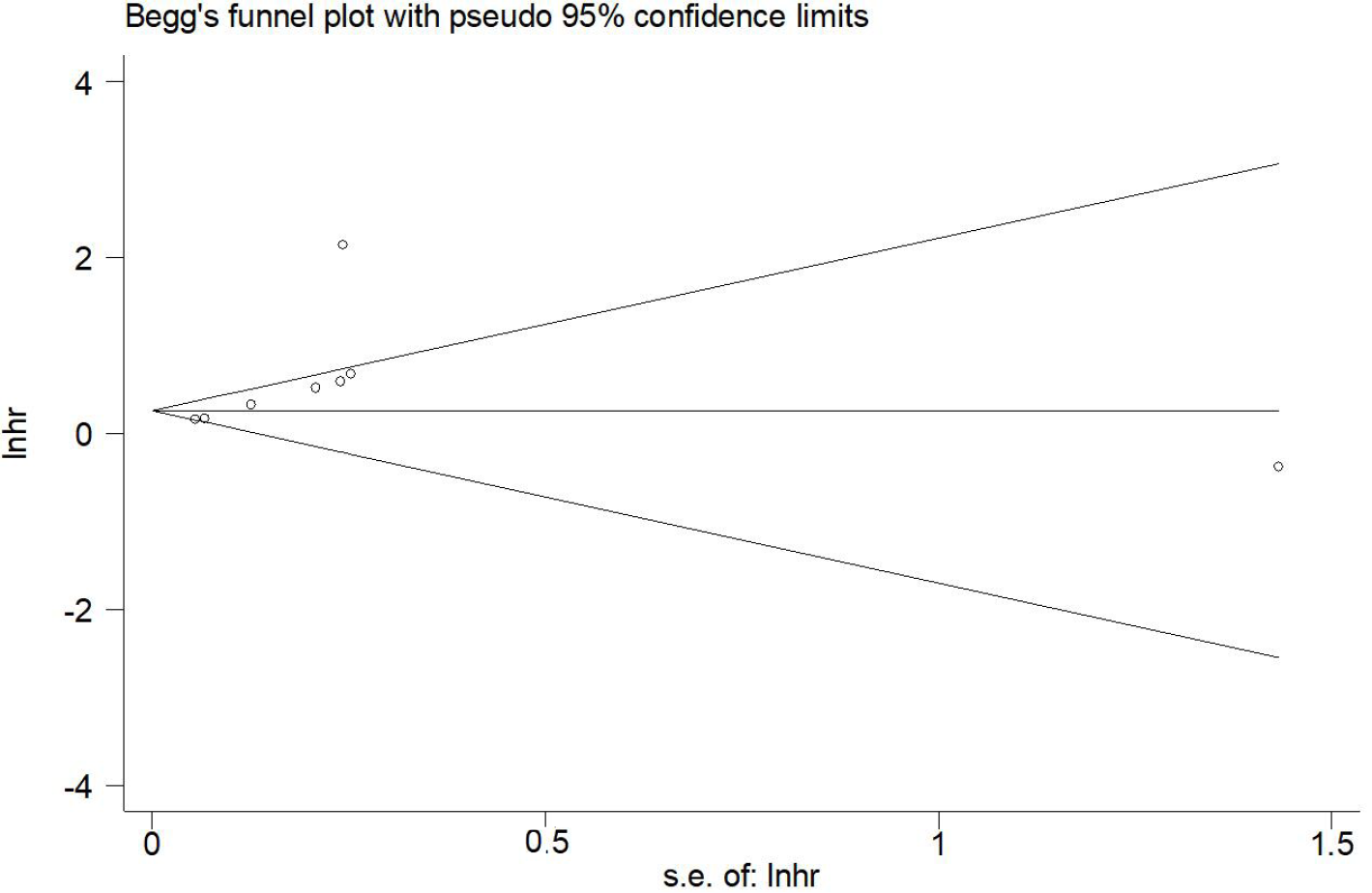
The sensitivity analysis and publication bias analysis for OS were further conducted to assess the stability and reliability of the pooled results. The sensitivity analysis showed that the results of this meta-analysis was stable and none of included studies had a significant impact on the results (Figure 4). Begg’s funnel plot was basically symmetrical (Figure 5) and the P value of Egger’s test was 0.109, which indicated that no potentially unpublished articles existed.
The current meta-analysis demonstrated that YKL-40 was a relatively reliable and valuable prognostic indicator for colorectal carcinoma patients after including nine relevant studies. However, whether the YKL-40 shows high prognostic value in all groups of colorectal carcinoma patients is still needed to be further verified.
Actually, the specific mechanisms by which YKL-40 affects the disease progression, therapeutic effect and long-term survival are still not very clear now. The study conducted by Faibish et al[34] manifested that YKL-40 could affect the invasion of tumor cells through the regulation of matrixmetallo proteinase-2 (MMP-2) expression, adhesion to extracellular matrix (ECM), cytoskeleton rearrangement and contractility. Besides, Jeet et al[35] demonstrated that the knockdown of YK-40 gene in the bone metastatic C4-2B cells could decrease the ability of migration and invasion. Furthermore, YKL-40 could also promote the chemotaxis of macrophages and angiogenesis accompanied by increased IL-8 and monocytechemoattractantprotein-1 secretion through the mitogen-activated protein kinase signaling pathway[36].
Bian et al[37] performed a meta-analysis by including 41 publications involving a total of 7762 patients with solid cancers and demonstrated that elevated serum/plasma YKL-40 was significantly associated with worse OS (HR = 1.44, 95%CI: 1.33-1.56). Actually, in addition to the predictive role for prognosis, YKL-4 might also play an important role in the diagnosis of colorectal cancer. Fuksiewicz et al[22] indicated that YKL-40 Levels were more valuable in diagnosing rectal cancer [area under the Receiver Operating Characteristic (ROC) curve: 769] than CEA (area under the ROC curve: 0.728) in early stage patients. Besides, YKL-40 showed a much higher value in predicting the recurrence of Chinese colorectal cancer (area under the ROC curve: 0.907, comparing with the 0.714 of CEA and 0.759 of CA199)[38]. Therefore, more investigation about the diagnostic role of YKL-40 is still valuable.
Although we demonstrated that YKL-40 was predictive for OS and PFS in colorectal carcinoma patients in overall, whether it could be applied as a reliable prognostic indicator in all colorectal cancer patients is still needed to further explored by more high-quality studies. According to the subgroup analysis, YKL-40 was not related with OS (P = 0.796) or PFS (P = 0.655) in rectal cancer patients. However, in the study conducted by Fuksiewicz et al[22], the Kaplan-Meier survival curves showed obvious differences of OS (P = 0.040) and PFS (P = 0.044) between patients with elevated (> 44.6 ng/mL) and normal serum concentration (≤ 44.6 ng/mL)of YKL-40. After calculating the HR with 95%CI according to the survival curves using the method introduced by Tierney et al[31], different results were observed, which might be explained by the bias caused by the statistical method and small sample size. Besides, Tarpgaard et al[27] manifested negative association between plasma YKL-40 and PFS according to the multivariate Cox analysis, but the positive results were observed in the univariate Cox analysis (HR = 1.11, 95%CI: 1.03-1.20, P = 0.006). Thus, based on the original data presented in the articles, we still believe that YKL-40 might show high prognostic value in these subgroups of colorectal cancer patients. However, more prospective studies with high-quality are needed to verify this conjecture.
There are several limitations in the current meta-analysis. First, all included studies are retrospective and the overall sample size is relatively small, which might cause some bias. Second, due to the lack of specific data, we failed to conduct more subgroup analysis based on other important parameters such as the disease stage, age and thresholds of serum/plasma concentration of YKL-40. Third, the comparison of expression status in tissues and serum/plasma levels in predicting long-term survival of colorectal carcinoma patients was not performed.
In overall, elevated serum/plasma concentration of YKL-40 or positive expression in tumor cells was related with poor survival of colorectal carcinoma patients. YKL-40 might serve as a novel and reliable indicator for the evaluation of prognosis in colorectal cancer.
The predictive role of YKL-40 for long-term survival in colorectal cancer patients has been gradually investigated in recent years.
Whether it is a reliable and valuable prognostic indicator in patients with colorectal carcinoma has not been certified.
To verify the prognostic value of serum/plasma concentration of YKL-40 or expression status of YKL-40 in tumor cells in colorectal carcinoma.
Several electronic databases were searched to identify relevant articles. The hazard ratio with 95% confidence interval was combined to the evaluate the association between YKL-40 and overall survival (OS) and progression-free survival (PFS).
YKL-40 was significantly associated with poor OS (P < 0.001) and PFS (P = 0.001). Subgroup analysis stratified by the treatment, tumor type and source of YKL-40 showed similar results.
Elevated serum/plasma concentration of YKL-40 or positive expression in tumor cells was related with worse prognosis of colorectal carcinoma patients.
YKL-40 might serve as a novel and reliable indicator for the evaluation of prognosis in colorectal cancer.
Provenance and peer review: Unsolicited article; externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Leyva-Vazquez M, Moreno-Gómez-Toledano R S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Chang SH, Patel N, Du M, Liang PS. Trends in Early-onset vs Late-onset Colorectal Cancer Incidence by Race/Ethnicity in the United States Cancer Statistics Database. Clin Gastroenterol Hepatol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 2. | Miller KD, Ortiz AP, Pinheiro PS, Bandi P, Minihan A, Fuchs HE, Martinez Tyson D, Tortolero-Luna G, Fedewa SA, Jemal AM, Siegel RL. Cancer statistics for the US Hispanic/Latino population, 2021. CA Cancer J Clin. 2021;71:466-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 256] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 3. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64542] [Article Influence: 16135.5] [Reference Citation Analysis (176)] |
| 4. | Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, Alexander DD, Choti MA, Poston G. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 279] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 5. | Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandalà M, Cervantes A, Arnold D; ESMO Guidelines Working Group. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi64-vi72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 647] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 6. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Gunn A, Hoffe S, Hubbard J, Hunt S, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Johnson-Chilla A, Gurski LA. NCCN Guidelines Insights: Rectal Cancer, Version 6.2020. J Natl Compr Canc Netw. 2020;18:806-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 331] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 7. | Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Garrido-Laguna I, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wuthrick E, Gregory KM, Freedman-Cass DA. NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J Natl Compr Canc Netw. 2018;16:359-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 685] [Article Influence: 97.9] [Reference Citation Analysis (1)] |
| 8. | Johansen JS, Schultz NA, Jensen BV. Plasma YKL-40: a potential new cancer biomarker? Future Oncol. 2009;5:1065-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Johansen JS, Høyer PE, Larsen LA, Price PA, Møllgård K. YKL-40 protein expression in the early developing human musculoskeletal system. J Histochem Cytochem. 2007;55:1213-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Ringsholt M, Høgdall EV, Johansen JS, Price PA, Christensen LH. YKL-40 protein expression in normal adult human tissues--an immunohistochemical study. J Mol Histol. 2007;38:33-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Johansen JS, Jensen BV, Roslind A, Nielsen D, Price PA. Serum YKL-40, a new prognostic biomarker in cancer patients? Cancer Epidemiol Biomarkers Prev. 2006;15:194-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 236] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 12. | Böckelmann LC, Felix T, Calabrò S, Schumacher U. YKL-40 protein expression in human tumor samples and human tumor cell line xenografts: implications for its use in tumor models. Cell Oncol (Dordr). 2021;44:1183-1195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Pelloski CE, Mahajan A, Maor M, Chang EL, Woo S, Gilbert M, Colman H, Yang H, Ledoux A, Blair H, Passe S, Jenkins RB, Aldape KD. YKL-40 expression is associated with poorer response to radiation and shorter overall survival in glioblastoma. Clin Cancer Res. 2005;11:3326-3334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Johansen JS, Christensen IJ, Riisbro R, Greenall M, Han C, Price PA, Smith K, Brünner N, Harris AL. High serum YKL-40 levels in patients with primary breast cancer is related to short recurrence free survival. Breast Cancer Res Treat. 2003;80:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Krogh M, Christensen I, Bouwhuis M, Johansen JS, Nørgaard P, Schmidt H, Hansson J, Suciu S, Eggermont AM, Bastholt L; Nordic Melanoma Group and EORTC Melanoma Group. Prognostic and predictive value of YKL-40 in stage IIB-III melanoma. Melanoma Res. 2016;26:367-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Boisen MK, Madsen CV, Dehlendorff C, Jakobsen A, Johansen JS, Steffensen KD. The Prognostic Value of Plasma YKL-40 in Patients With Chemotherapy-Resistant Ovarian Cancer Treated With Bevacizumab. Int J Gynecol Cancer. 2016;26:1390-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Vom Dorp F, Tschirdewahn S, Niedworok C, Reis H, Krause H, Kempkensteffen C, Busch J, Kramer G, Shariat SF, Nyirady P, Rübben H, Szarvas T. Circulating and Tissue Expression Levels of YKL-40 in Renal Cell Cancer. J Urol. 2016;195:1120-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Bertini I, Cacciatore S, Jensen BV, Schou JV, Johansen JS, Kruhøffer M, Luchinat C, Nielsen DL, Turano P. Metabolomic NMR fingerprinting to identify and predict survival of patients with metastatic colorectal cancer. Cancer Res. 2012;72:356-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 19. | Lehtomäki K, Mustonen H, Kellokumpu-Lehtinen PL, Joensuu H, Hermunen K, Soveri LM, Boisen MK, Dehlendorff C, Johansen JS, Haglund C, Osterlund P. Lead Time and Prognostic Role of Serum CEA, CA19-9, IL-6, CRP, and YKL-40 after Adjuvant Chemotherapy in Colorectal Cancer. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Cintin C, Johansen JS, Christensen IJ, Price PA, Sørensen S, Nielsen HJ. Serum YKL-40 and colorectal cancer. Br J Cancer. 1999;79:1494-1499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 144] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Cintin C, Johansen JS, Christensen IJ, Price PA, Sørensen S, Nielsen HJ. High serum YKL-40 level after surgery for colorectal carcinoma is related to short survival. Cancer. 2002;95:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Fuksiewicz M, Kotowicz B, Rutkowski A, Achinger-Kawecka J, Wagrodzki M, Kowalska MM. The Assessment of Clinical Usage and Prognostic Value of YKL-40 Serum Levels in Patients With Rectal Cancer Without Distant Metastasis. Technol Cancer Res Treat. 2018;17:1533033818765209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Hermunen K, Soveri LM, Boisen MK, Mustonen HK, Dehlendorff C, Haglund CH, Johansen JS, Osterlund P. Postoperative serum CA19-9, YKL-40, CRP and IL-6 in combination with CEA as prognostic markers for recurrence and survival in colorectal cancer. Acta Oncol. 2020;59:1416-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Liu X, Zhang Y, Zhu Z, Ha M, Wang Y. Elevated pretreatment serum concentration of YKL-40: an independent prognostic biomarker for poor survival in patients with colorectal cancer. Med Oncol. 2014;31:85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Oh IH, Pyo JS, Son BK. Prognostic Impact of YKL-40 Immunohistochemical Expression in Patients with Colorectal Cancer. Curr Oncol. 2021;28:3139-3149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Peltonen R, Gramkow MH, Dehlendorff C, Osterlund PJ, Johansen JS, Isoniemi H. Elevated serum YKL-40, IL-6, CRP, CEA, and CA19-9 combined as a prognostic biomarker panel after resection of colorectal liver metastases. PLoS One. 2020;15:e0236569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Tarpgaard LS, Guren TK, Glimelius B, Christensen IJ, Pfeiffer P, Kure EH, Sorbye H, Ikdahl T, Yilmaz M, Johansen JS, Tveit KM. Plasma YKL-40 in patients with metastatic colorectal cancer treated with first line oxaliplatin-based regimen with or without cetuximab: RESULTS from the NORDIC VII Study. PLoS One. 2014;9:e87746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Yang F, Liu X, Peng Q. The expression and significance of YKL-40, RSK4 and cyclinD1 in colon cancer. Zhongguo Laonianxue Zhazhi. 2021;41:3938-3943. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 29. | Zhang X, Tan R, Lam WC, Yao L, Wang X, Cheng CW, Liu F, Chan JC, Aixinjueluo Q, Lau CT, Chen Y, Yang K, Wu T, Lyu A, Bian Z. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Extension for Chinese Herbal Medicines 2020 (PRISMA-CHM 2020). Am J Chin Med. 2020;48:1279-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 30. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12634] [Article Influence: 842.3] [Reference Citation Analysis (0)] |
| 31. | Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4738] [Cited by in RCA: 4951] [Article Influence: 275.1] [Reference Citation Analysis (0)] |
| 32. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46470] [Article Influence: 2112.3] [Reference Citation Analysis (3)] |
| 33. | Wang Y, Li J, Chang S, Dong Y, Che G. Risk and Influencing Factors for Subsequent Primary Lung Cancer After Treatment of Breast Cancer: A Systematic Review and Two Meta-Analyses Based on Four Million Cases. J Thorac Oncol. 2021;16:1893-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 34. | Faibish M, Francescone R, Bentley B, Yan W, Shao R. A YKL-40-neutralizing antibody blocks tumor angiogenesis and progression: a potential therapeutic agent in cancers. Mol Cancer Ther. 2011;10:742-751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 35. | Jeet V, Tevz G, Lehman M, Hollier B, Nelson C. Elevated YKL40 is associated with advanced prostate cancer (PCa) and positively regulates invasion and migration of PCa cells. Endocr Relat Cancer. 2014;21:723-737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Kawada M, Seno H, Kanda K, Nakanishi Y, Akitake R, Komekado H, Kawada K, Sakai Y, Mizoguchi E, Chiba T. Chitinase 3-like 1 promotes macrophage recruitment and angiogenesis in colorectal cancer. Oncogene. 2012;31:3111-3123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 37. | Bian B, Li L, Yang J, Liu Y, Xie G, Zheng Y, Zeng L, Zeng J, Shen L. Prognostic value of YKL-40 in solid tumors: a meta-analysis of 41 cohort studies. Cancer Cell Int. 2019;19:259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 38. | Ye HM, Lu YZ, Liang XM, Lin YZ, Li Y, Zhang ZY, Tzeng CM. Clinical significance of combined testing of YKL-40 with CEA in Chinese colorectal cancer patients. Clin Lab. 2014;60:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |