Published online Mar 6, 2022. doi: 10.12998/wjcc.v10.i7.2174
Peer-review started: November 21, 2021
First decision: December 9, 2021
Revised: December 26, 2021
Accepted: January 22, 2022
Article in press: January 22, 2022
Published online: March 6, 2022
Processing time: 100 Days and 16.2 Hours
Thoracic surgery for radical resection of lung tumor requires deep anesthesia which can lead to an adverse inflammatory response, loss of hemodynamic stability, and decreased immune function. Herein, we evaluated the feasibility and benefits of ultrasound-guided paravertebral nerve block anesthesia, in combination with general anesthesia, for thoracic surgery for lung cancer. The block was performed by diffusion of anesthetic drugs along the paravertebral space to achieve unilateral multi-segment intercostal nerve and dorsal branch nerve block.
To evaluate the application of ultrasound-guided paravertebral nerve block anesthesia for lung cancer surgery to inform practice.
The analysis was based on 140 patients who underwent thoracic surgery for lung cancer at our hospital between January 2018 and May 2020. Patients were randomly allocated to the peripheral + general anesthesia (observation) group (n = 74) or to the general anesthesia (control) group (n = 66). Patients in the observation group received ultrasound-guided paravertebral nerve block anesthesia combined with general anesthesia, with those in the control group receiving an epidural block combined with general anesthesia. Measured outcomes included the operative and anesthesia times, as well as the mean arterial pressure (MAP), heart rate (HR), and blood oxygen saturation (SpO2) measured before surgery, 15 min after anesthesia (T1), after intubation, 5 min after skin incision, and before extubation (T4).
The dose of intra-operative use of remifentanil and propofol and the postoperative use of sufentanil was lower in the observation group (1.48 ± 0.43 mg, 760.50 ± 92.28 mg, and 72.50 ± 16.62 mg, respectively) than control group (P < 0.05). At the four time points of measurement (T1 through T4), MAP and HR values were higher in the observation than control group (MAP, 90.20 ± 9.15 mmHg, 85.50 ± 7.22 mmHg, 88.59 ± 8.15 mmHg, and 90.02 ± 10.02 mmHg, respectively; and HR, 72.39 ± 8.22 beats/min, 69.03 ± 9.03 beats/min, 70.12 ± 8.11 beats/min, and 71.24 ± 9.01 beats/min, respectively; P < 0.05). There was no difference in SpO2 between the two groups (P > 0.05). Postoperative levels of epinephrine, norepinephrine, and dopamine used were significantly lower in the observation than control group (210.20 ± 40.41 pg/mL, 230.30 ± 65.58 pg/mL, and 54.49 ± 13.32 pg/mL, respectively; P < 0.05). Similarly, the postoperative tumor necrosis factor-α and interleukin-6 levels were lower in the observation (2.43 ± 0.44 pg/mL and 170.03 ± 35.54 pg/mL, respectively) than control group (P < 0.05). There was no significant difference in the incidence of adverse reactions between the two groups (P > 0.05).
Ultrasound-guided paravertebral nerve block anesthesia improved the stress and hemodynamic response in patients undergoing thoracic surgery for lung cancer, with no increase in the rate of adverse events.
Core Tip: Ultrasound-guided paravertebral nerve block anesthesia has good indication for lung cancer surgery, with little effect on patients’ stress and hemodynamic responses.
- Citation: Zhen SQ, Jin M, Chen YX, Li JH, Wang H, Chen HX. Ultrasound-guided paravertebral nerve block anesthesia on the stress response and hemodynamics among lung cancer patients. World J Clin Cases 2022; 10(7): 2174-2183
- URL: https://www.wjgnet.com/2307-8960/full/v10/i7/2174.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i7.2174
Lung cancer is a common malignant tumor of the chest, with radical excision recommended to lower the mortality risk. Although this treatment is effective, the need for general anesthesia, even for minimally invasive surgery, has been associated with pain and patient restlessness during the perioperative period, leading to abnormal hemodynamic fluctuations. Therefore, effective anesthesia methods would be needed to improve patient outcomes during surgical treatment for lung cancer[1,2].
General anesthesia combined with epidural anesthesia is generally used for surgery. However, this approach carries a risk for postoperative complications, including serious negative effects on respiratory and circulatory function[3]. As an alternative form of anesthesia, ultrasound-guided paravertebral nerve block anesthesia has been used in clinical practice in recent years. By diffusion of anesthetic drugs along the paravertebral space, unilateral multi-segment intercostal nerve and dorsal branch nerve can be blocked, with ultrasound-guidance ensuring accurate localization of application[4-6]. Therefore, the aim of this study was to evaluate the application of ultrasound-guided paravertebral nerve block anesthesia for lung cancer surgery to inform practice.
The study sample included 140 patients [85 men; mean age 54.02 ± 9.11 (range, 45-80) years] with lung cancer treated at our hospital between January 2018 and May 2020. Inclusion criteria were as follows: Pathological diagnosis of non-small cell lung cancer; elective surgical treatment performed at our hospital; American Society of Anesthesiologists physical status grade I or II; and provision of informed consent by the patient and family. The exclusion criteria were: coagulation disorders, cardiovascular and cerebrovascular diseases, acute and chronic infections, liver and kidney dysfunction; cognitive impairment; and obesity.
After enrollment, patients were allocated to either the observation (n = 74) or control (n = 66) group, according to the odd and even number at the end of their medical record number, respectively. The clinical data for the two groups is summarized in Table 1.
| Clinical data | Observation group (n = 74) | Control group (n = 66) | t/χ2 | P value |
| Sex | 1.134 | 0.287 | ||
| Male | 48 (64.86) | 37 (56.06) | ||
| Female | 26 (35.14) | 29 (43.94) | ||
| Age (yr) | 54.40 ± 9.92 | 53.19 ± 8.82 | 0.759 | 0.449 |
| Body mass index (kg/m2) | 22.27 ± 3.02 | 22.14 ± 2.83 | 0.262 | 0.794 |
| Karnofsky score (Points) | 82.20 ± 9.92 | 83.03 ± 8.95 | -0.517 | 0.606 |
| ASA | 0.078 | 0.780 | ||
| I | 42 (56.76) | 39 (59.09) | ||
| II | 32 (43.24) | 27 (40.91) | ||
| TNM | 0.586 | 0.444 | ||
| I | 44 (59.46) | 35 (53.03) | ||
| II | 30 (40.54) | 31 (46.97) | ||
| Pathological type | 0.023 | 0.88 | ||
| Adenocarcinoma | 48 (64.86) | 42 (63.64) | ||
| Squamous cell carcinoma | 26 (35.14) | 24 (36.36) |
Patients in the control group received an epidural block combined with general anesthesia. General anesthesia was induced using vecuronium (0.1 mg/kg), etomidate (0.2 mg/kg), midazolam (0.1 mg/kg), and sufentanil (0.5 μg/kg). The thoracic 6-7 space was selected for epidural puncture, with placement of a cephalic tube. Epidural anesthesia was maintained using 0.375% ropivacaine, injected intermittently (8-15 mL). The surgery proceeded once anesthesia was established. During the surgery, target-controlled infusion of propofol was used to maintain anesthesia, with intermittent intravenous injection of sufentanil and cis-atracurium. Symptomatic treatment was selected according to each patient’s condition during the surgery.
For patients in the observation group, ultrasound-guided paravertebral nerve block anesthesia was used before induction of general anesthesia. The puncture was performed 2-3 cm lateral to the lower edge of the 4th thoracic spinous process on the operative side, using a probe frequency of 8 MHz. The position of the puncture needle was adjusted under the ultrasound guidance to penetrate the skin, intercostal external muscle, and intercostal internal muscle for location in the paravertebral space. Once the needle was positioned, 0.375% ropivacaine (2 mg/kg) was injected and a catheter then inserted through a puncture needle toward the paravertebral space at a depth of 3-4 cm. The needle was then withdrawn and the catheter was fixed in place. Once the level of anesthesia was stable, general anesthesia was induced as per the control group.
The stress response was evaluated using a fasting blood sample (5 mL). The serum was separated by centrifugation (3000 r/min). The concentrations of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were determined by enzyme-linked immunosorbent assay, with the concentrations of epinephrine, norepinephrine, and dopamine determined by radio-immunity turbidimetry. The reagents provided by Shanghai Ximei Biotechnology Co., Ltd. were used as per the manufacturer’s instructions.
Measured outcomes were operative and anesthesia time, the mean arterial pressure (MAP), heart rate (HR), blood oxygen saturation (SpO2), quantified at baseline, before surgery (T0), and at 15 min after anesthesia (T1), after intubation (T2), 5 min after skin incision (T3), and before extubation (T4).
The stress response and hemodynamic data were reported as a mean ± SD. Between-group differences were evaluated using Student’s t-test, with repeated analysis of variance used to evaluate differences between time points of assessment. The sex distribution within each group and adverse events were reported as a count (%), with between-group differences evaluated using a χ2 test. All analyses were performed using SPSS (version 22.0), with a P value of 0.05 deemed significant.
Operative time, anesthesia time, and intra-operative volume of blood loss are reported for the two groups in Table 2, with no between-group differences (P > 0.05). However, the use of intra-operative remifentanil and propofol and of postoperative sufentanil was significantly less in the observation than the control group (P < 0.05).
| Group | Cases | Operation time (min) | Anesthesia time (min) | Intraoperative blood loss (mL) | Intraoperative remifentanil dosage (mg) | Intraoperative propofol dosage (mg) | Postoperative sufentanil use (μmg) |
| Observation group | 74 | 175.59 ± 23.39 | 183.39 ± 21.15 | 180.40 ± 33.59 | 1.48 ± 0.43 | 760.50 ± 92.28 | 72.50 ± 16.62 |
| Control group | 66 | 176.60 ± 24.45 | 185.50 ± 24.44 | 177.80 ± 35.52 | 2.18 ± 0.50 | 920.40 ± 91.15 | 90.40 ± 17.80 |
| t | -0.250 | -0.548 | 0.445 | -8.905 | -10.294 | -6.152 | |
| P value | 0.803 | 0.585 | 0.657 | 0.000 | 0.000 | 0.000 |
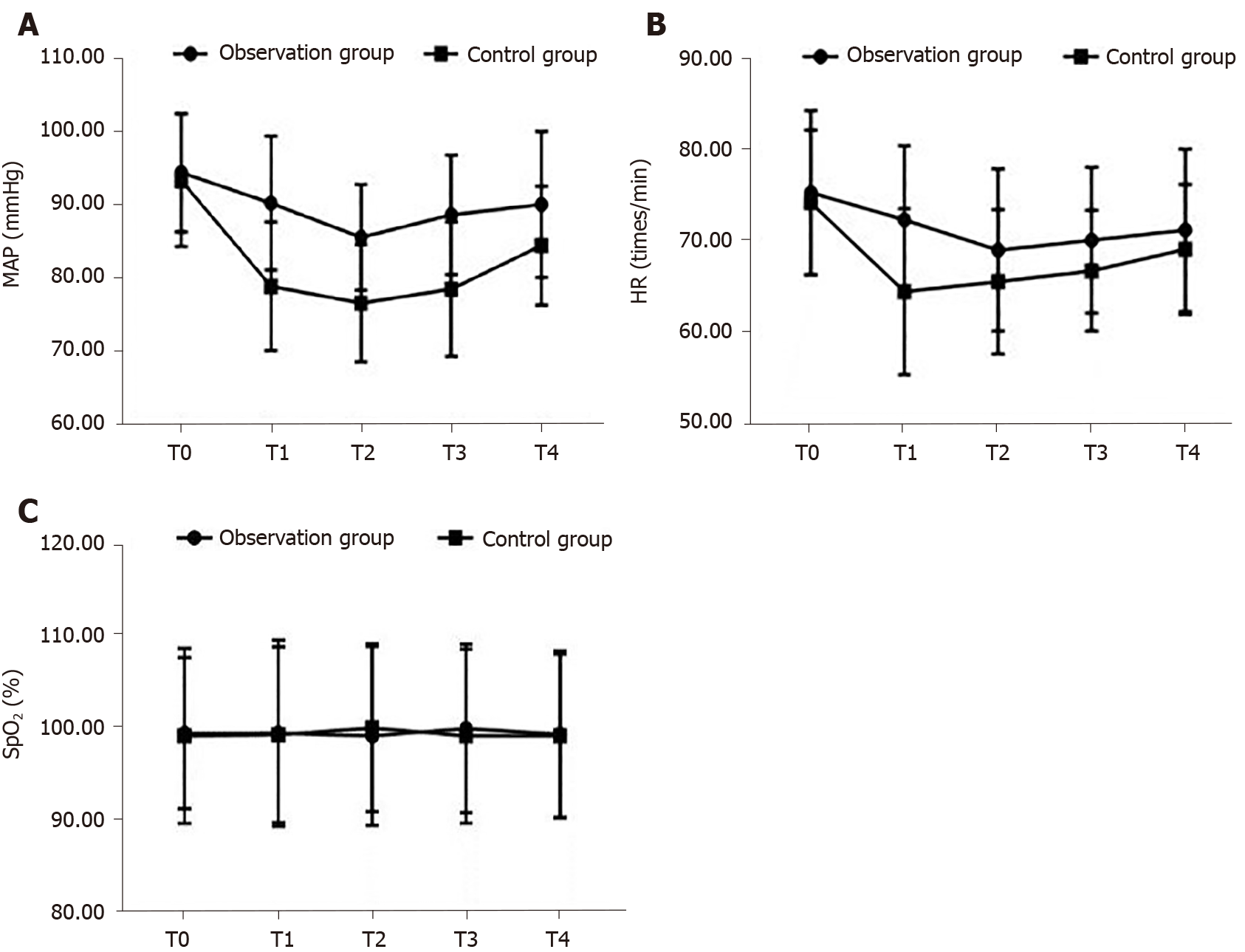
The changes in hemodynamic variables from baseline and between groups are reported in Table 3. The MAP and HR were lower at T1 through T4, compared to values at baseline T0 (P < 0.05) in both groups. However, these values at T1 through T4 were higher for the observation than control group (P < 0.05). There were no between-group differences in SpO2 (Figure 1).
| Index | Group | Cases | T0 | T1 | T2 | T3 | T4 |
| MAP (mmHg) | Observation group | 74 | 94.40 ± 8.11 | 90.20 ± 9.15a | 85.50 ± 7.22a | 88.59 ± 8.15a | 90.02 ± 10.02a |
| Control group | 66 | 93.32 ± 9.05 | 78.80 ± 8.81a | 76.50 ± 8.03a | 78.45 ± 9.21a | 84.40 ± 8.11a | |
| Ftime × group = 20.022; Ftime = 13.282; Fgroup = 6.261. | |||||||
| Ptime × group = 0.000; Ptime = 0.000; Pgroup = 0.000. | |||||||
| HR (beats/min) | Observation group | 74 | 75.40 ± 9.11 | 72.39 ± 8.22a | 69.03 ± 9.03a | 70.12 ± 8.11a | 71.24 ± 9.01a |
| Control group | 66 | 74.30 ± 8.06 | 64.40 ± 9.21a | 65.50 ± 8.03a | 66.72 ± 6.72a | 69.11 ± 7.22a | |
| Ftime × group=17.281; Ftime = 9.822; Fgroup = 7.201. | |||||||
| Ptime × group=0.000; Ptime = 0.000; Pgroup = 0.000. | |||||||
| SpO2 (%) | Observation group | 74 | 99.20 ± 8.21 | 99.32 ± 10.11 | 99.03 ± 9.72 | 99.83 ± 9.15 | 99.20 ± 9.04 |
| Control group | 66 | 99.02 ± 9.50 | 99.15 ± 9.54 | 99.89 ± 9.12 | 99.01 ± 9.45 | 99.01 ± 8.89 | |
| Ftime × group = 2.291; Ftime = 1.822; Fgroup = 0.782. | |||||||
| Ptime × group = 0.103; Ptime = 0.322; Pgroup = 0.554. | |||||||
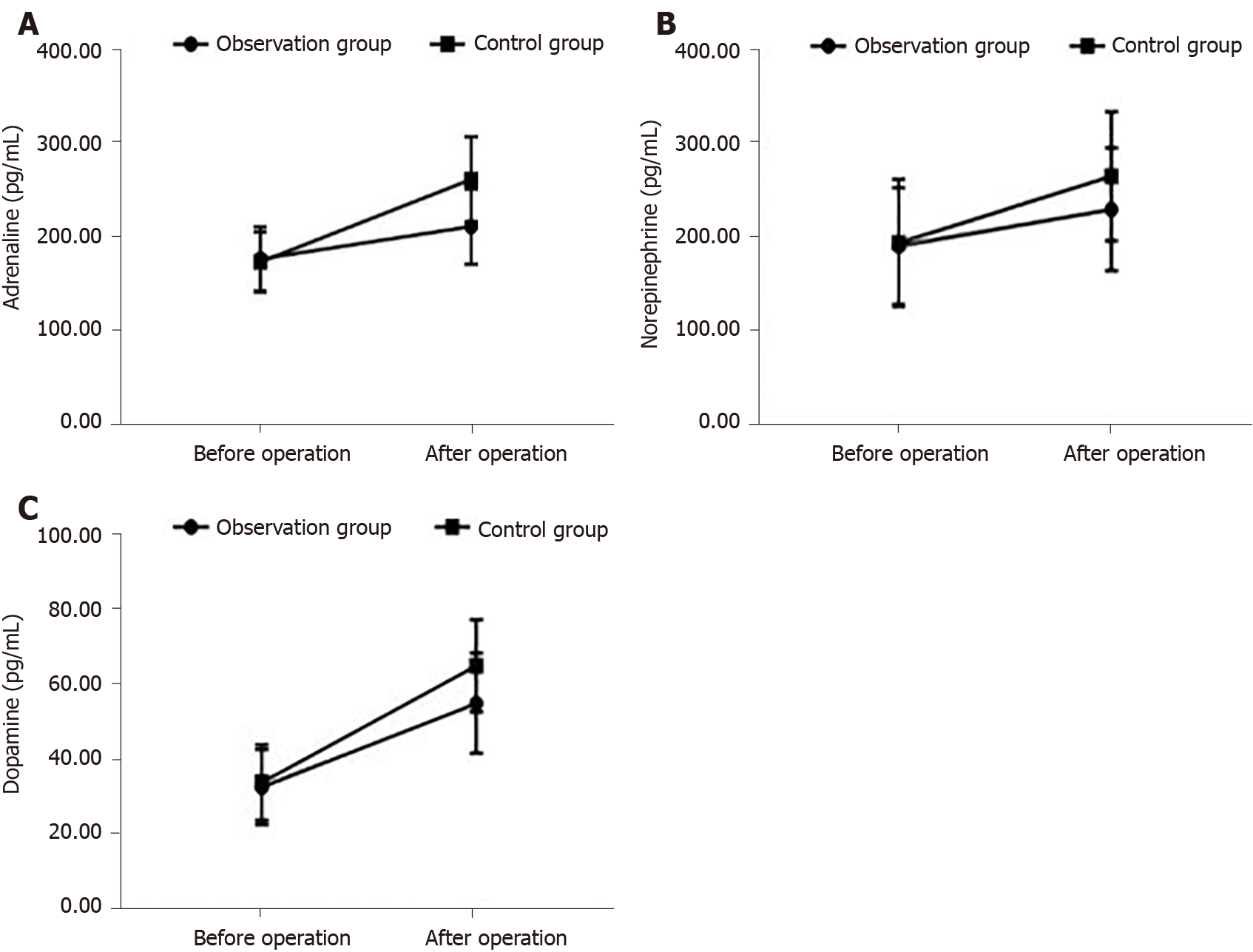
Levels of epinephrine, norepinephrine and dopamine are reported for both groups at each time point in Table 4. The postoperative levels of epinephrine, norepinephrine and dopamine were higher after surgery than at baseline for both groups (P < 0.05). However, these levels were lower in the observation than control group at all time points, T1 through T4 (P < 0.05; Figure 2).
| Group | Cases | Adrenaline (pg/mL) | Norepinephrine (pg/mL) | Dopamine (pg/mL) | |||
| Pre-operation | Post-operation | Pre-operation | Post-operation | Pre-operation | Post-operation | ||
| Observationgroup | 74 | 175.50 ± 34.43 | 210.20 ± 40.41a | 191.20 ± 62.23 | 230.30 ± 65.58a | 32.29 ± 9.92 | 54.49 ± 13.32a |
| Controlgroup | 66 | 172.29 ± 32.20 | 260.40 ± 45.59a | 194.40 ± 67.70 | 265.59 ± 68.82a | 33.50 ± 10.03 | 64.49 ± 12.25a |
| t | 0.568 | -6.907 | -0.291 | -3.105 | -0.717 | -4.605 | |
| P value | 0.571 | 0.000 | 0.771 | 0.002 | 0.475 | 0.000 | |
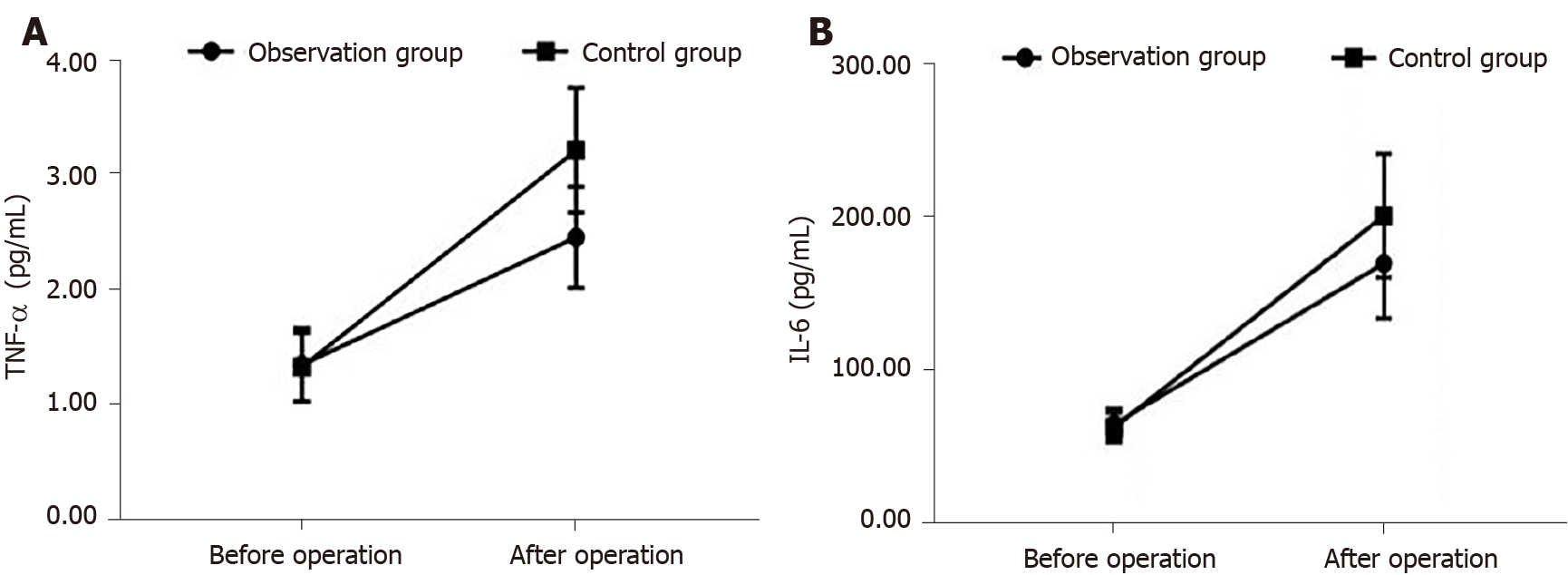
The levels of TNF-α and IL-6 are reported in Table 5. Again, levels of these inflammatory markers increased from baseline after surgery in both groups (P < 0.05). The postoperative TNF-α and IL-6 levels after surgery were lower in the observation than control group (P < 0.05; Figure 3).
There were two cases of nausea and vomiting, three cases of dizziness, and one case of hypotension in the observation group, for an overall incidence rate of 8.11%. In the control group, there were three cases of nausea and vomiting, four cases of dizziness, and three cases of hypotension, for an overall incidence rate of 15.15%. This difference in incidence rate of adverse reactions was not different (χ2 = 1.710, P = 0.191).
Thoracic surgeries are extremely complex and the operative time is long. As such, thoracic surgeries can easily have a negative impact on a patient’s respiratory and circulatory function. Moreover, the trauma from the surgery itself can lead to increased release of inflammatory mediators, which are likely to cause postoperative pain[7,8]. As such, effective perioperative anesthesia management is important to ensure respiratory stability and tissue oxygenation. This is of specific importance for lung cancer surgery. A decrease in alveolar oxygen content in the human body under physiological conditions may lead to hypoxic pulmonary vasoconstriction. With vasoconstriction, the pulmonary blood pressure on the unventilated side cannot return to the heart for oxygenation, leading to an increased volume of venous blood and, thus, a decrease in oxygen partial pressure and saturation. Low blood oxygen content will lead to an increase in acidic substances that can cause serious damage to important organs, such as the kidneys and brain during the surgery[9-11]. The negative effect of anesthesia on immune function is a further concern, increasing the risk for postoperative infection. Anesthetic drugs exert a direct negative effect on cellular immune activity, as well as an indirect regulatory effect on the neuroendocrine system of the human body, especially in cancer patients. In these patients, immune function is seriously constrained, with a reduction in the concentration of T cells and a weakened ability of the body to respond to antigen stimulation. Surgery as an external source of stress on the immune function will aggravate the deterioration of the immune response in cancer patients[12].
In recent years, there has been significant progress in the application of ultrasound technology in the field of anesthesia. Specifically, ultrasound-guidance for peripheral nerve block anesthesia has improved the accuracy of the technique and its overall success rate. At the same time, as ultrasound is a non-ionizing imaging source, it will not cause additional damage to the tissues of the body[13,14]. For thoracic surgery, the peripheral nerve block is performed in the paravertebral space. In the thoracic region, the paravertebral space is a wedge-shaped cavity located between the head and neck of the ribs. The outer boundary is the parietal pleura and the inner boundary is the posterolateral vertebral body, the intervertebral disc, and the contents. The posterior wall of the thoracic paravertebral space includes the transverse process ligament of the rib and its lateral extension, as well as the intercostal intima, extending from the lower edge of the upper transverse process to the upper edge of the lower transverse process. This area contains the intercostal nerve, dorsal branch of the spinal nerve, traffic branch, and blood vessel. Therefore, the anesthesia effect for surgery can be improved by a nerve block in this area[15].
Our findings show that peripheral nerve block anesthesia for thoracic surgery reduced the use of intraoperative remifentanil and propofol and postoperative use of sufentanil. Therefore, ultrasound-guided paravertebral nerve block anesthesia can reduce the overall use of anesthetics during lung cancer surgery. Ultrasound guidance allows for careful identification of anatomical structures to avoid injury during injection of the anesthetic drugs. During the surgery, the pleural pressure of the drug is injected to determine whether the puncture is successful to avoid the risk of vascular injury[16]. As a paravertebral nerve block improves anesthesia overall, it can lower the patient’s stress response during anesthesia, decreasing the release of harmful stimuli and pain substances in the body and, thus, reduce hemodynamic fluctuations. Moreover, blocking of the ipsilateral sensory and sympathetic nerves provides anesthesia without having an effect on the heart, resulting in more stable hemodynamics. At the same time, it can improve the myocardial oxygen supply and blood supply, which are generally reduced by the drugs used for general anesthesia. Moreover, as the area of nerve block area is limited, the potential for perioperative complications is decreased[17].
Generally, patients undergoing thoracotomy have a strong traumatic stress response, which increases blood levels of metabolic hormones, such as glucagon and cortisol, thus increasing the risk for glucose metabolism disorders during surgery. This is a significant issue when profound anesthesia is required, which can lead to difficulty in maintaining hemodynamic stability, including blood flow to the heart and brain[18]. Traditional thoracoscopic surgery uses a combination of general anesthesia and local infiltration anesthesia. The local infiltration anesthesia is important as general anesthesia cannot completely block sensation in the surgical area, which will cause an increase in the secretion of adrenal medullary hormones and synthesis of catecholamines, with resultant hemodynamic fluctuations. Therefore, patients are prone to restlessness during incision suture during thoracotomy. Some patients will also experience complications, such as uneven breathing and hypoxia due to pain, which delays their postoperative recovery. Local infiltration anesthesia, however, must be carefully monitored as a rapid rate of vascular injection can lead to high blood concentrations leading to drug poisoning, and the puncture site is prone to infection and nerve injury[19].
The MAP and HR at T1 through T4 were significantly higher in the observation than control group. These findings suggest that ultrasound-guided paravertebral nerve block anesthesia can improve stability of respiratory and circulatory functions compared to general anesthesia only in patients during lung cancer surgery. Postoperative epinephrine, norepinephrine, dopamine, TNF-α, and IL-6 Levels were significantly lower in the observation than control group, suggesting that ultrasound-guided paravertebral nerve block anesthesia can further significantly reduce the degree of stress response and inflammatory response during lung cancer surgery. Lung cancer is are common clinical malignant tumors. In recent years, the incidence of lung cancer has increased, with the age incidence progressively becoming younger and the mortality rate increasing. At present, surgical tumor resection is the only radical cure available. Thoracoscopic surgery is the most common surgical method for clinical treatment of lung and esophageal cancer. Compared to tradition thoracotomy, thoracoscopic surgery decreases operative time and trauma, improves visualization during surgery and postoperative recovery, and has an overall positive impact on postoperative quality of survival. It is for these advantages that thoracoscopic surgery has become an important method for thoracic surgical treatment. Studies have found[20] that in patients undergoing thoracoscopic radical surgery for lung cancer, general anesthesia combined with paraspinal nerve block plus postoperative intravenous analgesia has a significant analgesic effect while, at the same time, improving stability of the circulatory system. These findings are consistent with those of our study.
Overall, our study confirmed the positive effects of ultrasound-guided paravertebral nerve block anesthesia for lung cancer surgery, improving the overall convenience of the surgery, with fewer complications. The reliable and targeted anesthesia provided by this technique makes it feasible to use in a wide range of surgical applications, including lung cancer surgery. It is noteworthy, however, that ultrasound does not provide an image of the spatial conformation of the catheter. Resulting distortion and/or compromise of the paravertebral space by the catheter may lead to complications. More in-depth studies are needed to solve this issue.
Ultrasound-guided paravertebral nerve block anesthesia can be indicated for lung cancer surgery, with little effect on patients’ stress and hemodynamic responses.
Thoracic surgery for radical resection of lung tumor requires deep anesthesia which can lead to an adverse inflammatory response, loss of hemodynamic stability, and decreased immune function.
We evaluated the feasibility and benefits of ultrasound-guided paravertebral nerve block anesthesia in combination with general anesthesia for thoracic surgery for lung cancer.
This study aimed to evaluate the application of ultrasound-guided paravertebral nerve block anesthesia for lung cancer surgery to inform practice.
Patients in the control group received an epidural block combined with general anesthesia. For patients in the observation group, ultrasound-guided paravertebral nerve block anesthesia was used before induction of general anesthesia. The concentrations of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were determined by enzyme-linked immunosorbent assay, with the concentrations of epinephrine, norepinephrine, and dopamine determined by radio-immunity turbidimetry. The mean arterial pressure (MAP), heart rate (HR) and oxygen saturation (SpO2) were measured at baseline, before surgery and anesthesia, 15 min after anesthesia (T1), induction intubation, 5 min after skin incision and before extubation (T4) in both groups. The stress response and hemodynamic data were reported as a mean ± SD.
The use of intra-operative remifentanil and propofol and of postoperative sufentanil was significantly less in the observation than the control group. The MAP and HR at T1 through T4 were higher for the observation than control group. The postoperative levels of epinephrine, norepinephrine and dopamine were higher after surgery than at baseline for both groups. However, these levels were lower in the observation than control group at T1 through T4. The postoperative TNF-α and IL-6 levels after surgery were lower in the observation than in the control group. This difference in incidence rate of adverse reactions was not different.
Ultrasound-guided paravertebral nerve block anesthesia has good indication for lung cancer surgery, with little effect on patients’ stress and hemodynamic responses.
Large sample studies need to performed in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anesthesiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Guo MN, Ulas CH S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Chen X, Li M, Zheng R, Huang Q, Li Y, Zhu Y, Chen Z, Lin J. Effects of sevoflurane inhalation anesthesia on IL-6, TNF-α and MMP-9 expression and hemodynamics in elderly patients undergoing lobectomy for lung cancer. Cell Mol Biol (Noisy-le-grand). 2020;66:49-53. [PubMed] |
| 2. | Sen Y, Xiyang H, Yu H. Effect of thoracic paraspinal block-propofol intravenous general anesthesia on VEGF and TGF-β in patients receiving radical resection of lung cancer. Medicine (Baltimore). 2019;98:e18088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Cho E, Than TT, Kim SH, Park ER, Kim MY, Lee KH, Shin HJ. G3BP1 Depletion Increases Radiosensitisation by Inducing Oxidative Stress in Response to DNA Damage. Anticancer Res. 2019;39:6087-6095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Chibaya L, Karim B, Zhang H, Jones SN. Mdm2 phosphorylation by Akt regulates the p53 response to oxidative stress to promote cell proliferation and tumorigenesis. Proc Natl Acad Sci U S A. 2021;118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 5. | Correction to Supporting Information for Chibaya et al, Mdm2 phosphorylation by Akt regulates the p53 response to oxidative stress to promote cell proliferation and tumorigenesis. Proc Natl Acad Sci U S A. 2021;118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Zalewska-Ziob M, Adamek B, Kasperczyk J, Romuk E, Hudziec E, Chwalińska E, Dobija-Kubica K, Rogoziński P, Bruliński K. Activity of Antioxidant Enzymes in the Tumor and Adjacent Noncancerous Tissues of Non-Small-Cell Lung Cancer. Oxid Med Cell Longev. 2019;2019:2901840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Ciccarese F, Zulato E, Indraccolo S. LKB1/AMPK Pathway and Drug Response in Cancer: A Therapeutic Perspective. Oxid Med Cell Longev. 2019;2019:8730816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 8. | Sakaguchi M, Kitaguchi D, Morinami S, Kurashiki Y, Hashida H, Miyata S, Yamaguchi M, Sakai M, Murata N, Tanaka S. Berberine-induced nucleolar stress response in a human breast cancer cell line. Biochem Biophys Res Commun. 2020;528:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | D'Andrea R, Gambetti G, Querci L, Amodei B, Bianchini A. [Ultrasound-guided thoracic paravertebral block for closed loop ileostomy repair in severe COPD: a case report]. Braz J Anesthesiol. 2018;68:650-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Hung WT, Cheng YJ, Chen JS. Video-Assisted Thoracoscopic Surgery Lobectomy for Lung Cancer in Nonintubated Anesthesia. Thorac Surg Clin. 2020;30:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Huang QW, Li JB, Huang Y, Zhang WQ, Lu ZW. A Comparison of Analgesia After a Thoracoscopic Lung Cancer Operation with a Sustained Epidural Block and a Sustained Paravertebral Block: A Randomized Controlled Study. Adv Ther. 2020;37:4000-4014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Fujii T, Shibata Y, Ban Y, Shitaokoshi A, Takahashi K, Matsui S, Nishiwaki K. A single paravertebral injection via a needle vs. a catheter for the spreading to multiple intercostal levels: a randomized controlled trial. J Anesth. 2020;34:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Weiniger CF, Sharoni L. The use of ultrasound in obstetric anesthesia. Curr Opin Anaesthesiol. 2017;30:306-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Przkora R, Mora J, Balduyeu P, Meroney M, Vasilopoulos T, Solanki D. Ultrasound-Guided Regional Anesthesia Using a Head-Mounted Video Display: A Randomized Clinical Study. Pain Physician. 2021;24:83-87. [PubMed] |
| 15. | Matinian VV, Belousova EI, Saltanov AI. [Ultrasound guided catheterization of thoracic paravertebral space]. Anesteziol Reanimatol. 2014;59:57-58. [PubMed] |
| 16. | Echaniz G, Chan V, Maynes JT, Jozaghi Y, Agur A. Ultrasound-guided maxillary nerve block: an anatomical study using the suprazygomatic approach. Can J Anaesth. 2020;67:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Kamalanathan K, Knight T, Rasburn N, Joshi N, Molyneux M. Early Versus Late Paravertebral Block for Analgesia in Video-Assisted Thoracoscopic Lung Resection. A Double-Blind, Randomized, Placebo-Controlled Trial. J Cardiothorac Vasc Anesth. 2019;33:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | NeMoyer RE, Pantin E, Aisner J, Jongco R, Mellender S, Chiricolo A, Moore DF, Langenfeld J. Paravertebral Nerve Block With Liposomal Bupivacaine for Pain Control Following Video-Assisted Thoracoscopic Surgery and Thoracotomy. J Surg Res. 2020;246:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Oizumi H. [Thoracoscopic Surgery]. Kyobu Geka. 2018;71:838-842. [PubMed] |
| 20. | Chen N, Qiao Q, Chen R, Xu Q, Zhang Y, Tian Y. The effect of ultrasound-guided intercostal nerve block, single-injection erector spinae plane block and multiple-injection paravertebral block on postoperative analgesia in thoracoscopic surgery: A randomized, double-blinded, clinical trial. J Clin Anesth. 2020;59:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |