Published online Mar 6, 2022. doi: 10.12998/wjcc.v10.i7.2115
Peer-review started: November 29, 2021
First decision: January 12, 2022
Revised: January 17, 2022
Accepted: February 23, 2022
Article in press: February 23, 2022
Published online: March 6, 2022
Processing time: 92 Days and 21.5 Hours
The prognosis of borderline ovarian tumors (BOTs) has been the concern of clinicians and patients. It is urgent to develop a model to predict the survival of patients with BOTs.
To construct a nomogram to predict the likelihood of overall survival (OS) in patients with BOTs.
A total of 192 patients with histologically verified BOTs and 374 patients with epithelial ovarian cancer (EOC) were retrospectively investigated for clinical characteristics and survival outcomes. A 1:1 propensity score matching (PSM) analysis was performed to eliminate selection bias. Survival was analyzed by using the log-rank test and the restricted mean survival time (RMST). Next, univariate and multivariate Cox regression analyses were used to identify meaningful independent prognostic factors. In addition, a nomogram model was developed to predict the 1-, 3-, and 5-year overall survival of patients with BOTs. The predictive performance of the model was assessed by using the concordance index (C-index), calibration curves, and decision curve analysis (DCA).
For clinical data, there was no significant difference in body mass index, preoperative CA199 concentration, or tumor localization between the BOTs group and EOC group. Women with BOTs were significantly younger than those with EOC. There was a significant difference in menopausal status, parity, preoperative serum CA125 concentration, Federation International of gynecology and obstetrics (FIGO) stage, and whether patients accepted postoperative adjuvant therapy between the BOT and EOC group. After PSM, patients with BOTs had better overall survival than patients with EOC (P value = 0.0067); more importantly, the 5-year RMST of BOTs was longer than that of EOC (P value = 0.0002, 95%CI
Patients with BOTs had a better prognosis than patients with EOC. The nomogram we constructed might be helpful for clinicians in personalized treatment planning and patient counseling.
Core Tip: The recurrence and overall survival of borderline ovarian tumors (BOTs) after operation have been one of the main concerns of patients and clinicians. In this research, we firstly performed a 1:1 propensity score matching analysis by applying age as a matching variable, then we found that patients with BOTs had a better overall survival as compared to patients with epithelial ovarian cancer (EOC), more importantly, the 5-year restricted mean survival time of BOTs was longer than EOC. What’s more, we conducted a nomogram that could accurately predict 1, 3, 5-year overall survival in patients with BOTs.
- Citation: Gong XQ, Zhang Y. Develop a nomogram to predict overall survival of patients with borderline ovarian tumors. World J Clin Cases 2022; 10(7): 2115-2126
- URL: https://www.wjgnet.com/2307-8960/full/v10/i7/2115.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i7.2115
Borderline epithelial ovarian tumors (BOTs), a category of low-grade malignant potential carcinoma derived primarily from ovarian epithelial lesions, comprise 10%-20% of epithelial ovarian tumors[1]. BOTs have been classified into serous, mucinous, endometrioid, clear cell, Brenner (transitional cell), and mixed epithelial tumors. The major subtypes of BOTs are mucinous and serous following histological examination, accounting for approximately 30% and 67% of BOTs, respectively[2,3]. BOTs are characterized by cytological evidence of malignancy in which atypical epithelial cell proliferation and nuclear atypia are observed but with no obvious invasion to the stroma, which differs from epithelial ovarian cancer (EOC)[4,5].
Compared to EOC, patients with BOTs are reported to have a better prognosis[6-8]. Age is reported to be a significant risk factor for OS in EOC, and some studies have shown that BOT patients tend to have an earlier age at diagnosis than EOC patients, implying that age may be the reason for the different prognoses of the two groups[9]. Apart from age, the Federation international of gynecology and obstetrics (FIGO) stage of tumors is also demonstrated to be a risk factor that mainly influences prognosis and recurrence rates, and others are peritoneal spread, histology, genetic factors, and vascular invasion[10,11]. The 5-year overall survival rate of BOTs is 80%-95%, and the 20-year survival rate is 80%, but some patients will relapse and die from their disease[5,12,13]. Therefore, the recurrence and prognosis of BOTs after surgery are one of the main concerns of patients and gynecologic researchers.
FIGO classification is applied for BOT staging, as with malignant epithelial ovarian tumors. The diagnosis is primarily based on pathological results, and the main and initial treatment method is surgery. However, “semi malignant ovarian tumors” as BOTs were mentioned as early as 1929[14]. In addition, the diagnosis of BOTs cannot be determined before surgery because patients with BOTs have no specific clinical symptoms. Considering the above particular characteristics of BOTs, a prognostic model is urgently needed for physicians and patients in the course of therapy.
To date, nomograms are regarded as an important prognostic tool that has been applied to numerous types of cancer and nontumor clinical research and can be very valuable to clinicians and patients[15]. The use of nomograms has favorably been compared to traditional staging systems for many cancers, and thus, it has been proposed as an alternative or even as a new standard[16]. Although several studies have used nomograms to study the relapse of BOTs, there is no such model to investigate the survival of patients with BOTs.
In the present study, we retrospectively collected and analyzed single-center data of women diagnosed with BOTs and EOC after surgery. Next, we compared the basic characteristics and survival outcomes between BOTs and EOC. Then, we evaluated the prognostic risk factors for BOTs. Moreover, we established a nomogram based on 6 clinical variables and follow-up information to improve the predictive capacity for the prognosis of BOTs. We believe that the outcome gained from our study will provide more information for patient consultation and oncologists to evaluate the survival of patients with BOTs, ultimately contributing to the development of optimal therapeutic strategies.
Patients included in this research had histologically proven BOTs and were treated at the Department of Obstetrics and Gynecology, Tongji Hospital, between January 2012 and August 2020. The patients were staged (I–IV) according to the FIGO 2019 standards. The exclusion criteria were other benign or malignant tumors, especially cervical and ovarian tumors treated by preoperative chemotherapy and gynecological diseases (e.g., endometriosis and pelvic infection). A total of 563 individuals were ultimately enrolled, including 371 EOC patients and 192 BOTs patients. This study was approved by the Ethical Committee of Tongji Hospital of Tongji Medical College at Huazhong University of Science and Technology. Written informed consent was waived.
The clinical indices analyzed included patient age at diagnosis, body mass index, menopausal status and parity, the level of preoperative tumor marker (CA125, CA199) FIGO stage, surgical type, and postoperative adjuvant therapy. Overall survival (OS) was regarded as the time interval (in months) between the date of surgery and the date of death or censoring. Disease-free survival (DFS) was defined as the time interval (in months) between the date of surgery and the date of recurrence or censoring. The restricted mean survival time (RMST) described the average survival time of patients enrolled in the study during the follow-up period of truncation and was simply estimated by the area under the survival curve up to the specific time, which could be used to explain cumulative covariate effects, especially in the presence of nonproportionality.
Because this was a retrospective study and the operative approach was not assigned randomly, there were potentially confounding factors and selection biases between groups that could impact the outcomes of comparisons. To overcome the biases produced by disequilibrium between the two groups, PSM was conducted. The propensity score was calculated by the logistical regression model using age as covariates. PSM was performed as one-to-one matching between the BOTs and EOC groups with nearest-neighbor matching and a 0.1 caliper width using R (version 4.0.2).
We performed univariate and multivariate Cox regression analyses via clinical factors. To intensify the prognostic predictive ability of the clinical factors included for OS of BOT patients, a nomogram with the ‘rms’ R package was constructed. Bootstraps with 1000 resamples were used for these activities. The factors comprising the C-index, calibration plots, and DCA were adopted to measure the predictive effectiveness of the nomogram. The predicted results of the nomogram are displayed in the calibration curve, and the 45° line indicates the ideal prediction. During the internal validation of the nomogram, the total points of each patient in the validation cohort were calculated according to the established nomogram. Then, Cox regression in this cohort was performed using the total points as a factor, and finally, the C-index and calibration curve of validation were derived based on the regression analysis. P value < 0.05 was considered statistically significant.
Data were analyzed with SPSS (IBM version 19.0) and R (Version 4.0.2). Continuous variables were calculated as the mean ± standard deviation (SD) or median and range as appropriate and compared with Student’s t tests. Categorical variables were calculated as rates (%) and compared using the chi-square test or Fisher’s exact test, as appropriate. OS rates were determined using Kaplan–Meier survival curves and compared by the log-rank test. Univariate and multiple Cox regression analyses were used to assess independent prognostic factors among our clinical data. P value < 0.05 was considered statistically significant.
A total of 192 patients with BOTs and 371 patients with EOC who underwent primary resection were included in this study. Patient baseline characteristics before PSM and after PSM are presented in Table 1. Before PSM, there were no statistically significant differences in body mass index, tumor localizations, or the frequencies of elevated preoperative CA199 concentrations between the two groups. However, the age of diagnosis for BOTs (41 ± 15.2 years) was less than that of EOC (51 ± 11.8 years). The frequencies of postmenopausal status (25.5% vs 52.3%, P value < 0.0001), parity (74.1% vs. 90%, P value < 0.001), elevated preoperative CA125 concentration (55.7% vs 86.3%, P value < 0.0001), advanced FIGO stage (II-Ⅳ) (12.5% vs 59.3%, P value < 0.0001), and accepted adjuvant therapy (33.3% vs 78.7%, P value < 0.0001) were significantly lower in patients with BOTs than in patients with EOC. To overcome the effect of potentially confounding factors and selection biases on the comparison outcome, PSM was adopted. After 1:1 PSM analysis, a total of 192 patients with BOTs (mean diagnosed age 41 ± 15.3 years) and 192 patients with EOC (mean diagnosed age 45.8 ± 12.8 years) were matched. Except for menopausal status, the significantly different frequency trends between them were the same as the outcome before PSM.
| Variables | Before PSM | After PSM | ||||
| BOTs (n = 192) | EOC (n = 371) | P value | BOTs (n = 192) | EOC (n = 192) | P value | |
| Age at diagnosis (years) | 41.0 ± 15.2 | 51.0 ± 11.8 | < 0.0001 | 41.0 ± 15.2 | 45.8 ± 12.8 | 0.0008 |
| Body mass index (kg/m2) | 22.6 ± 3.2 | 23 ± 8.1 | 0.5159 | 22.6 ± 3.2 | 22.4 ± 3 | 0.5189 |
| Menopausal status (%) | < 0.0001 | 0.565 | ||||
| Premenopausal | 143 (74.5) | 176 (47.4) | 143 (74.5) | 138 (71.9) | ||
| Postmenopausal | 49 (25.5) | 194 (52.3) | 49 (25.5) | 54 (28.1) | ||
| Unknown | 0 (0) | 1 (0.3) | 0 (0) | 0 (0) | ||
| Parity (%) | < 0.0001 | < 0.0001 | ||||
| Nulliparous | 6 (3.1) | 0 (0) | 6 (3.1) | 0 (0) | ||
| Parous | 133 (69.3) | 334 (90) | 133 (69.3) | 164 (85.4) | ||
| Unknown | 53 (27.6) | 37 (10) | 53 (27.6) | 0 (0) | ||
| Preoperative CA125>35 IU/mL (%) | < 0.0001 | < 0.0001 | ||||
| Elevated | 107 (55.7) | 320 (86.3) | 107 (55.7) | 163 (84.9) | ||
| Normal | 76 (39.6) | 37 (10) | 76 (39.6) | 19 (9.9) | ||
| Unknown | 9 (4.7) | 14 (3.8) | 9 (4.7) | 10 (5.2) | ||
| Preoperative CA199>34 IU/mL (%) | 0.945 | 0.827 | ||||
| Elevated | 54 (28.1) | 104 (28) | 54 (28.1) | 58 (30.2) | ||
| Normal | 110 (57.3) | 209 (56.3) | 110 (57.3) | 104 (54.2) | ||
| Unknown | 28 (14.6) | 58 (15.6) | 28 (14.6) | 30 (15.6) | ||
| Tumor localizations | 0.153 | 0.196 | ||||
| LO | 44 (22.9) | 99 (26.7) | 44 (22.9) | 54 (28.1) | ||
| RO | 86 (44.8) | 165 (36.4) | 86 (44.8) | 69 (35.9) | ||
| Unknown | 62 (32.3) | 137 (36.9) | 62 (32.3) | 69 (35.9) | ||
| FIGO stage (%) | < 0.0001 | < 0.0001 | ||||
| Ⅰ | 168 (87.5) | 151 (40.7) | 168 (87.5) | 85 (44.3) | ||
| Ⅱ | 7 (3.6) | 34 (9.2) | 7 (3.6) | 16 (8.3) | ||
| Ⅲ | 17 (8.9) | 169 (45.6) | 17 (8.9) | 80 (41.7) | ||
| Ⅳ | 0 (0) | 17 (4.6) | 0 (0) | 11 (5.7) | ||
| Surgical type (%) | 0.0005 | 0.0005 | ||||
| BSO | 7 (3.6) | 4 (1.1) | 7 (3.6) | 1 (0.5) | ||
| HB | 13 (6.8) | 8 (2.2) | 13 (6.8) | 4 (2.1) | ||
| HBO | 5 (2.6) | 31 (8.4) | 5 (2.6) | 17 (8.9) | ||
| HBOA | 8 (4.2) | 15 (4) | 8 (4.2) | 11 (5.7) | ||
| HBOL | 77 (40.1) | 291 (78.4) | 77 (40.1) | 146 (76) | ||
| TR | 34 (17.7) | 6 (1.6) | 34 (17.7) | 5 (2.6) | ||
| USO | 48 (25) | 13 (3.5) | 48 (25) | 7 (3.6) | ||
| CS | 0 (0) | 3 (0.8) | 0 (0) | 1 (0.5) | ||
| Postoperative adjuvant therapy (%) | < 0.0001 | < 0.0001 | ||||
| Yes | 64 (33.3) | 292 (78.7) | 64 (33.3) | 156 (81.3) | ||
| No | 125 (65.1) | 78 (21) | 125 (65.1) | 36 (18.8) | ||
| Unknown | 3 (1.6) | 1 (0.3) | 3 (1.6) | 0 (0) | ||
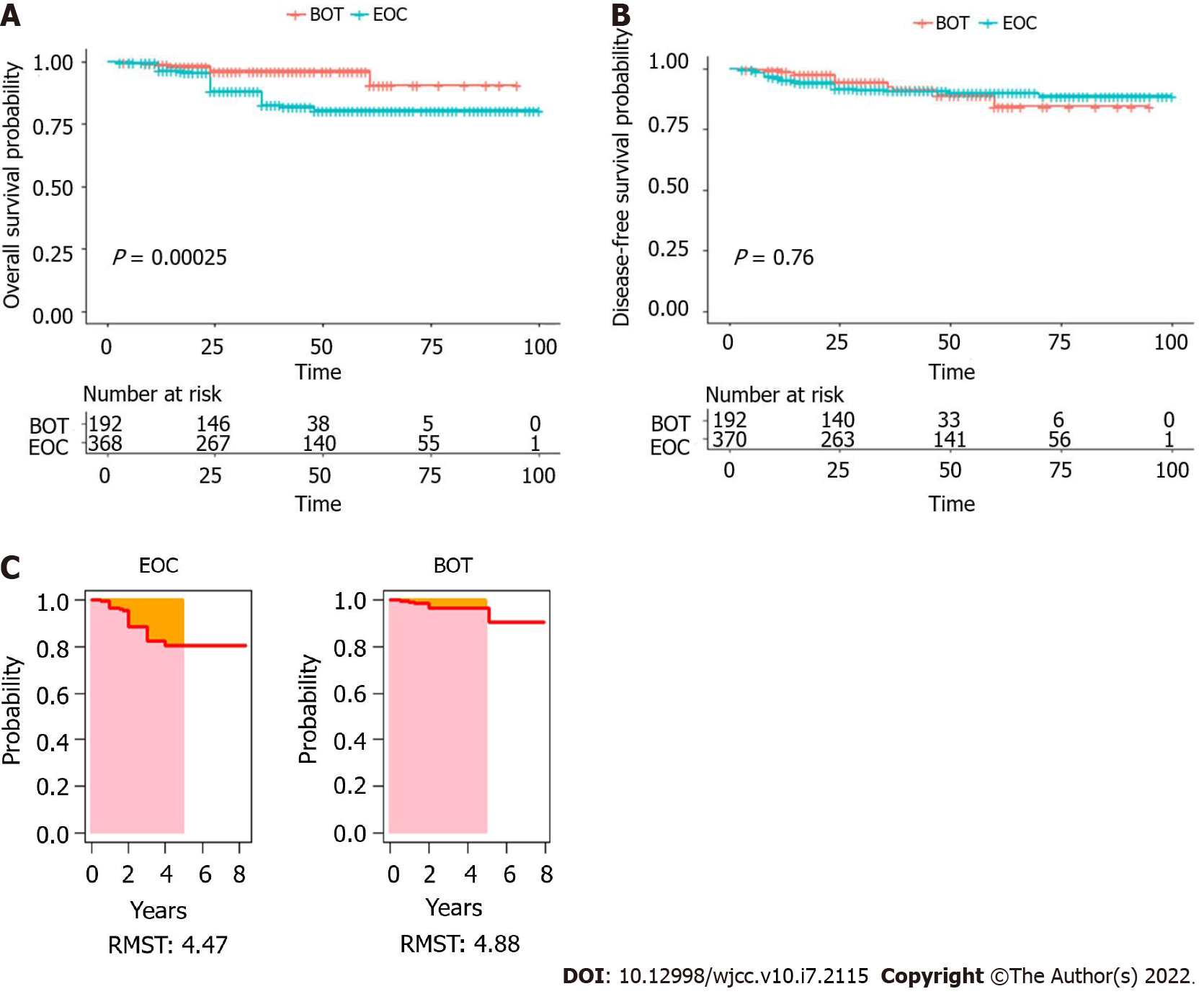
Before PSM, the median follow-up period for all patients was 41.9 mo (range, 3-100 mo). The 1-, 3- and 5-year OS rates of women with BOTs were 98.9%, 96.4%, and 90.8%, respectively, and the 1-, 3-, and 5-year DFS rates were 98.9%, 92.4%, and 84.6%, respectively. For the EOC group, the 1-, 3-, and 5-year OS rates of patients with BOTs were 96.7%, 82.7%, and 80.6%, respectively, and the 1-, 3-, and 5-year DFS rates were 95.3%, 91.0%, and 88.9%. In addition, the OS and DFS curves for BOTs and EOC patients are shown in Figure 1A and B. The P value of OS and DFS for the two groups were 0.00025 and 0.76, respectively. The RMST was used to analyze survival curves to perform comparisons between the BOTs group and the EOC group. Before PSM, for estimating the 5-year RMST, the mortality of EOC exceeded that of BOTs, with a P value equal to 0.041 and 95%CI (-0.509 to -0.997) (Figure 1C).
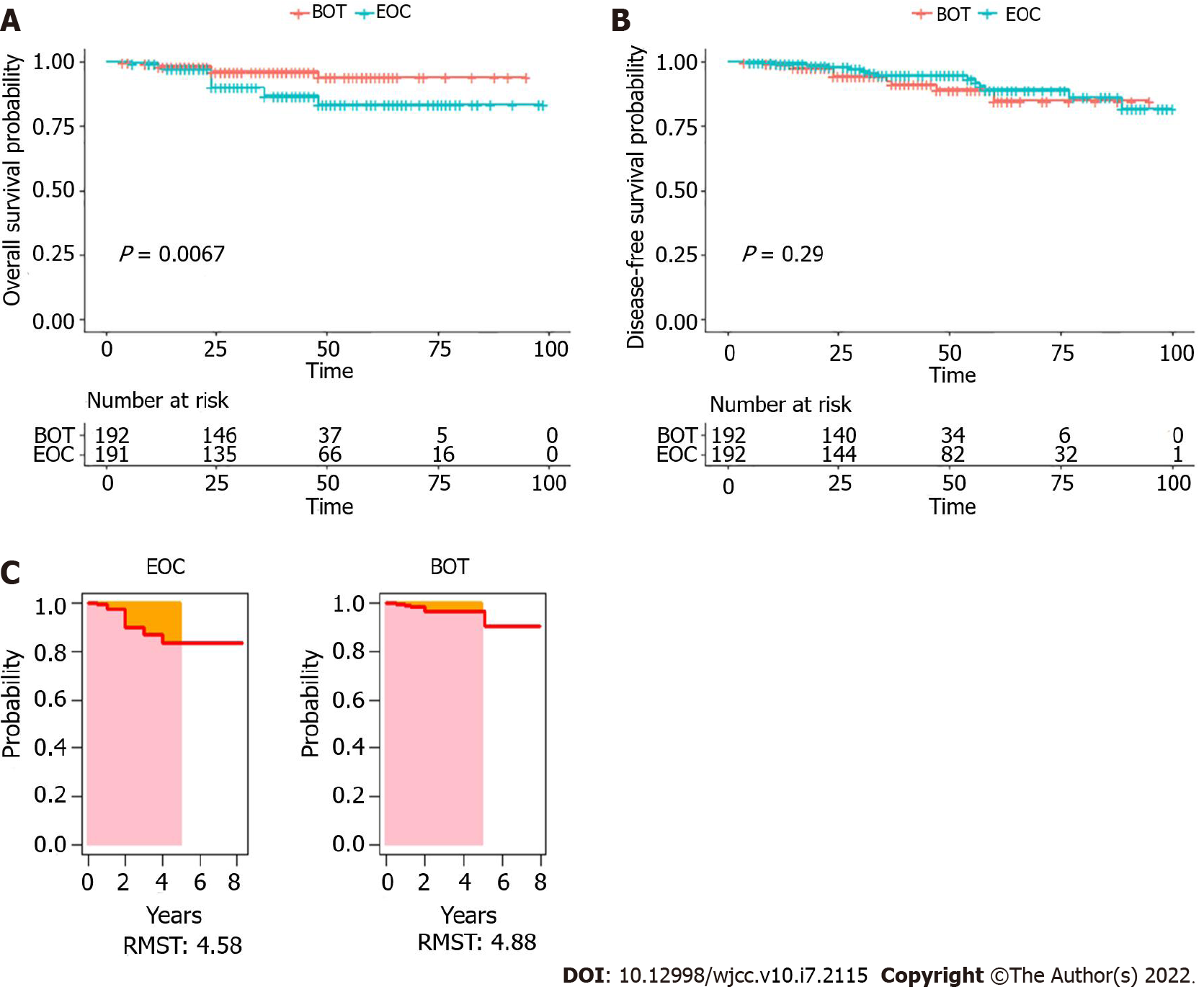
After PSM, the median follow-up period was 41.7 mo (range, 4-100 mo). The 1-, 3-, and 5-year OS rates of women with BOTs were 98.9%, 96.4%, and 90.8%, respectively, and the 1-, 3-, and 5-year DFS rates were 98.9%, 92.4%, and 84.6%, respectively. For the EOC group, the 1-, 3-, and 5-year OS rates were 97.3%, 86.9%, and 83.6%, respectively, and the 1-, 3-, and 5-year DFS rates were 98.8%, 95.0%, and 86.4%. The OS and DFS curves after PSM for patients from the two groups are shown in Figure 2A and B. The P values of OS and DFS for the two groups were 0.0067 and 0.29, respectively. Moreover, after PSM, the 5-year RMST of BOTs was also longer than that of EOC, with a P value equal to 0.002 and 95%CI (-1.137 to -0.263) (Figure 2C).
Univariate and multivariate analyses were performed to identify the predictors that influenced OS after PSM, and the results are shown in Table 2. Univariate Cox analysis demonstrated that diagnosed age was an independent risk factor for tumor OS (P value < 0.05). After multivariate analysis, age and the surgical type became independent risk factors for tumor OS (P value < 0.05).
| Variables | Univariate Cox analysis | Multivariate Cox analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age | 1.1 (1.1-1.2) | 3.3e-06 | 1.180 (1.093730-1.274) | 2.04e-05 |
| Surgical type | 1 (0.66-1.6) | 0.93 | 2.244 (1.164409-4.325) | 0.0157 |
| FIGO stage | 3.3e-08 (0-Inf) | 1 | 1.046e-08 (0-Inf) | 0.9990 |
| Tumor size | 1.6 (0.21-12) | 0.65 | 8.503 (0.006883-10503.344) | 0.5557 |
| CA125 | 1.7 (0.45-6.4) | 0.44 | 1.046 (0.203595-5.370) | 0.9574 |
| CA199 | 1.2 (0.49-3.1) | 0.66 | 2.156 (0.531390-8.746) | 0.2823 |
| BMI | 1.1 (0.88-1.4) | 0.4 | ||
| Menopausal status | 1.9 (0.42-8.4) | 0.41 | ||
| Parity | 0.31 (0.056-1.7) | 0.19 | ||
| Adjuvant therapy | 0.69 (0.14-3.4) | 0.65 | ||
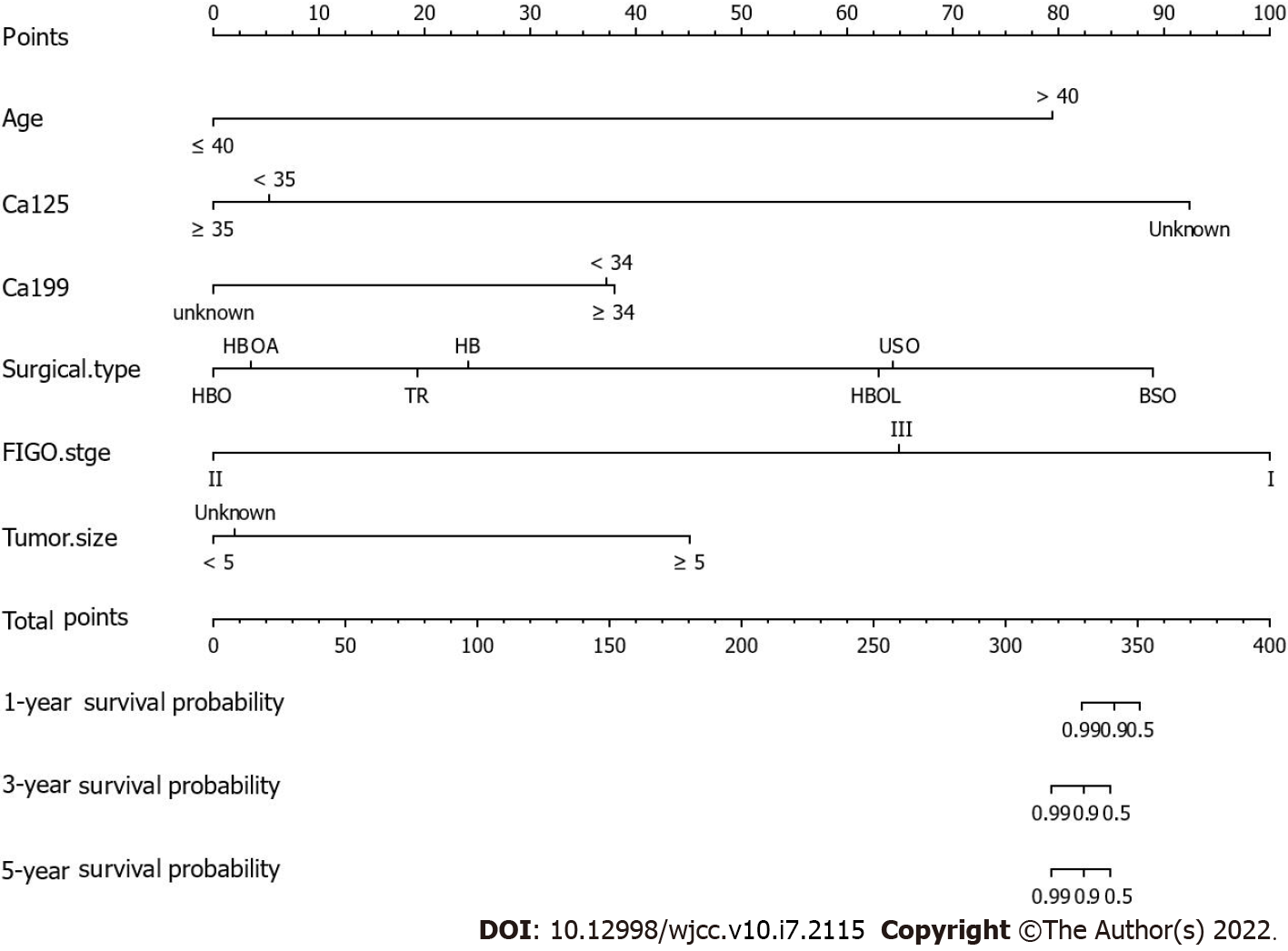
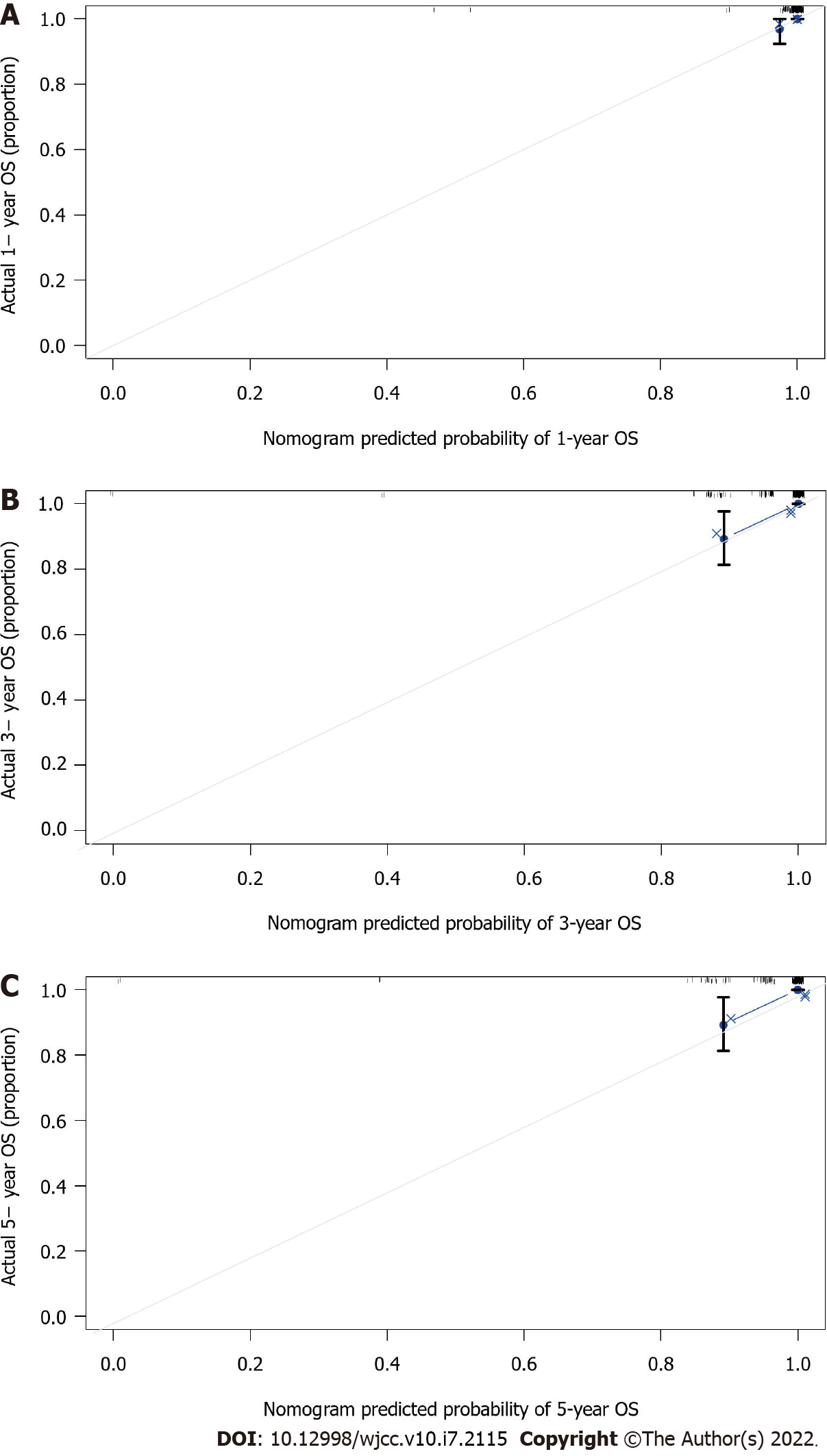
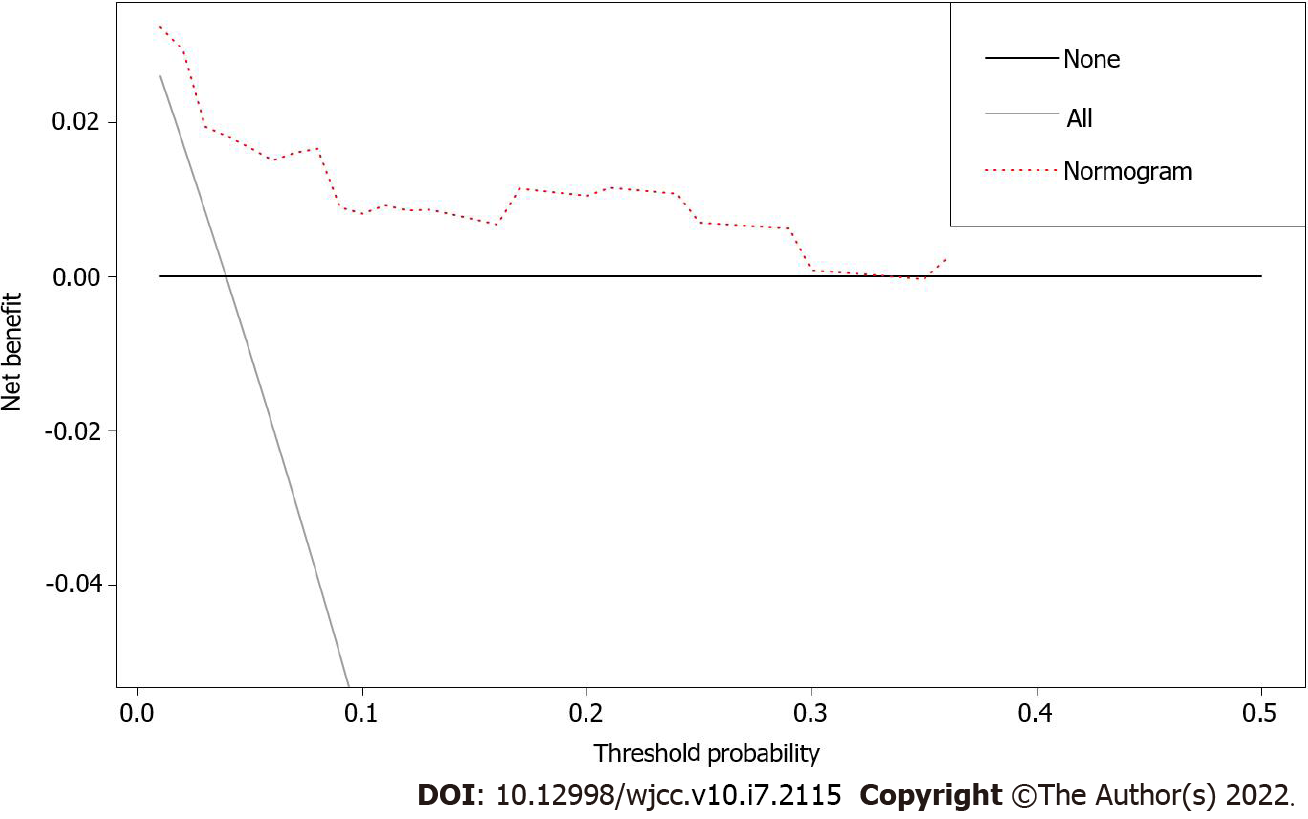
Nomograms are widely used as prognostic devices in oncology and medicine. To heighten the predictive capability of the model for the OS of patients with BOTs through a quantitative approach, we established a nomogram by using clinical factors included in multivariate Cox analysis to predict the 1-, 3-, and 5-year OS of BOT patients (Figure 3). The C-index (0.959, 95%CI: 0.0.8708-1.0472) and calibration plots at 1, 3, and 5 years showed that the nomogram was a valid tool (Figure 4A-C). In addition, the DCA showed that the nomogram yielded more important clinical applications for the prognostic prediction of women with BOTs than for those in the all-treatment cohort and no-treatment cohort. A net benefit was discovered for BOT patient survival risks (Figure 5), implying the good capability of our model.
Our results demonstrated that patients with BOTs had a significantly younger age and a better survival outcome than patients with EOC. In addition, the nomogram we constructed in the current study is the first to accurately predict the 1-, 3-, and 5-year OS of women with BOTs, and this model can be used as an aid to help clinicians assess the prognosis of patients with BOTs individually.
As mentioned earlier, several previous studies have shown that the median age at diagnosis of the BOT group was less than that of the EOC group[17,18], which was in accordance with our results. In addition, as age is reported to be associated with the prognosis of many diseases[6,19,20], and this study was retrospective, potentially confounding factors and selection biases may exist in the two groups. Moreover, our data showed that age at diagnosis was significantly different between the BOTs group and EOC group. Hence, PSM was performed by applying age as a matching variable.
As an important marker for ovarian cancer, CA125 has been widely studied in many studies to determine whether it should be applied to distinguish between benign and malignant tumors[21,22]. This study discovered that patients with BOTs had a lower proportion of elevated preoperative serum CA125 concentrations than EOC patients. However, Yang et al[4] found no apparent difference in preoperative serum CA125 Levels between BOTs and EOC. The probable reason for the difference is that their study population was BOT patients and stage I epithelial ovarian cancer patients, and the serum CA125 concentration was reported to have only 50% sensitivity for women with stage I ovarian cancer or with BOTs[23].
After PSM, we found that women with BOTs also had a more favorable 1-, 3-, and 5-year OS rate than women with EOC, and the 5-year RMST of BOTs was also longer than that of EOC, implying that BOTs patients truly had a better survival outcome than patients with EOC. These results coincide with those of previous studies showing that BOTs and EOC had significant differences in patient survival outcomes[6,13].
Notably, our results showed that age was an independent risk factor for OS in BOTs patients. Previously, a study including 801 patients with BOTs also showed that diagnosed age was important for overall survival[24]. In addition, although menopause was associated with age, menopausal status was not identified as a risk factor for OS. Similar results were discovered by Tal et al[11], who found that menopause was not an independent risk factor for the prognosis of women with BOTs.
To date, the standard surgical procedure for BOTs is comprehensive staging surgery. For young fertile patients, fertility-sparing surgery (unilateral salpingo-oophorectomy and contralateral ovarian biopsy to assess the opposite ovary) is a first-line treatment[5,25]. Systematic lymphadenectomy is not required routinely; it was only suggested when patients with BOTs had invasive peritoneal implants[26], but lymphatic resection is preferred for endometrial mucinous BOTs with microinvasion, peritoneal implantation, advanced FIGO staging, and pathology[27]. Consistent with these conclusions, we noted that surgical type was significantly associated with the OS of women with BOTs in multivariate Cox regression analysis.
Moreover, whether postoperative adjuvant therapy should proceed is a controversial issue. Chemotherapy is not recommended for patients in the early stage after surgery, and opinions vary among scholars as to whether chemotherapy is recommended for advanced patients. Wang et al[28] suggested that chemotherapy should be advised to patients with BOTs who have high risks, such as invasive implants or residual tumors, and they found that chemotherapy was related to poorer disease-specific survival. However, a retrospective study based on 364 patients with BOTs from 14 gynecological oncology departments in Turkey and Germany demonstrated that chemotherapy had no connection with overall survival[29], and the results of our study implied that postoperative adjuvant therapy was not a meaningful factor for patients with BOTs.
As a widely used tool in oncology, nomograms have been shown to predict overall outcomes in personalized patients well[15]. The variables included in our nomogram are regarded as important clinical characteristics of BOTs. CA125 and CA199 have been reported as considerable tumor markers that could predict malignancy[21,30]. In addition, Ayhan et al[31] found that high concentrations of serum CA125 and CA199 may imply larger tumor sizes, and tumor size has been recognized as a critical prognostic factor of many diseases, such as esophageal cancer, rectal cancer, and endometrioid endometrial cancer[32-34]. In addition, Song et al[35] showed that the FIGO stage was associated with BOT overall survival. More importantly, the present study found that age and surgical type were independent risk factors for BOT overall survival. Finally, the nomogram was internally validated using 1000 repetitions of bootstrap samples.
Despite the advantageous outcomes of our research, there are several limitations of this study. First, the nomogram was established based on data collected from a single center. Second, this was a retrospective study. The cohort was not representative of all patients with BOTs.
Considering its accuracy and clinical value, the current nomogram in the present study could provide an individualized evaluation of the prognosis of patients with BOTs. Our research will be beneficial for clinicians to make clinical treatment recommendations and patients to better know their therapeutic options, thus improving patients’ compliance and prognosis. Besides, we will collect more BOTs patients from different cohorts to validate the model from the external aspect in the future. Additional data and studies are needed to determine whether it can be applied to the total BOTs cohort.
Although patients with borderline ovarian tumors (BOTs) have been reported to have a better survival outcome as compared to patients with epithelial ovarian cancer (EOC), there are many risk factors of BOTs. Nomogram has been successfully applied to predict the prognosis of many cancers based on some meaningful prognostic factors, but there is no such model to study the 1, 3, 5 years’ survival of BOTs.
Served as “semi-malignant ovarian tumors”, the overall survival of BOTs after the operation has been paid high attention by the clinicians and patients. It was necessary to construct a prognostic model to assess the survival outcome of patients with BOTs.
This research aimed to develop a nomogram to predict the possibility of OS in patients with BOTs, thus contributing to making individualized treatment recommendations.
Totally 192 patients with BOTs and 374 patients with EOC were involved. Based on meaningful independent prognostic factors identified by univariate and multivariate Cox regression analyses, a nomogram model was developed to predict the 1-, 3-, and 5-year overall survival of patients with BOTs.
Compared to patients with EOC, patients with BOTs had better overall survival after 1:1 propensity score matching analysis (P value = 0.0067). We established a nomogram to predict the 1-, 3-, and 5-year OS of BOT patients. The C-index (0.959, 95% confidence interval 0.8708-1.0472) and calibration plots at 1, 3, and 5 years showed that the nomogram was a valid tool.
The current research constructed a nomogram that could accurately give a personalized prediction of the prognosis of patients with BOTs. The outcome gained from our study might provide convenience to patients and clinicians.
The nomogram developed by this study is the first to predict the 1-, 3-, and 5-year OS of women with BOTs. Moreover, it has been precisely assessed by internal validation. The results gained from our study will provide advice to make treatment planning.
Provenance and peer review: Unsolicited article; externally peer reviewed.
Peer-review model: Single blind
Specialty type: Obstetrics and gynecology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bredt LC, Cabezuelo AS S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Yilmaz E, Sahin N, Koleli I, Melekoglu R, Tanrikut E, Faydali S, Karaer A, Coskun EI. RETROSPECTIVE ANALYSIS OF BORDERLINE OVARIAN TUMORS: OUTCOMES AT A SINGLE CENTER. Acta Clin Croat. 2019;58:29-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Gilks CB. Subclassification of ovarian surface epithelial tumors based on correlation of histologic and molecular pathologic data. Int J Gynecol Pathol. 2004;23:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Solmaz Hasdemir P, Guvena T. Borderline ovarian tumors" A contemporary review of clinicopathological characteristics, diagnostic methods and therapeutic options. J BUON. 2016;21:780-786. [PubMed] |
| 4. | Yang S, Tang H, Xiao F, Zhu J, Hua T, Tang G. Differentiation of borderline tumors from type I ovarian epithelial cancers on CT and MR imaging. Abdom Radiol (NY). 2020;45:3230-3238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | du Bois A, Trillsch F, Mahner S, Heitz F, Harter P. Management of borderline ovarian tumors. Ann Oncol. 2016;27 Suppl 1:i20-i22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Matsuo K, Machida H, Mandelbaum RS, Grubbs BH, Roman LD, Sood AK, Gershenson DM. Mucinous borderline ovarian tumor versus invasive well-differentiated mucinous ovarian cancer: Difference in characteristics and outcomes. Gynecol Oncol. 2019;153:230-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Trimble CL, Kosary C, Trimble EL. Long-term survival and patterns of care in women with ovarian tumors of low malignant potential. Gynecol Oncol. 2002;86:34-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Flicek KT, VanBuren W, Dudiak K, Lahkman Y, Chen LW, Butler K, Menias CO. Borderline epithelial ovarian tumors: what the radiologist should know. Abdom Radiol (NY). 2021;46:2350-2366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Hanatani M, Yoshikawa N, Yoshida K, Tamauchi S, Ikeda Y, Nishino K, Niimi K, Suzuki S, Kawai M, Kajiyama H, Kikkawa F. Impact of age on clinicopathological features and survival of epithelial ovarian neoplasms in reproductive age. Int J Clin Oncol. 2020;25:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Longacre TA, McKenney JK, Tazelaar HD, Kempson RL, Hendrickson MR. Ovarian serous tumors of low malignant potential (borderline tumors): outcome-based study of 276 patients with long-term (> or =5-year) follow-up. Am J Surg Pathol. 2005;29:707-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 216] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Tal O, Ganer Herman H, Gluck O, Levy T, Kerner R, Bar J, Sagiv R. Characteristics and prognosis of borderline ovarian tumors in pre and postmenopausal patients. Arch Gynecol Obstet. 2020;302:693-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Fischerova D, Zikan M, Dundr P, Cibula D. Diagnosis, treatment, and follow-up of borderline ovarian tumors. Oncologist. 2012;17:1515-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 13. | Burger CW, Prinssen HM, Baak JP, Wagenaar N, Kenemans P. The management of borderline epithelial tumors of the ovary. Int J Gynecol Cancer. 2000;10:181-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Harter P, Gershenson D, Lhomme C, Lecuru F, Ledermann J, Provencher DM, Mezzanzanica D, Quinn M, Maenpaa J, Kim JW, Mahner S, Hilpert F, Baumann K, Pfisterer J, du Bois A. Gynecologic Cancer InterGroup (GCIG) consensus review for ovarian tumors of low malignant potential (borderline ovarian tumors). Int J Gynecol Cancer. 2014;24:S5-S8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173-e180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1119] [Cited by in RCA: 2396] [Article Influence: 239.6] [Reference Citation Analysis (0)] |
| 16. | Sternberg CN. Are nomograms better than currently available stage groupings for bladder cancer? J Clin Oncol. 2006;24:3819-3820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 17. | Eltabbakh GH, Natarajan N, Piver MS, Mettlin CJ. Epidemiologic differences between women with borderline ovarian tumors and women with epithelial ovarian cancer. Gynecol Oncol. 1999;74:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Skírnisdóttir I, Garmo H, Wilander E, Holmberg L. Borderline ovarian tumors in Sweden 1960-2005: trends in incidence and age at diagnosis compared to ovarian cancer. Int J Cancer. 2008;123:1897-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Qiu MJ, Yang SL, Wang MM, Li YN, Jiang X, Huang ZZ, Xiong ZF. Prognostic evaluation of esophageal cancer patients with stages I-III. Aging (Albany NY). 2020;12:14736-14753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 20. | Cai H, Liu B, Wang H, Sun G, Feng L, Chen Z, Zhou J, Zhang J, Zhang T, He M, Yang T, Guo Q, Teng Z, Xin Q, Zhou B, Zhang H, Xia G, Wang C. SP1 governs primordial folliculogenesis by regulating pregranulosa cell development in mice. J Mol Cell Biol. 2020;12:230-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Lertkhachonsuk AA, Buranawongtrakoon S, Lekskul N, Rermluk N, Wee-Stekly WW, Charakorn C. Serum CA19-9, CA-125 and CEA as tumor markers for mucinous ovarian tumors. J Obstet Gynaecol Res. 2020;46:2287-2291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 22. | Felder M, Kapur A, Gonzalez-Bosquet J, Horibata S, Heintz J, Albrecht R, Fass L, Kaur J, Hu K, Shojaei H, Whelan RJ, Patankar MS. MUC16 (CA125): tumor biomarker to cancer therapy, a work in progress. Mol Cancer. 2014;13:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 357] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 23. | Karadag B, Kocak M, Kayikcioglu F, Ercan F, Dilbaz B, Kose M, Haberal A. Risk for malignant and borderline ovarian neoplasms following basic preoperative evaluation by ultrasonography, ca125 level and age. Asian Pac J Cancer Prev. 2014;15:8489-8493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Obermair A, Tang A, Kondalsamy-Chennakesavan S, Ngan H, Zusterzeel P, Quinn M, Carter J, Leung Y, Janda M. Nomogram to predict the probability of relapse in patients diagnosed with borderline ovarian tumors. Int J Gynecol Cancer. 2013;23:264-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Guillaume A, Pirrello O. Preservation of fertility in surgery of benign and borderline malignant ovarian tumors. J Visc Surg. 2018;155 Suppl 1:S17-S21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Fadare O. Recent developments on the significance and pathogenesis of lymph node involvement in ovarian serous tumors of low malignant potential (borderline tumors). Int J Gynecol Cancer. 2009;19:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Tropé CG, Kaern J, Davidson B. Borderline ovarian tumours. Best Pract Res Clin Obstet Gynaecol. 2012;26:325-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Wang Y, Sun H, Yu A, Zhu T, Chen X. Association between chemotherapy and disease-specific survival in women with borderline ovarian tumors: A SEER-based study. Eur J Obstet Gynecol Reprod Biol. 2019;242:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Gungorduk K, Asicioglu O, Braicu EI, Almuheimid J, Gokulu SG, Cetinkaya N, Gungor T, Pakay G, Telli EU, Cuylan ZF, Toptas T, Bilgi A, Ozyurt R, Agacayak E, Ozdemir A, Yildirim N, Taskin S, Oge T, Erol O, Akman L, Turan A, Icen MS, Senol T, Ovali OI, Yucesoy B, Gungorduk O, Temizkan O, Sanci M, Simsek T, Meydanli MM, Harma M, Yasar L, Uysal AD, Karateke A, Ortac F, Ozalp SS, Sehouli J, Muallem MZ. The Impact of Surgical Staging on the Prognosis of Mucinous Borderline Tumors of the Ovaries: A Multicenter Study. Anticancer Res. 2017;37:5609-5616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Seckin KD, Karslı MF, Yucel B, Bestel M, Yıldırım D, Canaz E, Akbayır O. The utility of tumor markers and neutrophil lymphocyte ratio in patients with an intraoperative diagnosis of mucinous borderline ovarian tumor. Eur J Obstet Gynecol Reprod Biol. 2016;196:60-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Ayhan A, Guven S, Guven ES, Kucukali T. Is there a correlation between tumor marker panel and tumor size and histopathology in well staged patients with borderline ovarian tumors? Acta Obstet Gynecol Scand. 2007;86:484-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Cai D, Huang ZH, Yu HC, Wang XL, Bai LL, Tang GN, Peng SY, Li YJ, Huang MJ, Cao GW, Wang JP, Luo YX. Prognostic value of preoperative carcinoembryonic antigen/tumor size in rectal cancer. World J Gastroenterol. 2019;25:4945-4958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 33. | Mahdi H, Munkarah AR, Ali-Fehmi R, Woessner J, Shah SN, Moslemi-Kebria M. Tumor size is an independent predictor of lymph node metastasis and survival in early stage endometrioid endometrial cancer. Arch Gynecol Obstet. 2015;292:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Wu Z, Yu B. Tumor Size as a Critical Prognostic Factor in T1-2 Stage Esophageal Cancer. Gastroenterol Res Pract. 2020;2020:2796943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Song T, Lee YY, Choi CH, Kim TJ, Lee JW, Bae DS, Kim BG. Borderline ovarian tumor in women aged ≥ 65 years: impact on recurrence and survival. Eur J Obstet Gynecol Reprod Biol. 2015;184:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |