Published online Mar 6, 2022. doi: 10.12998/wjcc.v10.i7.2095
Peer-review started: August 28, 2021
First decision: November 17, 2021
Revised: December 1, 2021
Accepted: January 22, 2022
Article in press: January 22, 2022
Published online: March 6, 2022
Processing time: 185 Days and 17.1 Hours
The results of previous animal experiments and clinical studies have shown that there is a correlation between expression of betatrophin and blood lipid levels. However, there are still differences studies on the correlation and interaction mechanism between betatrophin, angiogenin-likeprotein3 (ANGPTL3) and lipoprotein lipase (LPL). In our previous studies, we found an increase in serum ANGPTL3 Levels in Chinese patients with coronary heart disease (CHD). Therefore, we retrospectively studied Kazakh CHD patients.
To explore the correlation between the betatrophin/ANGPTL3/LPL pathway and severity of coronary artery disease (CAD) in patients with CHD.
Nondiabetic patients diagnosed with CHD were selected as the case group; 79 were of Kazakh descent and 72 were of Han descent. The control groups comprised of 61 Kazakh and 65 Han individuals. The serum levels of betatrophin and LPL were detected by enzyme-linked immunosorbent assay (ELISA), and the double antibody sandwich ELISA was used to detect serum level of ANGPTL3. The levels of triglycerides, total cholesterol, and fasting blood glucose in each group were determined by an automatic biochemical analyzer. At the same time, the clinical baseline data of patients in each group were included.
Betatrophin, ANGPTL3 and LPL levels of Kazakh patients were significantly higher than those of Han patients (P = 0.031, 0.038, 0.021 respectively). There was a positive correlation between the Gensini score and total cholesterol (TC), triglycerides (TG), low- density lipoprotein cholesterol (LDL-C), betatrophin, and LPL in Kazakh patients (r = 0.204, 0.453, 0.352, 0.471, and 0.382 respectively), (P = 0.043, 0.009, 0.048, 0.001, and P < 0.001 respectively). A positive correlation was found between the Gensini score and body mass index (BMI), TC, TG, LDL-C, LPL, betatrophin in Han patients (r = 0.438, 0.195, 0.296, 0.357, 0.328, and 0.446 respectively), (P = 0.044, 0.026, 0.003, 0.20, 0.004, and P < 0.001). TG and betatrophin were the risk factors of coronary artery disease in Kazakh patients, while BMI and betatrophin were the risk factors in Han patients.
There was a correlation between the betatrophin/ANGPTL3/LPL pathway and severity of CAD in patients with CHD.
Core Tip: The correlation analysis of this study suggested that the Gensini score of Kazakh patients in the coronary heart disease (CHD) group was positively correlated with the levels of total cholesterol, triglyceride (TG), low-density lipoprotein cholesterol, betatrophin and lipoprotein lipase. Logistic regression analysis showed that TG and betatrophin are risk factors for CHD in Kazakh individuals, while betatrophin and body mass index are risk factors for Han individuals. Unlike previous studies, the lipoprotein lipase levels in the two CHD groups did not decrease but increased.
- Citation: Qin L, Rehemuding R, Ainiwaer A, Ma X. Correlation between betatrophin/angiogenin-likeprotein3/lipoprotein lipase pathway and severity of coronary artery disease in Kazakh patients with coronary heart disease. World J Clin Cases 2022; 10(7): 2095-2105
- URL: https://www.wjgnet.com/2307-8960/full/v10/i7/2095.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i7.2095
Coronary heart disease (CHD) is the leading cause of death worldwide, with high morbidity and mortality [1]. Coronary atherosclerosis is one of the important risk factors for CHD[2], and many studies have shown that dyslipidemia is an important risk factor for coronary atherosclerosis[3]. Betatrophin was discovered as a tumor-associated antigen in 2004, and it is expressed in liver and adipose tissue. Because the protein structure of betatrophin is similar to that of angiopoietin-like proteins (ANGPTLs), it was also named ANGPTL8[4]. Chen et al[5] found that it can inhibit the activity of lipoprotein lipase (LPL) and lead to increase of plasma triglyceride (TG) levels.
One of the other ANGPTL family members, ANGPTL3, has a dose-dependent inhibitory effect on the metabolism and transport function of LPL[6]. Because LPL can decompose TG, promote the transfer of cholesterol, phospholipids, and apolipoproteins between lipoproteins, and increase the binding and uptake of chylomicron (CM) residues to LPL receptors, the inhibition of LPL activity will lead to the increase of TG and CM levels, which can promote the development of arteriosclerosis[7]. However, there is still a dispute about the interaction mechanism between betatrophin, ANGPTL3 and LPL. Based on the differences in previous studies, it is necessary to conduct clinical research on CHD patients to explore their association. This study investigated Chinese Kazakh CHD patients. Exploration of the relationship between the betatrophin/ANGPT L3/LPL pathway and the severity of coronary artery disease (CAD) may provide new clinical research evidence for the regulation of lipids in CHD patients.
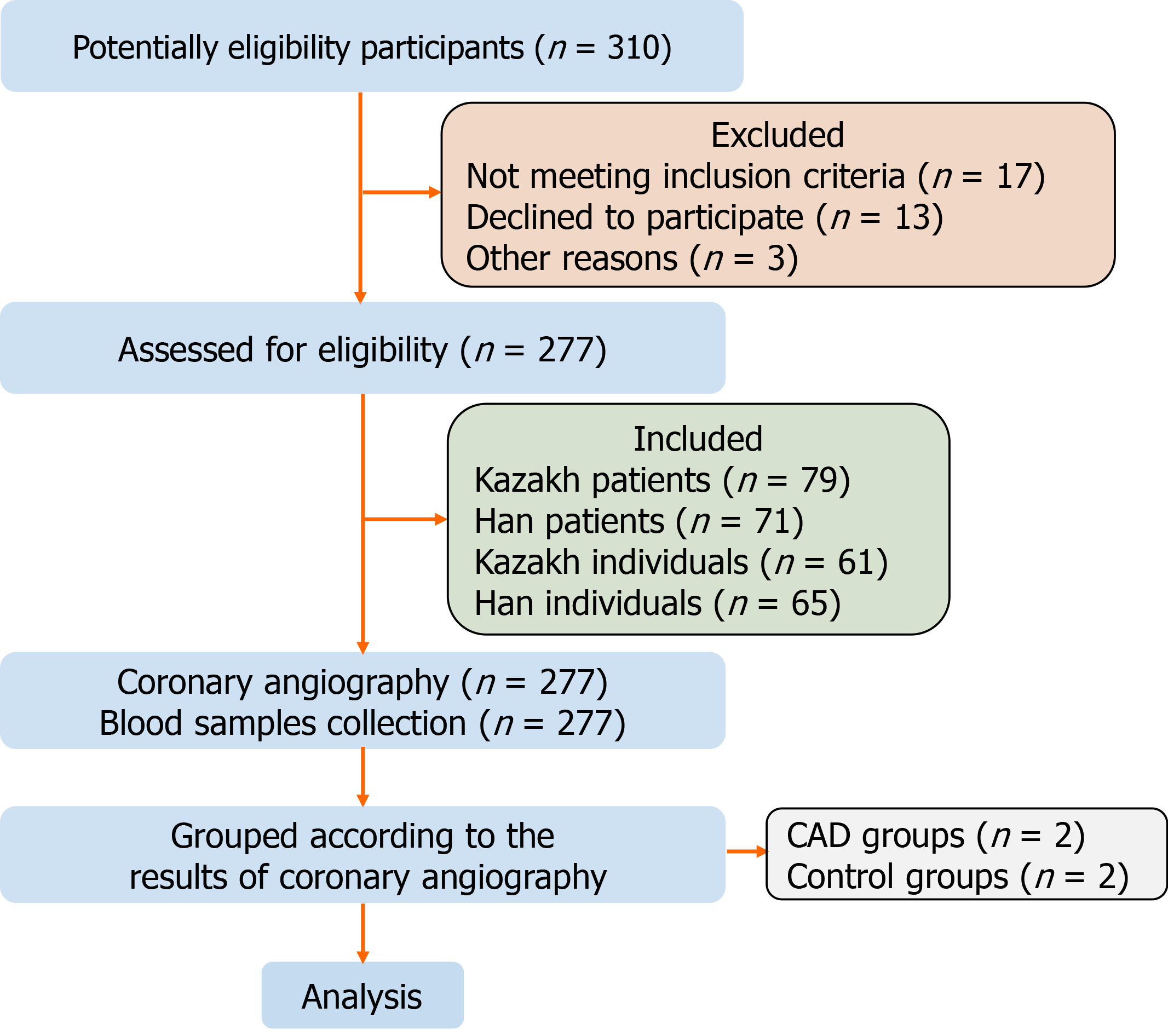
This case–control study involved 277 individuals in the First Affiliated Hospital of Shihezi University School of Medicine. From September 2017 to October 2020, 79 Kazakh and 72 Han patients with CHD confirmed by coronary angiography (CAG) were randomly included as the case groups. Sixty-one Kazakh and 65 Han individuals with normal CAG results were selected as the control group (Figure 1). The study was completed in the Immunology Laboratory of Shihezi University. The sample size met the design requirements of the case–control study, and the experiment and data analysis of the study adopted the double-blind principle.
Inclusion criteria: The diagnostic criteria for CHD followed the ACC/AHA 2014 guidelines. Moreover, the age range was 35–75 years. Participants completed CAG in the same hospital, and echocardiography (EPIQ 7C; Philips, Netherlands) was used to evaluate the cardiac function of patients. According to the evaluation standards of the New York Heart Association, patients with cardiac function grade I or II were included in the study.
Exclusion criteria: (1) According to the Medical Diagnosis and Treatment Standard of Diabetes formulated by the American Diabetes Association in 2019, patients diagnosed with type 1 or type 2 diabetes; (2) Patients with cardiomyopathy, congenital heart disease, severe valvular disease, pulmonary heart disease with pulmonary hypertension, and heart failure with cardiogenic shock were also excluded; (3) Patients with malignant tumors who were receiving radiotherapy and chemotherapy, patients with new cerebral hemorrhage, and patients who received surgery or thrombolytic therapy for cerebral infarction within 1 year; (4) Liver is the main synthetic organ of betatrophin and ANGPTL3, and liver failure may lead to abnormal synthetic function, so it was necessary to exclude patients diagnosed with liver failure. The diagnostic criteria for liver failure are based on the Guidelines for Diagnosis and Treatment of Liver Diseases in China. In order to prevent the occurrence of contrast medium nephropathy, the renal function of all selected cases was evaluated before CAG, and patients with renal failure were excluded (diagnostic criteria for renal failure: Blood urea nitrogen ≥ 21.42 mmol/L, serum creatinine > 442 mmol/L, and glomerular filtration rate < 5 mL/min); and (5) Patients who had quit smoking < 6 mo before the time of diagnosis, as well as those treated with statins, antiplatelet drugs, and nitrates with a withdrawal time < 2 wk.
CAG was performed by doctors with > 5 years of experience. Based on the double-blind design, the results of CAG were judged by three experienced cardiologists. The Gensini stenosis scoring system was used to evaluate the severity of coronary lesions[8], and the average value of the Gensini score calculated by the three cardiologists was taken as the final score. Mild lesions were defined as Gensini score ≤ 24. Those with a score ≥ 25 but < 53 were defined as moderate lesions, while those with a score ≥ 53 were defined as severe lesions. According to the Gensini scores, patients in the case groups were divided into three subgroups: mild, moderate and severe.
Detection of betatrophin: ELISA was selected to detect the level of betatrophin in serum (hz-EL-H2206c; Huzhen Biotechnology, China). The sensitivity of detection was 4 pg/mL, while the coefficient of variation was < 9%, and the coefficient of variation between batches was < 15%.
Detection of ANGPTL3: Serum ANGPTL3 was determined by the double antibody sandwich ELISA method (ab254510; Abcam, Cambridge, United Kingdom). The optical density (OD) was read by spectrophotometer (UV7; Mettler Todledo, Switzerland), and the results were calculated by the standard curve method.
Detection of LPL: The serum LPL level was also detected by ELISA (SP10920; Saibo Biotechnology, China). After the OD values were read by the spectrophotometer (UV7), the diluted concentration of each standard tube was used as the abscissa, and the measured OD value is used as the ordinate to construct the standard curve.
Blood samples collected from individuals in all groups were centrifuged for 5 min at 3500 rpm and the supernatant (serum) was used for the detection of biochemical indexes. The levels of total cholesterol (TC), TG, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), glucose (GLU), fasting plasma glucose (FPG) and fasting insulin (FINS) in serum samples of each group were determined by automatic biochemical analyzer (AU-2700; Olympus, Japan).
Homeostasis model assessment insulin resistance (HOMA-IR) was used to evaluate insulin resistance level. The function of an individual’s islet β cells was evaluated by HOMA-β. HOMA-IR = (FINS × FPG)/22.5, HOMA-β = 20 × FINS/(FP-3.5).
Data were analyzed by SPSS version 25.0. The data are expressed as mean ± SD, and the statistical differences between the data were compared by independent sample t-test. Differences between groups were analyzed by two-way analysis of variance, multigroup analysis of variance, correlation analysis by Spearman correlation, and risk factor assessment by ordered logistic regression analysis. P < 0.05 indicated that there were significant differences between the groups.
There was no significant difference in age, systolic blood pressure and diastolic blood pressure between Kazak and Han CHD groups and their respective control groups (P = 0.14, 0.24, and 0.15 respectively). Similarly, there was no significant difference in the levels of GLU, fructosamine, HOMA-IR and HOMA-β between Kazak and Han CHD groups and their respective control groups (P = 0.24, P =0.13, P =0.09, and P =0.11 respectively). In the comparison of blood lipid levels, there was no significant difference in HDL-C level between Kazak and Han CHD groups and their respective control groups (P = 0.26). BMI, TC, TG and LDL-C in the two groups were significantly higher than in the control groups (P = 0.03, P = 0.004, P = 0.006, and P =0.02 respectively) (Table 1).
| Variables | Kazakh | Han | ||||
| CHD (n = 79) | Non-CHD (n = 72) | P value | CHD (n = 61) | Non-CHD (n = 65) | P value | |
| Age (yr) | 52.27 ± 12.10 | 53.77 ± 12.24 | 0.16 | 53.78 ± 13.56 | 52.63 ± 11.73 | 0.14 |
| SBP (mmHg) | 135.13 ± 15.46 | 129.24 ± 13.42 | 0.27 | 129.44 ± 15.69 | 125.58 ± 16.81 | 0.24 |
| DBP (mmHg) | 84.85 ± 13.21 | 81.34 ± 12.52 | 0.18 | 81.41 ± 16.39 | 79.26 ± 12.14 | 0.15 |
| BMI | 27.43 ± 2.97 1,3 | 24.76 ± 3.22 | 0.007 | 26.74 ± 2.432 | 24.22 ± 3.37 | 0.03 |
| GLU (mmol/L) | 5.53 ± 0.62 | 54.06 ± 14.16 | 0.26 | 5.16 ± 0.37 | 4.73 ± 0.52 | 0.24 |
| Fructosamine (μmol/L) | 241.82 ± 23.42 | 235.89 ± 18.54 | 0.33 | 246.02 ± 21.42 | 239.71 ± 18.25 | 0.13 |
| HOMA-IR | 0.95 ± 0.11 | 0.93 ± 0.09 | 0.13 | 0.89 ± 0.06 | 0.87 ± 0.07 | 0.09 |
| HOMA-β | 0.81 ± 0.06 | 0.80 ± 0.09 | 0.15 | 0.78 ± 0.14 | 0.79 ± 0.07 | 0.11 |
| TC (mmol/L) | 5.37 ± 0.731,3 | 4.28 ± 0.34 | 0.005 | 5.01 ± 1.092 | 4.12 ± 0.76 | 0.004 |
| TG (mmol/L) | 1.36 ± 0.601,3 | 1.14 ± 0.56 | 0.007 | 1.15 ± 0.652 | 1.08 ± 0.37 | 0.006 |
| LDL-C (mmol/L) | 3.15 ± 0.621,3 | 2.56 ± 0.44 | 0.004 | 2.98 ± 1.022 | 2.87 ± 0.56 | 0.02 |
| HDL-C (mmol/L) | 1.85 ± 0.39 | 1.93 ± 0.2 3 | 0.21 | 1.87 ± 0.38 | 2.01 ± 0.36 | 0.26 |
The levels of betatrophin, ANGPTL3 and LPL in Kazak and Han CHD groups were higher than those in their respective control groups (P < 0.01, P = 0.022, and P = 0.043 respectively). The level of serum betatrophin and ANGPTL3 in the Kazakh CHD group was higher than that in the Han CHD group (P = 0.031, P = 0.038, P = 0.021 respectively) (Table 2).
| Variables | Kazakh | Han | ||||
| CHD (n = 79) | Non-CHD (n = 72) | P value | CHD (n = 61) | Non-CHD (n = 65) | P value | |
| Betatrophin (pg/mL) | 435.32 ± 60.361,2 | 243.21 ± 62.731 | < 0.001 | 408.26 ± 57.452 | 219.73 ± 59.37 | 0.031 |
| ANGPTL3 (ng/mL) | 3.42 ± 1.631,2 | 2.69 ± 1.331 | 0.022 | 3.27 ± 1.452 | 2.52 ± 1.53 | 0.038 |
| LPL (ng/mL) | 56.37 ± 13.271,2 | 42.37 ± 13.161 | 0.043 | 54.52 ± 14.522 | 41.42 ± 12.26 | 0.021 |
In the Kazakh and Han CHD groups, the levels of serum betatrophin, ANGPTL3 and LPL in patients with severe CAD were significantly higher than those in patients with moderate and mild CAD (P < 0.001). The levels of serum betatrophin and ANGPTL3 in the Kazakh CHD group with severe CAD were significantly higher than those in the Han CHD group with severe CAD (P < 0.001). However, there was no significant difference in serum LPL levels in patients with mild CAD (P = 0.16) (Table 3).
| Groups | n | Betatrophi (pg/mL) | ANGPTL3 (ng/mL) | LPL (ng/mL) | |
| Kazakh | Gensini ≤ 24 | 32 | 356.86 ± 58.61 | 2.34 ± 0.57 | 50.22 ± 12.27 |
| 25 ≤ Gensini < 53 | 22 | 380.03 ± 61.56 | 3.56 ± 1.03 | 57.59 ± 10.41 | |
| Gensini ≥ 53 | 18 | 452.74 ± 62.241,2 | 5.94 ± 1.461,2 | 59.35 ± 13.191,2 | |
| Han | Gensini ≤ 24 | 35 | 326.07 ± 50.56 | 2.00 ± 1.32 | 50.16 ± 13.483 |
| 25 ≤ Gensini < 53 | 21 | 369.64 ± 53.44 | 3.33 ± 0.83 | 54.39 ± 12.333 | |
| Gensini ≥ 53 | 9 | 422.39 ± 59.141 | 5.19 ± 1.211 | 57.60 ± 12.351 |
There was a positive correlation between the Gensini score and TC, TG, LDL-C, betatrophin and LPL levels in Kazakh patients in the CHD group (r = 0.204, r = 0.453, r = 0.352, r = 0.471, and r = 0.382 respectively). There was a positive correlation between the Gensini score, BMI, TC, TG, LDL-C, betatrophin and LPL levels in Han patients in the CHD group (r = 0.438, r = 0.195, r = 0.296, r = 0.357, r = 0.446 and r = 0.328). Logistic regression analysis revealed that TG and serum betatrophin were risk factors for coronary atherosclerosis in Kazakh patients, and BMI and serum betatrophin were risk factors for coronary atherosclerosis in Han patients. In the Kazakh CHD group, the Gensini score was taken as the dependent variable, and TC, TG, LDL-C, betatrophin, and LPL as the independent variables in logistic regression analysis. Similarly, the Gensini score was taken as the dependent variable, and BMI, TC, TG, LDL-C, serum betatrophin and LPL as the independent variables in the logistic regression analysis of the Han CHD group (Tables 4 and 5).
| Group | TC (mmol/L) | TG (mmol/L) | LDL-C (mmol/L) | Betatrophin (pg/mL) | LPL (ng/mL) | BMI (ng/mL) | |
| Kazakh | r | 0.204 | 0.453 | 0.352 | 0.471 | 0.382 | 0.097 |
| P value | 0.043 | 0.009 | 0.048 | 0.001 | 0.001 | 0.261 | |
| Han | r | 0.195 | 0.296 | 0.357 | 0.446 | 0.328 | 0.438 |
| P value | 0.026 | 0.003 | 0.20 | 0.001 | 0.004 | 0.044 |
| Variables | B value | P value | Exp (B) | |
| Kazakh | TG | 3.292 | 0.03 | 3.632 |
| Betatrophin | 1.258 | 0.043 | 1.802 | |
| Han | BMI | 5.635 | 0.01 | 2.457 |
| Betatrophin | 1.170 | 0.036 | 1.615 |
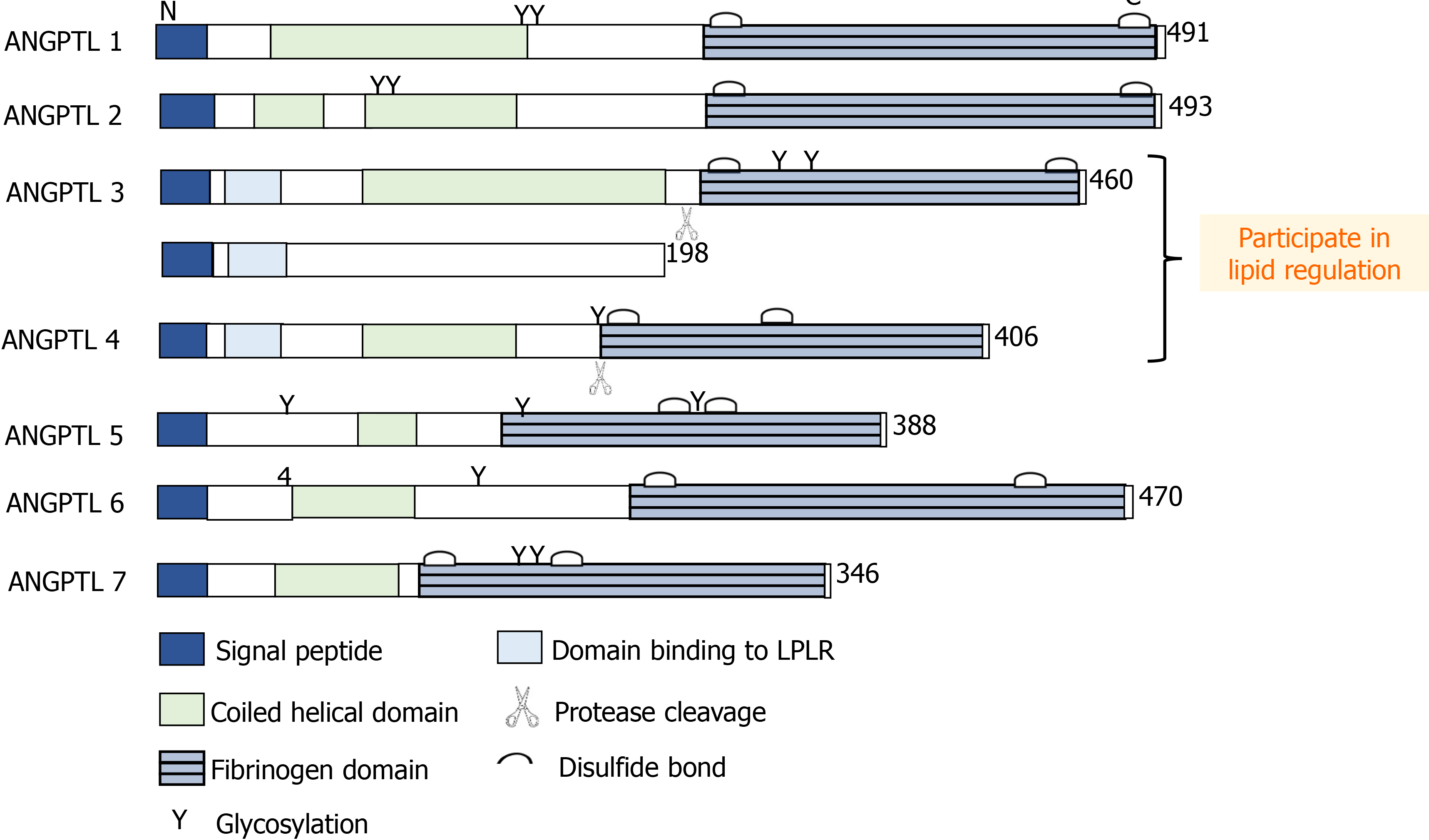
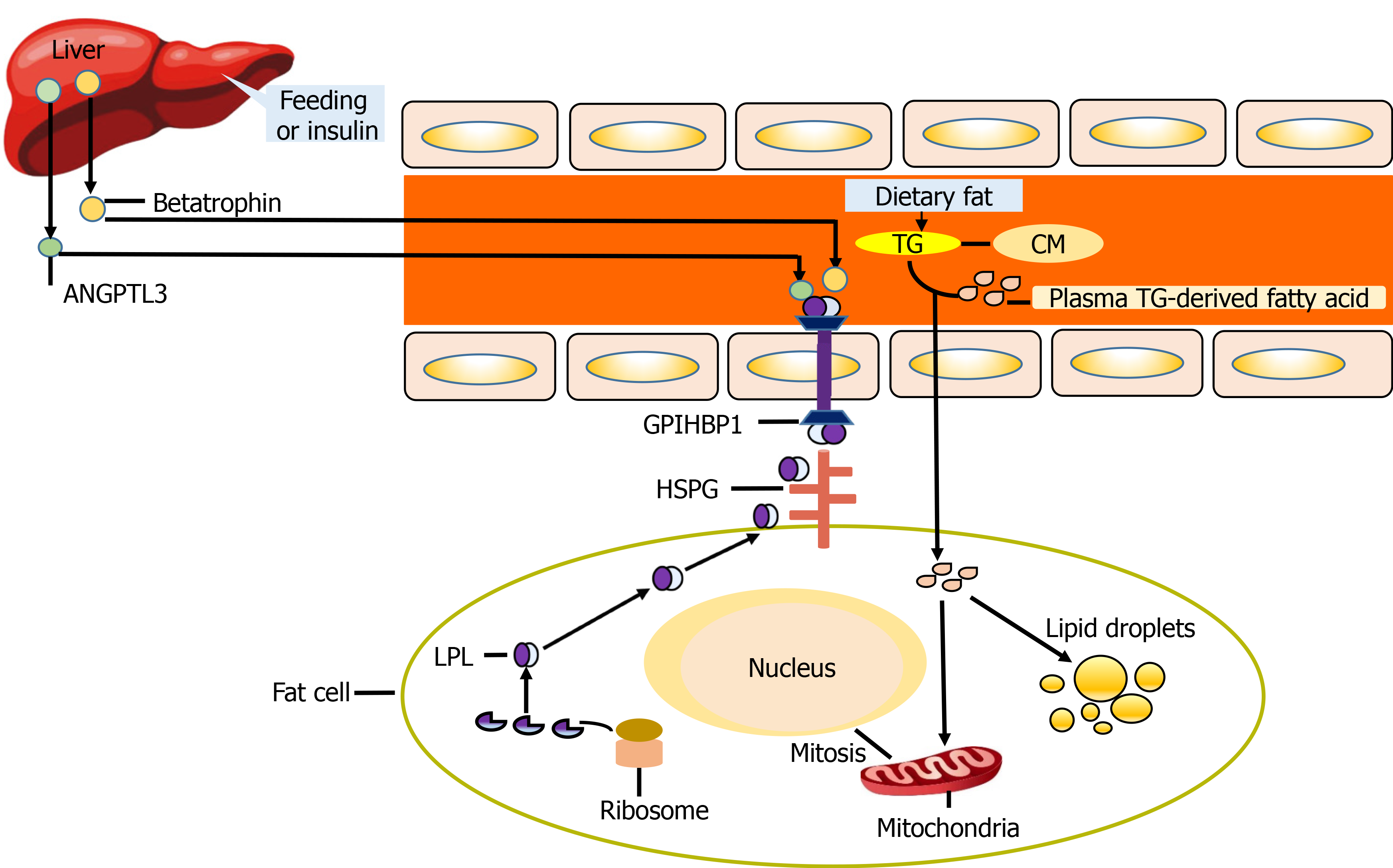
The results of this study suggest that the betatrophin/ANGPTL3/LPL pathway is related to the severity of CHD. In addition, the results showed that the levels of LPL and ANGPTL3 in CHD patients increased, and their increasing trends were consistent. According to previous research, the functional difference of ANGPTL family members lies in the difference between C-terminal and N-terminal domains (Figure 2). Betatrophin was recognized as an atypical new member of the ANGPTL family because of its lack of a fibrinogen-like domain in the C terminus[5]. However, betatrophin is closely related to ANGPTL3, which can promote the cleavage of ANGPTL3 and indirectly reduce the inhibitory effect of ANGTPL3 on LPL[9-11]. Jiao et al[12] used ApoE(-/-) mice to prove that betatrophin is highly expressed during the development of arteriosclerosis. Luo et al[13] believe that betatrophin plays an important role as a regulator in metabolic disorders. Chi et al[14] verified that betatrophin promotes the combination of ANGPTL3 and LPL. The above studies have shown the synergistic function between betatrophin and ANGPTL3 and play a role together in the process of lipid regulation (Figure 3). In previous clinical studies, Leiherer et al[15] included 201 CAD patients for an 8-year follow-up study, and the results showed that elevated levels of betatrophin have predictive value for the occurrence of cardiovascular events. Moreover, the study by Fadaei et al[16] revealed that the higher circulating levels of betatorphin in patients with CHD are related to BMI, TG and endothelial dysfunction. In a previous study based on Chinese CHD patients, Jiao et al[17] also showed that the circulating full-length betatrophin levels in nondiabetic CHD patients were increased, and they could be used as an independent risk factor for CHD. Our findings also showed similar patterns and the levels of betatrophin in the CHD group were higher than in the control group, and we also found that the level of betatrophin was positively correlated with the severity of coronary artery stenosis. However, the difference from previous studies was that the serum levels of LPL and ANGPTL3 showed a consistent increase.
ANGPTL3 has become a new target for the treatment of patients with dyslipidemia. Recent studies have confirmed the safety and effectiveness of using monoclonal antibodies or antisense oligonucleotides to inhibit ANGPTL3, and the goal of lipid regulation has been achieved[18-20]. The current evidence supports that ANGPTL3 inhibits the activity of LPL in a manner dependent on betatrophin activation, and there is consistency between them. The results of our study also showed that the level of ANGPTL3 in Kazakh and Han CHD patients was significantly higher than that in their respective control groups. Moreover, the level of serum in the Kazakh CHD group was higher than that in the Han CHD group. After the severity of CAD was stratified according to the Gensini score, there was no significant difference in serum LPL levels between patients with moderate CAD and the control group, and the same result was found in patients with mild CAD. Based on the above results, we consider that severe coronary lesions may be one of the factors that induce the compensatory increase of betatrophin levels in the circulation. The combination of increased betatrophin and ANGPTL3 indirectly reduces the inhibition of LPL by ANGPTL3, allowing activated LPL to participate in lipid regulation.
Kazakh individuals are mainly descendants of Turks and medieval Mongolians, living in the mountains and pastures of Northern Xinjiang. They have their specific dietary characteristics, such as high-salt and high-fat diet, and less intake of vegetables and fruits. Risk factors related to cardiovascular disease in the Kazakh population include obesity, hypertension and metabolic syndrome[21-23], leading to the high incidence of CHD in this population[24]. The correlation analysis showed that there was a positive correlation between the Gensini score and TC, TG, LDL-C, betatrophin and LPL levels of Kazakh patients with CHD. Logistic regression analysis confirmed that TG and betatrophin were risk factors for CHD in Kazakh patients, and BMI and betatrophin were risk factors in Han patients. As a key enzyme in TG metabolism[25,26], LPL can hydrolyze TG carried by CM and very low density lipoprotein into glycerol and fatty acids. When the activity of LPL is inhibited by ANGPTL3, it may lead to accumulation of TG. However, the results of this study showed that the levels of LPL in Kazakh and Han CHD patients were significantly higher than those in their respective control groups, and there was no significant decrease. According to our analysis, the reasons for the above results may be as follows. At a certain point in time, the level of LPL does not represent the characteristics of change over time, and the level of LPL may show a downward trend with disease progression. The increase in lipid level stimulates the liver to synthesize betatrophin, and the increase of betatrophin promotes cleavage of ANGPTL3, which leads to a decrease in the inhibitory effect of ANGPTL3 on LPL. After the activation of LPL, the increase in its activity and level promote lipid metabolism. Based on the above process, the increase of betatrophin, ANGPTL3 and LPL levels in this pathway may be consistent, but the increased ANGPTL3 may be the product of cleavage. Patients with acute coronary syndrome may induce expression of betatrophin, ANGPTL3 and LPL in the short term due to the combined effects of atherosclerotic plaque rupture, activation of inflammatory pathways, and stress. However, for relatively stable CHD patients, there may be differences with the above results.
Detection of the betatrophin/ANGPTL3/LPL pathway may be used in clinical practice as one of the assessment tools of CAD severity in CHD patients. The detection of this pathway in CHD patients with acute coronary occlusion may have more diagnostic value. Betatrophin can also be used as a target for new lipid regulation treatments to provide clues for the development of new drugs[27]. Regarding the other functions of betatrophin, progress has been made not only in the field of lipid metabolism, but also in the exploration of betatrophin gene polymorphism[28] and inflammation-related pathways[29]. The results of Catalano et al[30] revealed that betatrophin specifically regulates TG-rich lipoproteins through the LPL pathway. Therefore, the expression of betatrophin and its participation in lipid regulation pathways still need more exploration.
There is a positive correlation between the betatrophin/ANGPTL3/LPL pathway and the severity of CAD in Kazakh patients with CHD. This pathway is involved in the lipid regulation of CHD, and betatrophin has the possibility of being used as a diagnostic marker of CHD.
Lipid metabolism plays an essential role in the pathogenesis of atherosclerosis, a major cause for coronary heart disease (CHD). Lipid regulation therapy can reduce major adverse cardiovascular events. Although previous studies have shown that betatrophin, angiogenin-like protein 3 and lipoprotein lipase are jointly involved in lipid regulation, the interaction and mechanism of action between them are still controversial.
The purpose of this study was to explore the correlation between the betatrophin/ angiogenin-likeprotein3 (ANGPTL3) / lipoprotein lipase (LPL) pathway and severity of coronary artery disease in patients with CHD. The detection of this pathway in CHD patients with acute coronary occlusion may have more diagnostic value. Betatrophin can also be used as a target for new lipid regulation treatments to provide clues for the development of new drugs.
The betatrophin/ANGPTL3/LPL pathway is related to the severity of CHD. In addition, the results showed that the levels of LPL and ANGPTL3 in CHD patients increased, and their increasing trends were consistent. The detection of this pathway can be used as one of the non-invasive tools to evaluate the severity of coronary artery disease (CAD) lesions in patients with CHD. Not only that, betatrophin may also serve as a new target for lipid regulation therapy.
This case–control study involved 277 individuals. Nondiabetic patients diagnosed with CHD were selected as the case group; 79 were of Kazakh descent and 72 were of Han descent. The control groups comprised of 61 Kazakh and 65 Han individuals. The serum levels of betatrophin and LPL were detected by enzyme-linked immunosorbent assay (ELISA), and the double antibody sandwich ELISA was used to detect serum level of ANGPTL3. The data are expressed as average ± standard deviation, and the statistical differences between the data were compared by independent sample t-test. Differences between groups were analyzed by two-way analysis of variance, multigroup analysis of variance, correlation analysis by Spearman correlation, and risk factor assessment by ordered logistic regression analysis.
The betatrophin/ANGPTL3/LPL pathway is positively correlated with the severity of CAD. The levels of serum betatrophin and ANGPTL3 in the Kazakh CHD group with severe CAD were significantly higher than those in the Han CHD group with severe CAD. However, there was no significant difference in serum LPL levels in patients with mild CAD. Logistic regression analysis revealed that TG and serum betatrophin were risk factors for coronary atherosclerosis in Kazakh patients, and BMI and serum betatrophin were risk factors for coronary atherosclerosis in Han patients. Other expression mechanisms of betatrophin in lipid regulation still need to be explored. The inflammatory response and autophagy mediated by betatrophin in atherosclerosis may become a new research direction.
There was a correlation between the betatrophin/ANGPTL3/LPL pathway and severity of CAD in patients with CHD.
The expression of betatrophin in other tissues and its new mechanism of lipid regulation, as well as the inflammatory response and autophagy of atherosclerosis mediated by betatrophin may become new research directions. Not only that, betatrophin can also be used as a target for new lipid regulation treatments to provide clues for the development of new drugs.
We thank all medical staff and technicians of medical centers who agreed to participate in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gaman MA S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Walli-Attaei M, Joseph P, Rosengren A, Chow CK, Rangarajan S, Lear SA, AlHabib KF, Davletov K, Dans A, Lanas F, Yeates K, Poirier P, Teo KK, Bahonar A, Camilo F, Chifamba J, Diaz R, Didkowska JA, Irazola V, Ismail R, Kaur M, Khatib R, Liu X, Mańczuk M, Miranda JJ, Oguz A, Perez-Mayorga M, Szuba A, Tsolekile LP, Prasad Varma R, Yusufali A, Yusuf R, Wei L, Anand SS, Yusuf S. Variations between women and men in risk factors, treatments, cardiovascular disease incidence, and death in 27 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. 2020;396:97-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 221] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 2. | Lawler PR, Kotrri G, Koh M, Goodman SG, Farkouh ME, Lee DS, Austin PC, Udell JA, Ko DT. Real-world risk of cardiovascular outcomes associated with hypertriglyceridaemia among individuals with atherosclerotic cardiovascular disease and potential eligibility for emerging therapies. Eur Heart J. 2020;41:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 3. | Duran EK, Aday AW, Cook NR, Buring JE, Ridker PM, Pradhan AD. Triglyceride-Rich Lipoprotein Cholesterol, Small Dense LDL Cholesterol, and Incident Cardiovascular Disease. J Am Coll Cardiol. 2020;75:2122-2135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 207] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 4. | Yi P, Park JS, Melton DA. Betatrophin: a hormone that controls pancreatic β cell proliferation. Cell. 2013;153:747-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 358] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 5. | Chen YQ, Pottanat TG, Siegel RW, Ehsani M, Qian YW, Zhen EY, Regmi A, Roell WC, Guo H, Luo MJ, Gimeno RE, Van't Hooft F, Konrad RJ. Angiopoietin-like protein 8 differentially regulates ANGPTL3 and ANGPTL4 during postprandial partitioning of fatty acids. J Lipid Res. 2020;61:1203-1220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 6. | Graham MJ, Lee RG, Brandt TA, Tai LJ, Fu W, Peralta R, Yu R, Hurh E, Paz E, McEvoy BW, Baker BF, Pham NC, Digenio A, Hughes SG, Geary RS, Witztum JL, Crooke RM, Tsimikas S. Cardiovascular and Metabolic Effects of ANGPTL3 Antisense Oligonucleotides. N Engl J Med. 2017;377:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 475] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 7. | Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H, Watts GF, Borén J, Fazio S, Horton JD, Masana L, Nicholls SJ, Nordestgaard BG, van de Sluis B, Taskinen MR, Tokgözoglu L, Landmesser U, Laufs U, Wiklund O, Stock JK, Chapman MJ, Catapano AL. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459-2472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1507] [Cited by in RCA: 2444] [Article Influence: 349.1] [Reference Citation Analysis (0)] |
| 8. | Rampidis GP, Benetos G, Benz DC, Giannopoulos AA, Buechel RR. A guide for Gensini Score calculation. Atherosclerosis. 2019;287:181-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 182] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 9. | Kovrov O, Kristensen KK, Larsson E, Ploug M, Olivecrona G. On the mechanism of angiopoietin-like protein 8 for control of lipoprotein lipase activity. J Lipid Res. 2019;60:783-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 10. | Jin N, Matter WF, Michael LF, Qian Y, Gheyi T, Cano L, Perez C, Lafuente C, Broughton HB, Espada A. The Angiopoietin-Like Protein 3 and 8 Complex Interacts with Lipoprotein Lipase and Induces LPL Cleavage. ACS Chem Biol. 2021;16:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Zhang R, Zhang K. An updated ANGPTL3-4-8 model as a mechanism of triglyceride partitioning between fat and oxidative tissues. Prog Lipid Res. 2021;85:101140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 12. | Jiao X, Yang Y, Li L, Yu H, Li J, Du Y, Zhang J, Hu C, Qin Y. Angiopoietin-like protein 8 accelerates atherosclerosis in ApoE-/- mice. Atherosclerosis. 2020;307:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Luo M, Peng D. ANGPTL8: An Important Regulator in Metabolic Disorders. Front Endocrinol (Lausanne). 2018;9:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Chi X, Britt EC, Shows HW, Hjelmaas AJ, Shetty SK, Cushing EM, Li W, Dou A, Zhang R, Davies BSJ. ANGPTL8 promotes the ability of ANGPTL3 to bind and inhibit lipoprotein lipase. Mol Metab. 2017;6:1137-1149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 15. | Leiherer A, Ebner J, Muendlein A, Brandtner EM, Zach C, Geiger K, Fraunberger P, Drexel H. High betatrophin in coronary patients protects from cardiovascular events. Atherosclerosis. 2020;293:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Fadaei R, Shateri H, DiStefano JK, Moradi N, Mohammadi M, Emami F, Aghajani H, Ziamajidi N. Higher circulating levels of ANGPTL8 are associated with body mass index, triglycerides, and endothelial dysfunction in patients with coronary artery disease. Mol Cell Biochem. 2020;469:29-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Jiao X, He J, Yang Y, Yang S, Li J, Qin Y. Associations between circulating full-length angiopoietin-like protein 8 levels and severity of coronary artery disease in Chinese non-diabetic patients: a case-control study. Cardiovasc Diabetol. 2018;17:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Ruscica M, Zimetti F, Adorni MP, Sirtori CR, Lupo MG, Ferri N. Pharmacological aspects of ANGPTL3 and ANGPTL4 inhibitors: New therapeutic approaches for the treatment of atherogenic dyslipidemia. Pharmacol Res. 2020;153:104653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 19. | Dewey FE, Gusarova V, Dunbar RL, O'Dushlaine C, Schurmann C, Gottesman O, McCarthy S, Van Hout CV, Bruse S, Dansky HM, Leader JB, Murray MF, Ritchie MD, Kirchner HL, Habegger L, Lopez A, Penn J, Zhao A, Shao W, Stahl N, Murphy AJ, Hamon S, Bouzelmat A, Zhang R, Shumel B, Pordy R, Gipe D, Herman GA, Sheu WHH, Lee IT, Liang KW, Guo X, Rotter JI, Chen YI, Kraus WE, Shah SH, Damrauer S, Small A, Rader DJ, Wulff AB, Nordestgaard BG, Tybjærg-Hansen A, van den Hoek AM, Princen HMG, Ledbetter DH, Carey DJ, Overton JD, Reid JG, Sasiela WJ, Banerjee P, Shuldiner AR, Borecki IB, Teslovich TM, Yancopoulos GD, Mellis SJ, Gromada J, Baras A. Genetic and Pharmacologic Inactivation of ANGPTL3 and Cardiovascular Disease. N Engl J Med. 2017;377:211-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 645] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 20. | Kersten S. ANGPTL3 as therapeutic target. Curr Opin Lipidol. 2021;335-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 21. | Liu F, Du GL, Song N, Ma YT, Li XM, Gao XM, Yang YN. Hyperuricemia and its association with adiposity and dyslipidemia in Northwest China: results from cardiovascular risk survey in Xinjiang (CRS 2008-2012). Lipids Health Dis. 2020;19:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 22. | Zhang J, Zhou X, Xing Q, Li Y, Zhang L, Zhou Q, Lu Y, Zhai M, Bao J, Tang B. Sudden cardiac death in the Kazakh and Han peoples of Xinjiang, China: A comparative cross-sectional study. Medicine (Baltimore). 2019;98:e18126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Zhang XH, Zhang M, He J, Yan YZ, Ma JL, Wang K, Ma RL, Guo H, Mu LT, Ding YS, Zhang JY, Liu JM, Li SG, Niu Q, Rui DS, Guo SX. Comparison of Anthropometric and Atherogenic Indices as Screening Tools of Metabolic Syndrome in the Kazakh Adult Population in Xinjiang. Int J Environ Res Public Health. 2016;13:428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Mao L, Zhang X, Hu Y, Wang X, Song Y, He J, Yang W, Ma J, Yan Y, Mu L, Zhang J, Wang K, Guo H, Ma R, Guo S. Nomogram Based on Cytokines for Cardiovascular Diseases in Xinjiang Kazakhs. Mediators Inflamm. 2019;2019:4756295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Kersten S. New insights into angiopoietin-like proteins in lipid metabolism and cardiovascular disease risk. Curr Opin Lipidol. 2019;30:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 26. | Abu-Farha M, Ghosh A, Al-Khairi I, Madiraju SRM, Abubaker J, Prentki M. The multi-faces of Angptl8 in health and disease: Novel functions beyond lipoprotein lipase modulation. Prog Lipid Res. 2020;80:101067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 27. | Morelli MB, Chavez C, Santulli G. Angiopoietin-like proteins as therapeutic targets for cardiovascular disease: focus on lipid disorders. Expert Opin Ther Targets. 2020;24:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 28. | Alenad A, Alenezi MM, Alokail MS, Wani K, Mohammed AK, Alnaami AM, Sulimani M, Zargar S, Clerici M, Al-Daghri NM. Association of ANGPTL8 (Betatrophin) Gene Variants with Components of Metabolic Syndrome in Arab Adults. Sci Rep. 2020;10:6764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Zhang Y, Guo X, Yan W, Chen Y, Ke M, Cheng C, Zhu X, Xue W, Zhou Q, Zheng L, Wang S, Wu B, Liu X, Ma L, Huang L, Huang K. ANGPTL8 negatively regulates NF-κB activation by facilitating selective autophagic degradation of IKKγ. Nat Commun. 2017;8:2164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 30. | Catalano-Iniesta L, Sánchez Robledo V, Iglesias-Osma MC, Galán Albiñana A, Carrero S, Blanco EJ, Carretero-Hernández M, Carretero J, García-Barrado MJ. Evidences for Expression and Location of ANGPTL8 in Human Adipose Tissue. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |