Published online Feb 26, 2022. doi: 10.12998/wjcc.v10.i6.2023
Peer-review started: September 4, 2021
First decision: November 7, 2021
Revised: November 16, 2021
Accepted: January 14, 2022
Article in press: January 14, 2022
Published online: February 26, 2022
Processing time: 172 Days and 6.4 Hours
A congenital intrahepatic portosystemic shunt (IPSVS) is a rare vascular abnormality that is characterized by an anomalous intrahepatic venous tract that connects the intrahepatic portal vein with the hepatic venous system. Hepatic encephalopathy is an indication for IPSVS embolization, which is technically challenging because rapid blood flow through shunts can induce the migration of embolization material to systemic veins. This case report discusses the efficacy of percutaneous balloon-occluded retrograde transvenous obliteration for treating patients with IPSVSs.
A 75-year-old woman presented with a six-month history of repeated hepatic encephalopathy due to an IPSVS without liver cirrhosis. We successfully embolized the IPSVS using percutaneous balloon-occluded retrograde trans
Balloon-occluded retrograde transvenous obliteration with detachable coils can be effective for the endovascular treatment of an IPSVS.
Core Tip: Portosystemic venous shunts are generally formed in patients with hepatic fibrosis and cirrhosis due to portal hypertension. Adult cases of congenital intrahepatic portosystemic shunt (IPSVS) are extremely rare. Hepatic encephalopathy is an indication for IPSVS embolization. Minimally invasive treatments that use interventional techniques, such as transcatheter embolization, are being utilized increasingly recently; however, shunt embolization is technically challenging because rapid blood flow through shunts can induce the migration of embolization material to systemic veins. Balloon-occluded retrograde transvenous obliteration with detachable coils can be effective for the endovascular treatment of an IPSVS.
- Citation: Saito H, Murata S, Sugihara F, Ueda T, Yasui D, Miki I, Hayashi H, Kumita SI. Successful embolization of an intrahepatic portosystemic shunt using balloon-occluded retrograde transvenous obliteration: A case report. World J Clin Cases 2022; 10(6): 2023-2029
- URL: https://www.wjgnet.com/2307-8960/full/v10/i6/2023.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i6.2023
An intrahepatic portosystemic shunt (IPSVS) is a rare vascular abnormality that is characterized by an anomalous intrahepatic venous tract that connects the intrahepatic portal vein with the hepatic venous system. An IPSVS was first reported by Raski et al[1] in 1964. Since then, reports of these abnormalities have increased with the development of imaging modalities[2-4]. An IPSVS connects the portal and systemic venous circulation; it has a diameter of > 1 mm, and at least some part of it is located inside the liver. This condition may be congenital or acquired secondary to portal hypertension[1,5,6]. Treatment is required when an IPSVS causes hepatic encephalopathy[3]. Recently, transcatheter embolization using balloon-occluded retrograde transvenous obliteration (B-RTO) has been used to treat symptomatic IPSVSs. B-RTO is a well-known method for treating gastric varices or hepatic encephalopathy in patients with hepatic fibrosis and cirrhosis. A mixture of 5% ethanolamine oleate (Oldamin; Takeda Pharmaceutical, Osaka, Japan) and iopamidol (Iopamidol; Schering, Osaka, Japan) (5% EOI), is usually injected as a liquid embolic agent to treat gastric varices. However, in cases of IPSVSs, it is difficult to prevent migration of the 5% EOI because the shunt length is short, and blood flow through the shunt is rapid, even when the flow is controlled with a balloon catheter. Herein, we present a case of a symptomatic IPSVS that was embolized using B-RTO with detachable coils.
The patient was a 75-year-old unemployed Asian woman who was 170 cm tall and weighed 50 kg. She presented with a six-month history of repeated episodes of unconsciousness.
The patient consulted a doctor because of a loss of consciousness. Hepatic encephalopathy due to IPSVS was diagnosed because laboratory tests and ultrasound showed elevated ammonia levels and IPSVS, respectively. Since hepatic encephalopathy did not improve with drug treatment, embolization was performed.
The patient had no history of liver disease or trauma and was receiving treatment for pulmonary stenosis. She had a past medical history of hypertension, breast cancer surgery, and thyroid cancer surgery, and she was taking Bisoprolol (2.5 mg), Olmesartan Medoxomil (20 mg), Azelnidipine (16 mg), and Eszopiclon (2 mg). She did not have any known allergies.
The patient’s personal and family history was not significant.
The patient demonstrated flapping tremors. However, there was no hepatomegaly, splenomegaly, abdominal tenderness, edema, or ascites present.
Laboratory tests revealed an abnormally high ammonia level (214 μg/dL; normal range, 27–73 μg/dL). Other laboratory test results were as follows: White blood cell count, 41 × 102/μL; red blood cell count, 442 × 104/μL; hemoglobin level, 14.0 g/dL; hematocrit level, 41.9%; platelet count, 18.5 × 104/μL; C-reactive protein, 0.09 mg/dL; aspartate aminotransferase level, 45 U/L; alanine aminotransferase level, 43 U/L; total bilirubin level, 0.8 mg/dL; direct bilirubin level, 0.3 mg/dL; total protein level, 6.5 g/dL; albumin level, 3.5 g/dL; glutamyl transferase level, 36 U/L; prothrombin time, 13.1 s (86.3% of normal); international normalized ratio, 1.14; creatinine level, 0.65 mg/dL; and blood urea nitrogen level, 16.7 mg/dL. The patient tested negative for serum HBs Ag, anti-HBc, and anti-HCV antibodies in a viral screening.
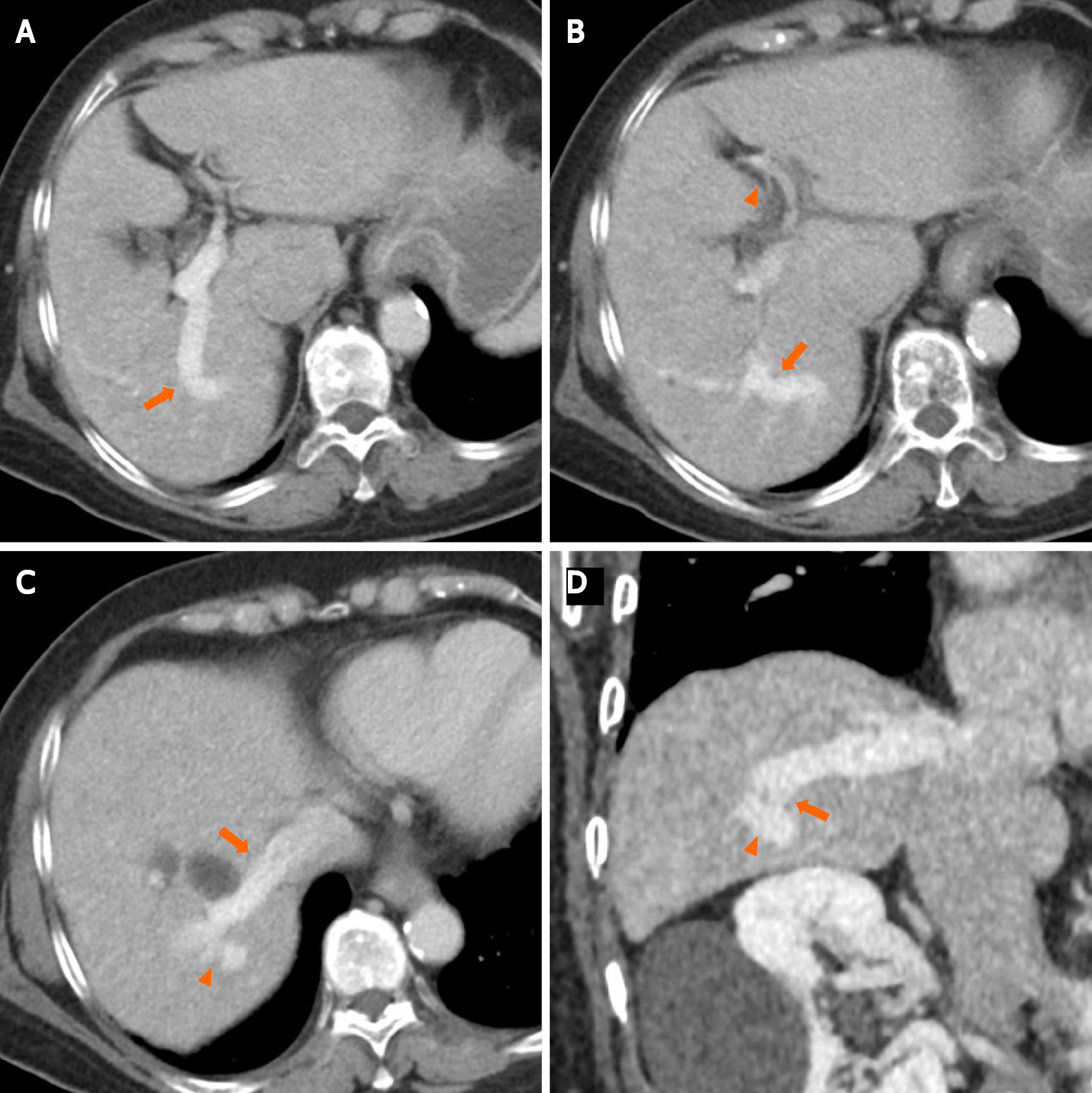
Contrast-enhanced computed tomography (CECT) revealed multiple anomalous vessels that were communicating with the dilated right portal and hepatic veins (Figure 1). The shunt measured 14 mm in diameter, and the left portal vein was narrow due to blood flow steal. The morphology and CT attenuation value of the liver were normal, and no cystic formation was observed in the liver.
The patient was diagnosed with hepatic encephalopathy secondary to an IPSVS in the right lobe of the liver; therefore, we performed an IPSVS embolization.
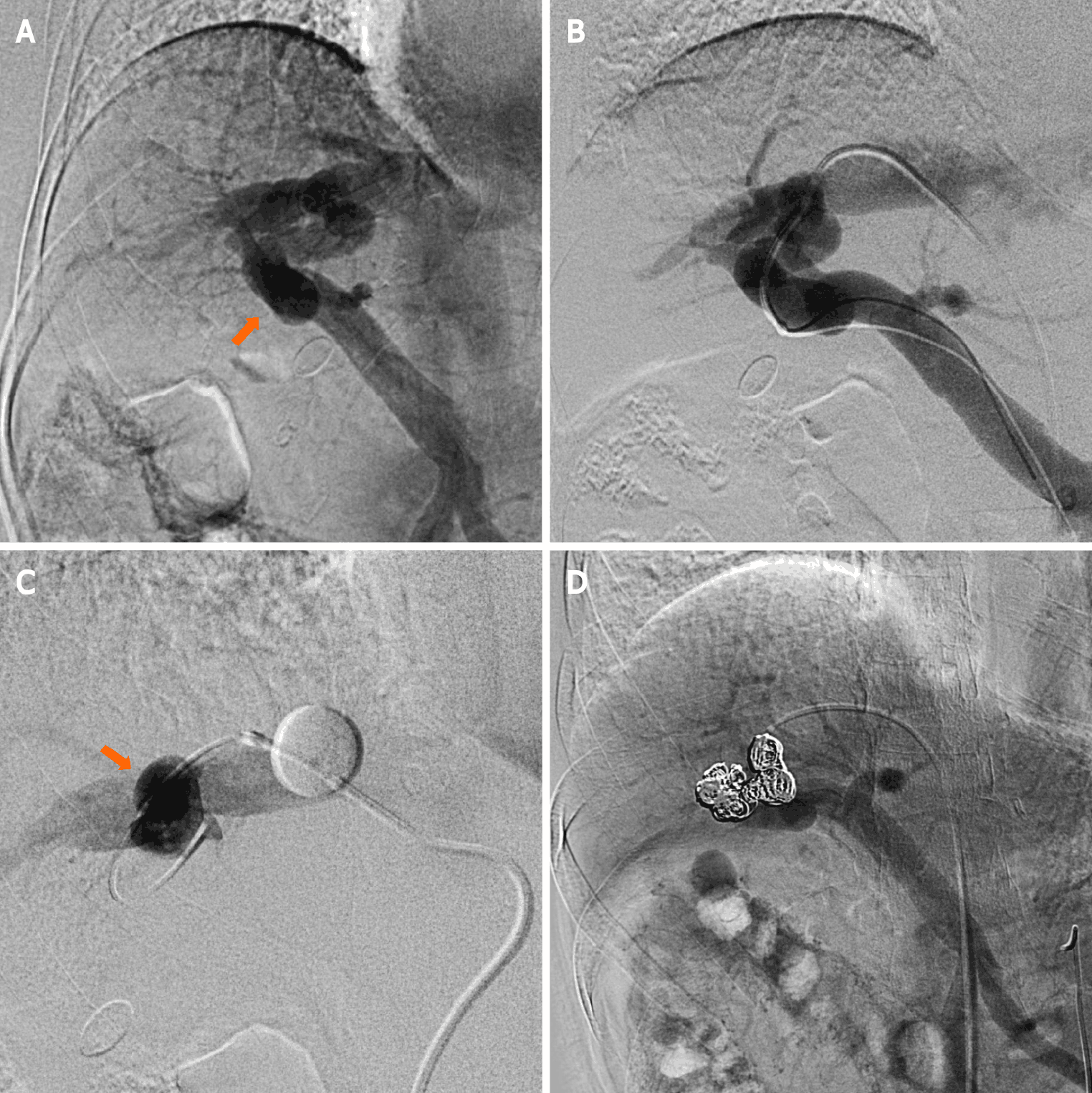
The patient provided consent for the publication of this case report and any additional related information. Subsequently, the following procedures were performed under local anesthesia: We inserted a 4-Fr shepherd-hook catheter (Terumo Clinical Supply, Tokyo, Japan) into the right femoral artery. Next, we performed superior mesenteric arterial portography, and the presence of an IPSVS between the right portal and hepatic veins was confirmed (Figure 2A). We inserted an 8-Fr sheath introducer (Medikit, Tokyo, Japan) into the right femoral vein to facilitate the insertion of a 6-Fr shepherd-hook catheter with a 20-mm-diameter balloon (Terumo Clinical Supply, Tokyo, Japan). Further, an 8-Fr sheath introducer (Medikit, Tokyo, Japan) was inserted into the right femoral vein to facilitate the insertion of a 6-Fr shepherd-hook catheter with a 20-mm-diameter balloon (Terumo Clinical Supply, Tokyo, Japan) into the right hepatic vein. Subsequently, coaxial passage of a 0.20-inch microcatheter (Masters Parkway; ASAHI INTECC J-sales, Inc., Tokyo, Japan) through the IPSVS into the portal vein was performed. Three IPSVSs were observed following the injection of contrast media via the microcatheter in the portal vein (Figure 2B). The pre-embolization pressures of both the portal and right hepatic veins were 230 mm H2O. The balloon catheter was inflated to decrease the hepatofugal blood flow in the IPSVS and avoid coil migration into the systemic venous circulation (Figure 2C). Re-injection of contrast media via the microcatheter in the portal vein showed hepatofugal blood flow stasis in the IPSVS. The microcatheter in the IPSVS was replaced, and all three IPSVSs were embolized with ten interlocking detachable coils (Boston Scientific, Watertown, MA, United States; interlock diameters: 10–12 mm, 6–8 mm, and 4–6 mm; length: 30 cm, 20 cm, and 15 cm).
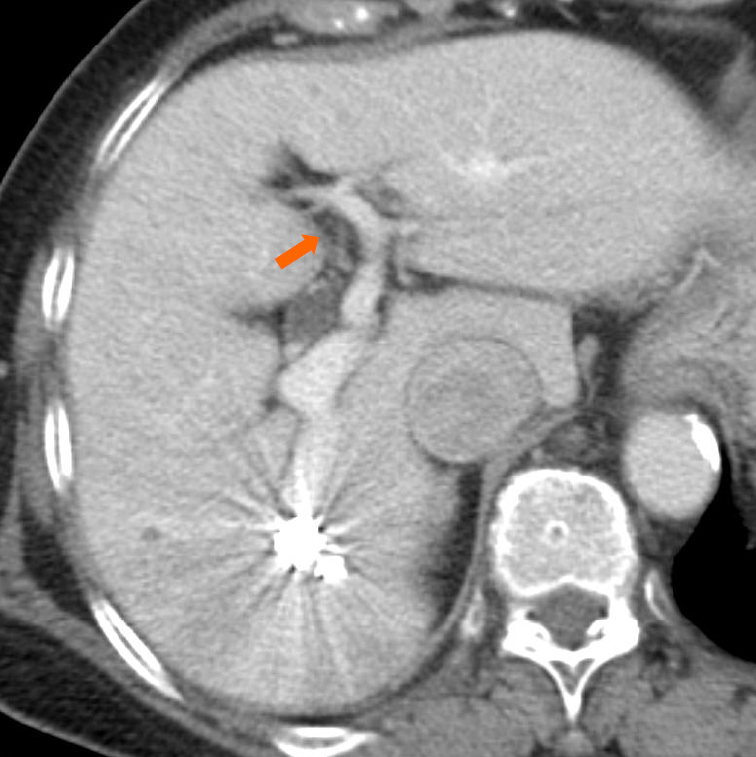
Superior mesenteric arterial portography was performed after balloon deflation, and it revealed sufficient IPSVS embolization (Figure 2D). The patient’s liver function was normal, and the right hepatic venous pressure decreased to 155 mm H2O after the embolization. There were no procedural complications. The serum ammonia level normalized to 55 μg/dL on the first post-intervention day, and a CECT scan that was performed one week after the procedure revealed sufficient embolization of the IPSVS and expansion of the left portal vein (Figure 3). Currently (14 months post-intervention), the patient has no hepatic encephalopathy symptoms, and her clinical condition is good.
Portal-systemic venous shunts are formed in patients with hepatic fibrosis and cirrhosis due to portal hypertension[7]. While it is unclear how these shunts are formed in patients without cirrhosis, portal hypertension, trauma, and portal vein aneurysm ruptures are considered possible causes[6]. For patients with no history of these conditions, however, IPSVS a congenital cause is considered. Between the third and fourth months of fetal life, development of the intra- and extrahepatic portal venous systems occurs through selective persistence of the vitelline and umbilical systems[6]. The cause of congenital IPSVSs is supposedly persistence of communication between the cranial and caudal hepatic sinusoids formed by the vitelline and umbilical veins[6]. This communication generally closes during the fetal stage or after birth[6,8]; therefore, adult cases of congenital IPSVSs are extremely rare.
IPSVSs are categorized into four morphologic types as follows[3]: Type I is characterized by a single, large tube with a consistent diameter connecting the right portal vein to the inferior vena cava; type II by a localized peripheral shunt wherein there are communications (single or multiple) between the peripheral branches of the portal and hepatic veins in a hepatic segment; type III by a connection between the peripheral portal and hepatic veins through an aneurysm; and type IV by multiple diffuse communications between the peripheral portal and hepatic veins in both lobes. Our patient had features characteristic of type II IPSVS.
Patients with an IPSVS with hepatic encephalopathy have increased blood flow through the shunt that requires treatment[3]. Traditionally, shunts are surgically occluded[1]; in recent years, however, less invasive treatments that incorporate interventional techniques, including transcatheter embolization, are increasingly being used[6,9].
It is technically challenging to embolize large shunts under rapid blood flow conditions. Therefore, selecting appropriate embolic materials and blood flow control are important for the success of the procedure. When selecting embolic materials, detachable coils, such as the ones used in the present case, and the Amplatazer Vascular PlugTM (AVP) are preferred over pushable coils because detachable coils and AVPs can be advanced or withdrawn freely before being released, resulting in accurate and safe placement. Conversely, pushable coils cannot be withdrawn after the coils have been pushed from the cartridge into the introducer catheter. Consequently, these coils cannot be replaced, which results in an increased risk of migration to systemic veins. In addition, it is more difficult to use liquid embolus material, such as the 5% EOI, which is usually employed for portosystemic shunts in the treatment of cirrhosis, to prevent migration to systemic veins than metallic embolus materials, even when the flow is controlled using a balloon catheter. In this case, we opted to use detachable coils because embolization of large shunts with an AVP requires a large delivery system, and it is sometimes technically challenging. Furthermore, the risk of migration remains despite the use of detachable coils or AVPs. Occlusion of the hepatic vein, which communicates with the IPSVS through inflation of a balloon catheter, can decrease blood flow to the IPSVS and prevent coil migration. Moreover, detachable coils of various lengths and diameters are available, and this may enable their use in the treatment of lesions with large or high-flow shunts[10].
Balloon-occluded retrograde transvenous obliteration with detachable coils can be effective for the endovascular treatment of IPSVSs.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gencdal G, Ridola L S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Raskin NH, Price JB, Fishman RA. Portal-systemic encephalopathy due to congenital intrahepatic shunts. N Engl J Med. 1964;270:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 112] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Itai Y, Kurosaki Y, Saida Y, Niitsu M, Kuramoto K. CT and MRI in detection of intrahepatic portosystemic shunts in patients with liver cirrhosis. J Comput Assist Tomogr. 1994;18:768-773. [PubMed] |
| 3. | Park JH, Cha SH, Han JK, Han MC. Intrahepatic portosystemic venous shunt. AJR Am J Roentgenol. 1990;155:527-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 184] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 4. | Grattagliano A, Rapaccini GL, Camaldo G, Pompili M, Marino P, Mastromatteo AM, Cotroneo AR, Gasbarrini G. Spontaneous intrahepatic portosystemic venous shunt in a patient with cirrhosis: diagnosis by combined color Doppler and pulsed Doppler ultrasonography. Liver. 1997;17:307-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Takayasu K, Moriyama N, Shima Y, Muramatsu Y, Goto H, Yamada T, Makuuchi M, Yamasaki S, Okazaki N. Spontaneous portal-hepatic venous shunt via an intrahepatic portal vein aneurysm. Gastroenterology. 1984;86:945-948. [PubMed] |
| 6. | Tanoue S, Kiyosue H, Komatsu E, Hori Y, Maeda T, Mori H. Symptomatic intrahepatic portosystemic venous shunt: embolization with an alternative approach. AJR Am J Roentgenol. 2003;181:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Watanabe A. Portal-systemic encephalopathy in non-cirrhotic patients: classification of clinical types, diagnosis and treatment. J Gastroenterol Hepatol. 2000;15:969-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 108] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Meyer WW, Lind J. The ductus venosus and the mechanism of its closure. Arch Dis Child. 1966;41:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 72] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Pocha C, Maliakkal B. Spontaneous intrahepatic portal-systemic venous shunt in the adult: case report and review of the literature. Dig Dis Sci. 2004;49:1201-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Reidy JF, Qureshi SA. Interlocking detachable platinum coils, a controlled embolization device: early clinical experience. Cardiovasc Intervent Radiol. 1996;19:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |