Published online Feb 26, 2022. doi: 10.12998/wjcc.v10.i6.2015
Peer-review started: September 2, 2021
First decision: November 19, 2021
Revised: November 29, 2021
Accepted: January 19, 2022
Article in press: January 19, 2022
Published online: February 26, 2022
Processing time: 174 Days and 11.8 Hours
Bone deficiency and soft tissue atrophy in the absence of maxillary lateral incisors are among the most challenging problems for implant clinicians. Autologous bone grafting is the gold standard for bone augmentation, but not without limitations. Platelet-rich fibrin (PRF), a biodegradable autologous biomaterial, has been widely used for bone and soft tissue management. Moreover, titanium plate is an advantageous barrier due to its good space-maintaining ability. However, there is a lack of literature on implant site development using titanium plate and PRF for congenitally missing maxillary lateral incisors.
The patient was a 19-year-old girl with a congenitally missing tooth (#12). She underwent implant placement and simultaneous autologous bone grafting with titanium plate and PRF. At the follow-up visit 15 d post-procedure, the vascularization of soft tissue was visible. There was no swelling or pain after the surgery. Six months postoperatively, bone regeneration was evident. Subsequently, the definitive restoration was placed, and the patient was satisfied with the esthetic outcomes.
Implant site development using titanium plate and PRF for congenitally missing maxillary lateral incisors is a feasible procedure. In this case, the labial bone plate was displaced but remained connected to the base bone, ensuring blood supply. The titanium plate fixed the labial bone plate and maintained the osteogenic space, while the PRF provided growth factors and leukocytes for bone and soft tissue regeneration. Furthermore, the procedure reduced the surgical complexity and adverse reactions, displaying outstanding esthetic outcomes.
Core Tip: The procedure reported in this paper reduced the surgical complexity and adverse reactions, besides displaying outstanding esthetic outcomes by: (1) Displacement of the labial bone plate that remained connected to the base bone, ensuring blood supply; (2) Fixing the labial bone plate and maintaining the osteogenic space with a titanium plate; and (3) Providing growth factors and leukocytes for bone and soft tissue regeneration by leukocyte-platelet rich fibrin.
- Citation: Zhang TS, Mudalal M, Ren SC, Zhou YM. Implant site development using titanium plate and platelet-rich fibrin for congenitally missed maxillary lateral incisors: A case report. World J Clin Cases 2022; 10(6): 2015-2022
- URL: https://www.wjgnet.com/2307-8960/full/v10/i6/2015.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i6.2015
Missing maxillary lateral incisors is a common congenital and developmental anomaly that affects the esthetics due to their position on the denture. Patients are offered several treatment options in such cases, including dental implant treatment, orthodontic space closure, or prosthetic rehabilitation. Dental implant treatment is a popular choice because it offers maximum restoration of tooth function and esthetics. Sufficient quality and quantity of alveolar bone and soft tissue are essential at the implant recipient sites, especially in the esthetic zone for this treatment. Most studies indicated that labial bone and soft tissue thickness should exceed 2 mm to ensure the best outcome and esthetics for implants[1]. Conversely, an extensive bone and soft tissue deficiency with congenitally absent maxillary lateral incisors poses a challenge for dental implant treatment.
Autologous bone grafting is the gold standard for bone augmentation but not without its limitations, such as low blood supply, unpredictable resorption, and donor site morbidity, contributing to research intensification for suitable alternatives[2]. Some studies have reported reconstruction in severe bone deficiency using autologous bone with bone substitute materials in the first procedure to expand the available bone volume and reduce the resorption of autologous bone[3,4]. An adequate blood supply is essential in this procedure, and space creation/maintenance is necessary for bone ingrowth. In addition, primary closure is crucial to ensure uneventful healing[5]. Nevertheless, perfect primary closure may not always occur, especially with the incidence of soft tissue atrophies due to the congenitally missing maxillary lateral incisors. Platelet-rich fibrin (PRF), a biodegradable autologous biomaterial, promotes angiogenesis and bone and soft tissue regeneration and prevents infection since it contains platelets, growth factors, and leukocytes[6,7]. Meanwhile, titanium plate is an advantageous barrier due to its good space-maintaining ability.
In this case report, a procedure was designed to restore a congenitally missing maxilla lateral incisor. First, the labial bone plate was displaced but remained connected to the base bone for bone augmentation using a titanium plate to create/maintain the space. Then, PRF was applied for angiogenesis, bone and soft tissue regeneration, and infection prevention.
A 19-year-old girl visited the Department of Oral Implantology with congenitally absent tooth #12.
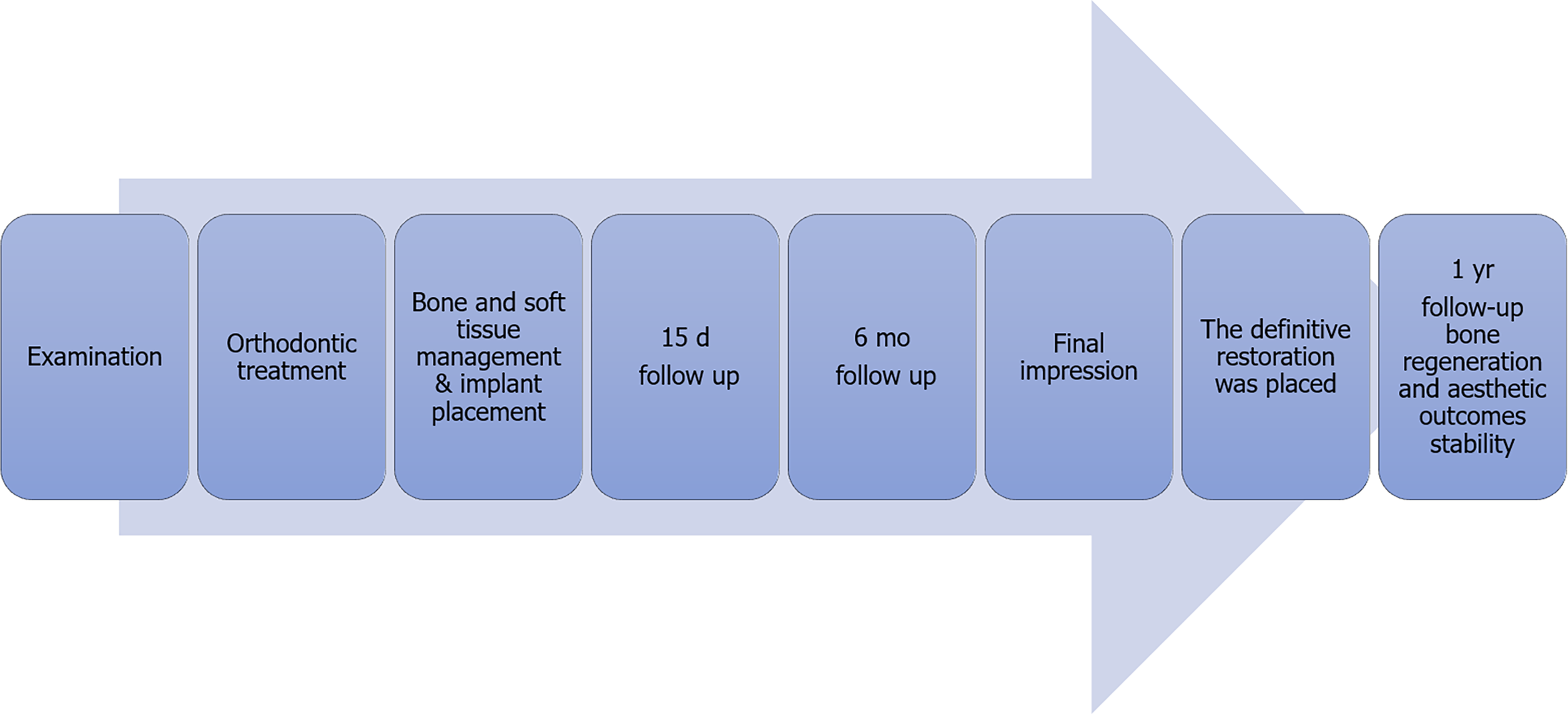
The clinical examination found that the spacing was in the maxillary anterior region, and tooth #12 was missing. In addition, keratinized gingiva atrophy and alveolar crest absorption were observed in the edentulous space. After consultation with orthodontists, an interdisciplinary treatment plan was drawn up (Figure 1).
The patient denied any systemic diseases, and her family history was unremarkable.
Family history was unremarkable.
Physical examination revealed no remarkable findings.
Complete blood count and common urine analysis were performed, which showed no abnormalities.
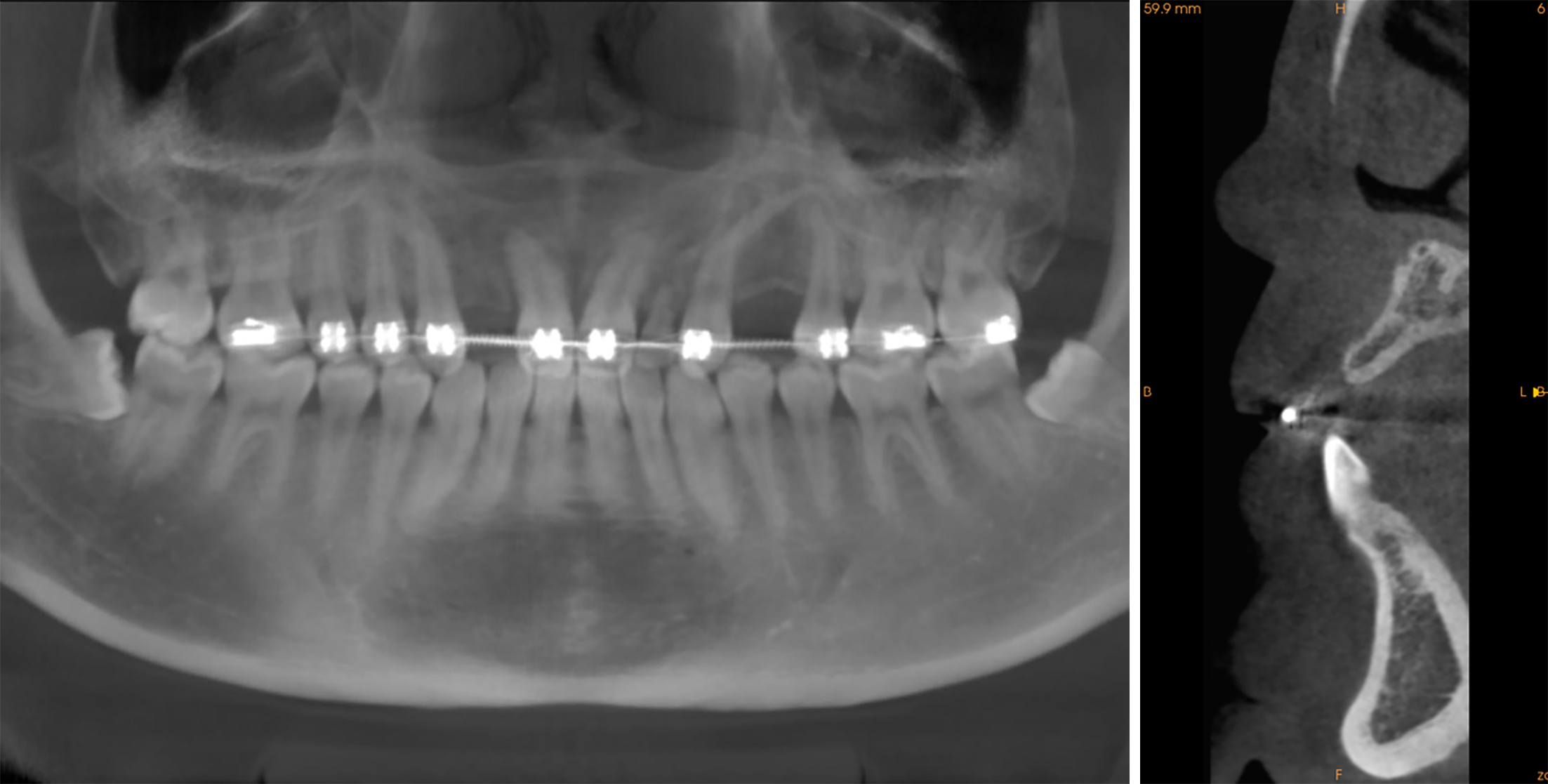
Cone-beam computed tomography (CBCT) showed no residual root and other abnormal conditions, although substantial alveolar bone was lost at the edentulous space (buccal bone thickness = 3.0 mm; alveolar crest height = 12.8 mm) (Figure 2).
Tooth #12 congenital absence.
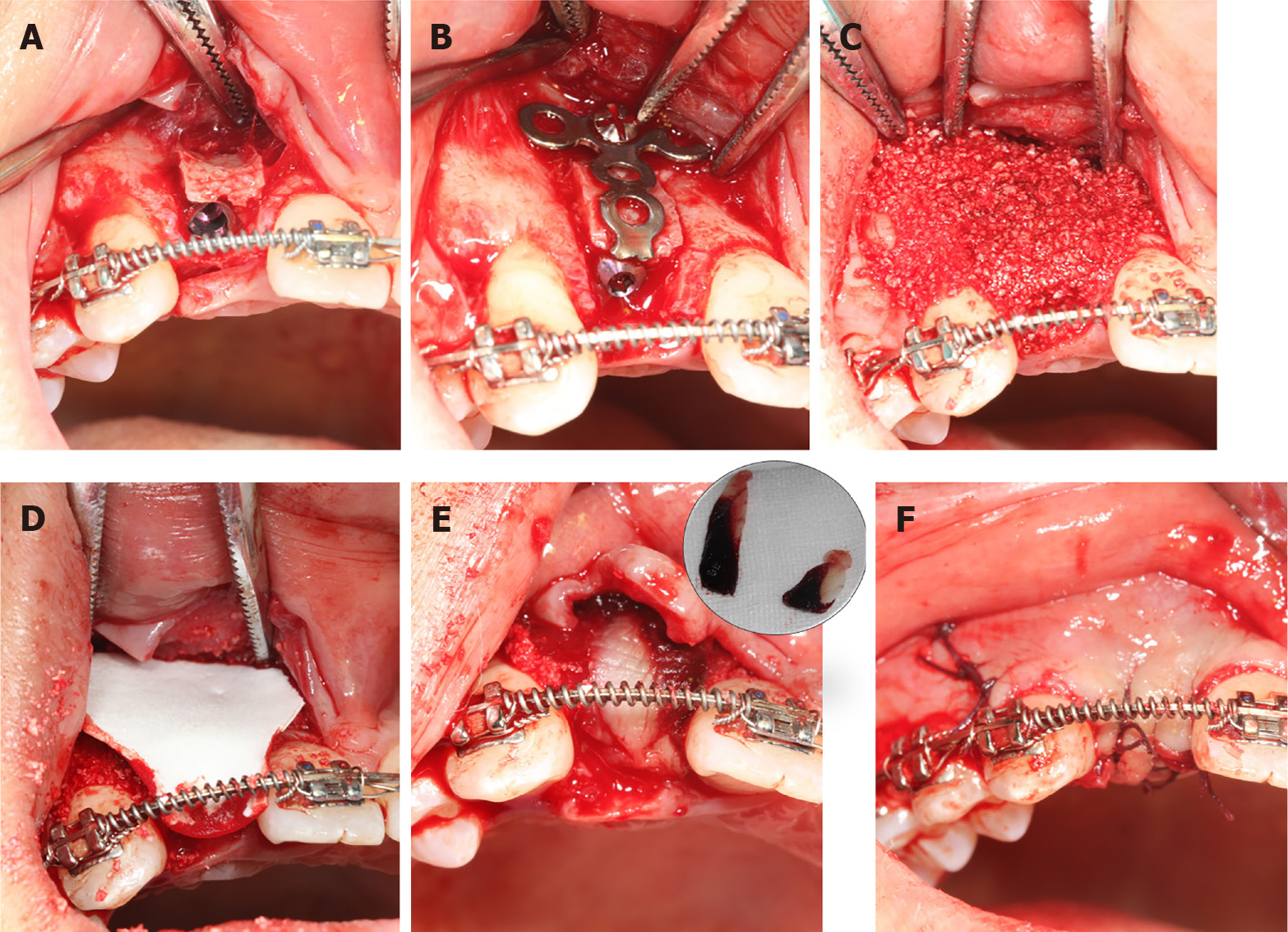
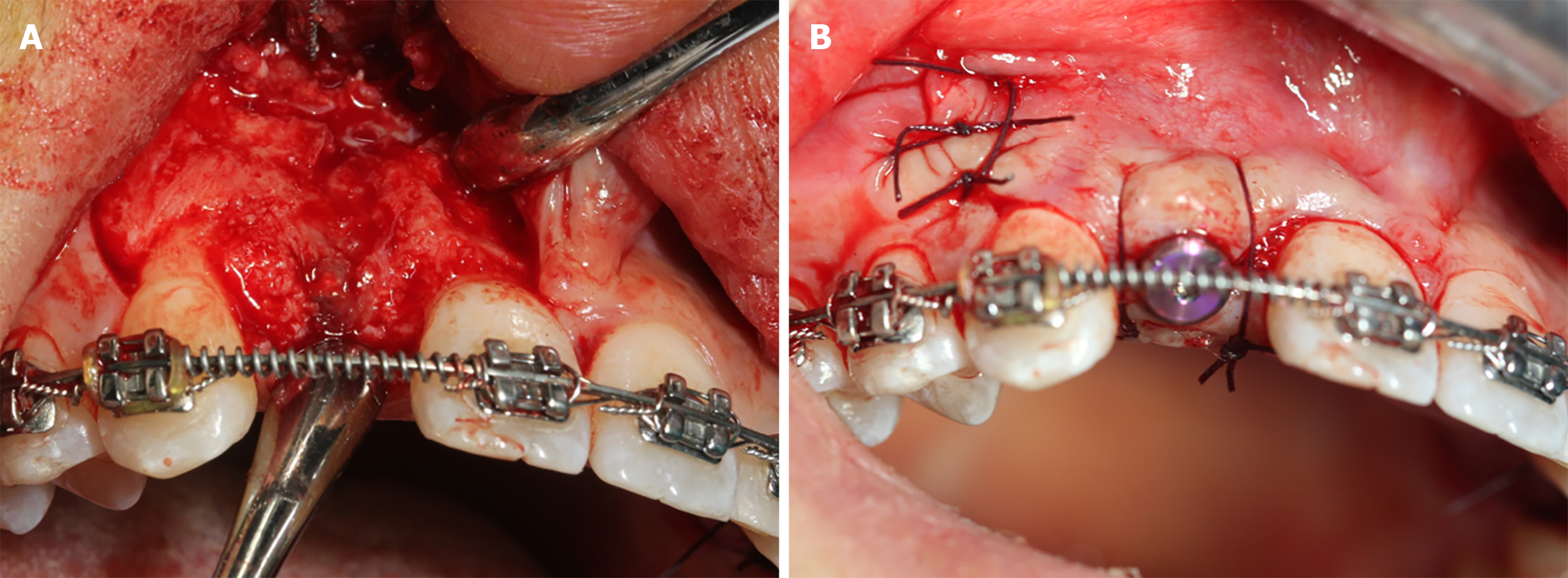
Pre-operatively, the patient rinsed her mouth with 0.12% chlorhexidine solution every 3 min, thrice. Then, two PRF clots were established using the standard protocol (two whole blood samples were collected in two glass-coated 10 mL plastic tubes without anticoagulant and immediately centrifuged at 3000 rpm for 10 min). Subsequently, one PRF clot was mixed with the xenograft bone substitutes, while the other was pressed into the membranes with a sterile dry gauze to cover the bone granulates. Following local anaesthesia, the #12 alveolar ridge crest mucoperiosteum was excised angularly, followed by flap surgery. First, the bone was expanded to form a receptor site for implant using the ridge splitting set (Helmut, Zepf, Germany) without any bone removal. Then, the labial bone plate was displaced carefully, ensuring that its base remained attached. Next, an implant (Nobel replace 3.5 mm × 13 mm) was inserted at the prepared site (Figure 3A), and the bone block was then fixed with a titanium plate to maintain the bone block (Figure 3B), which was grafted with the PRF and xenograft bone substitute mixture (Bio-oss, 2.5 g, Geistlich) (Figure 3C). Finally, resorbable and PRF membranes were used to double cover the defect site (Figure 3D and E), and the recipient site was loosely sutured (Figure 3F).
For antibiotic therapy, 500 mg azithromycin was prescribed twice daily for 5 d. Additionally, the patient was instructed to avoid chewing in the surgical area and continue using mouthwash with chlorhexidine 0.12% for 10 d. The sutures were removed after 15 d.
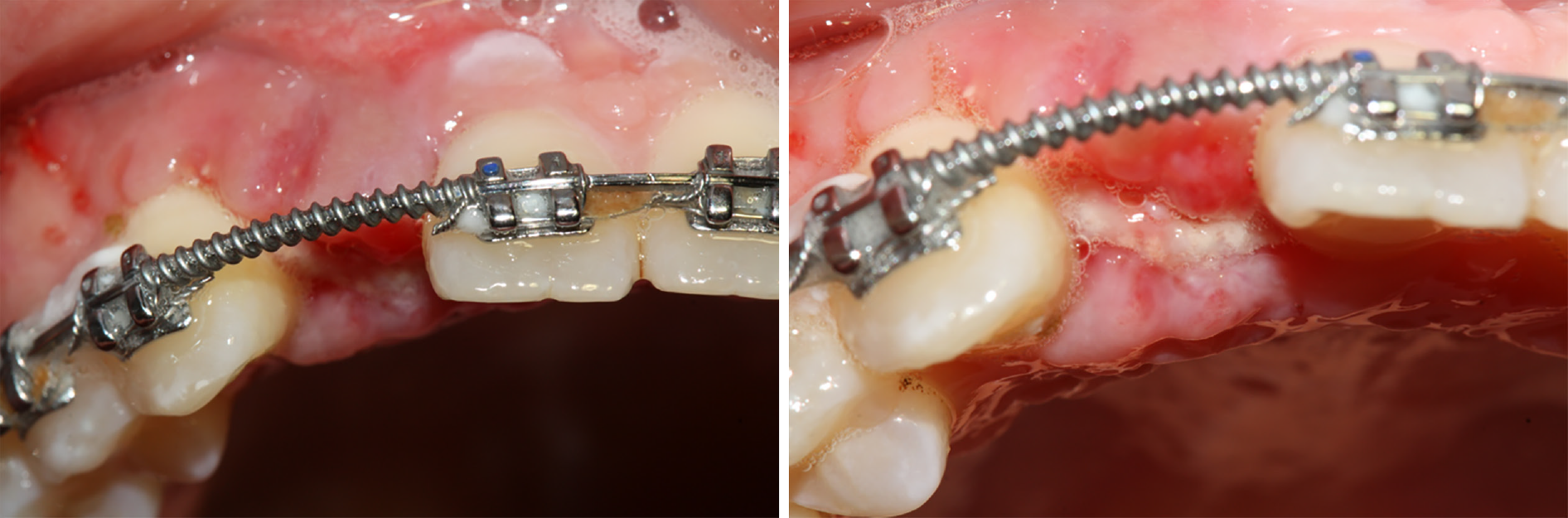
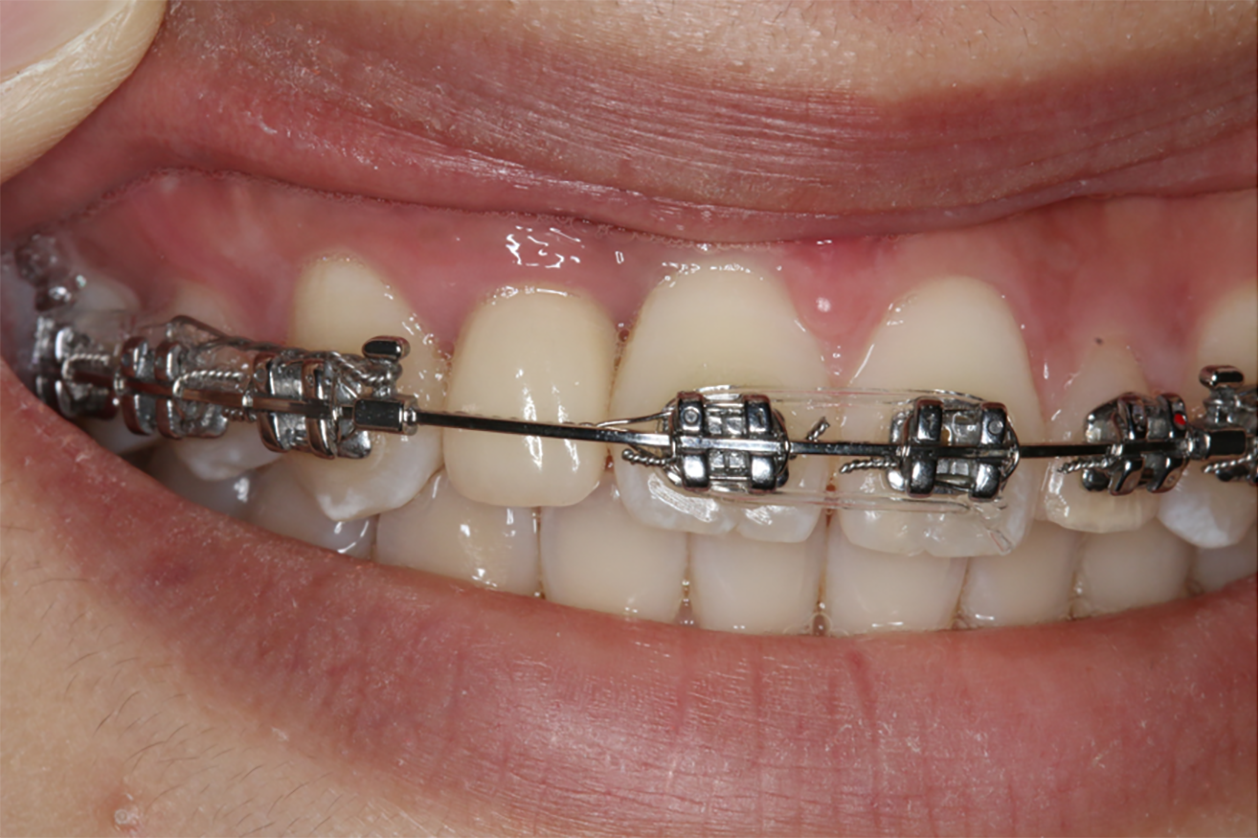
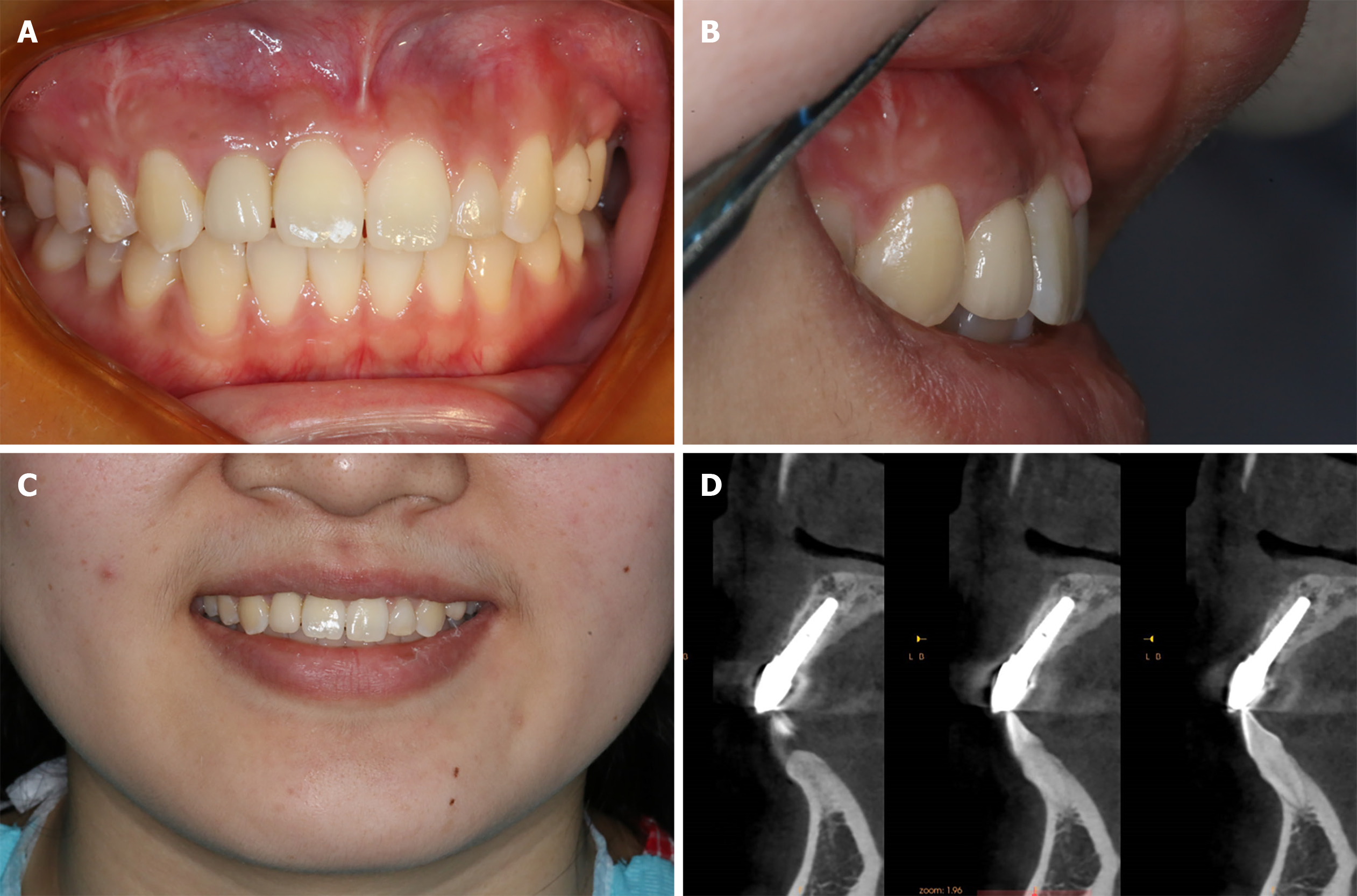
The patient denied any swelling and pain after the surgery. Furthermore, the vascularization of soft tissue at the surgery site was visible at the follow-up visit on day 15 (Figure 4). Later, implant osseointegration was evident after a healing period of 6 mo. During the second surgery, the area was explored using the same flap design. Upon reopening the surgical site for titanium plate and healing abutment replacement, it was found that the shoulder of the implant was surrounded by bone, and the titanium plate was covered by the new bone (Figure 5A), indicating that the bone defect had completely regenerated. Then, the recipient site was sutured (Figure 5B). After 14 d of gingiva stabilization, the sutures were removed, and a final impression was taken to construct a conventional permanent super-structure for restoration. Subsequently, the definitive restoration was placed (Figure 6). Later, a 1-year follow-up revealed the integration of soft tissue and tooth with the adjacent tooth (Figure 7A-C). Apart from that, CBCT showed that the bone around the implant was stable (Figure 7D). Thus, the patient was satisfied with the esthetic and functional outcomes.
Dental implant treatment is often selected based on their functional and esthetic outcomes in congenitally missing maxillary lateral incisors with available space. However, insufficient bone and soft tissue become obstacles to successful implant treatment. An adequate supporting bone around the implant is essential for the long-term stability and esthetic results of the implant. Some studies proposed combining autologous bone with bone substitute materials for the reconstruction of severe alveolar ridge defects to reduce autologous bone resorption. Titanium plate effectively prevents connective tissue colonization and has good mechanical strength to maintain the osteogenic space during the alveolar ridge reconstruction[8,9]. Meanwhile, Strauss et al[10] reported that PRF with Bio-Oss had an outstanding ability in promoting osteogenesis due to its abundant growth factors.
In this report, the labial bone plate was first displaced, ensuring that the base of the labial bone plate was attached to the basal bone for blood supply. Afterwards, a titanium plate was placed to fix the labial bone plate and maintain the bone formation space. Then, the bone substitute materials and PRF were mixed to cover the bone defect. Upon reopening of the surgical site for titanium plate removal and replacement of healing abutment, it was found that the implant shoulder was surrounded by bone, and the titanium plate was covered by the new bone, indicating that the bone defect had completely regenerated. In addition, CBCT displayed adequate supporting bone around the implant during the 1-year follow-up, and the labial bone plate exceeded 2 mm.
In the esthetic zone, sufficient soft tissue is mandatory for successful implant outcomes. Primary closure is vital to ensure uneventful healing and the soft tissue abundance ensures the esthetic results and long-term health of the implant. Some studies suggested that obtaining primary closure through the relaxation of incision or connective tissue free flap may disrupt the blood supply, accompanied by higher surgical complexity[11]. Recently, concentrated platelets have been recommended as an efficient strategy for wound healing[12,13]. PRF, a second-generation platelet concentrate, contains various growth factors, platelets, and leukocytes[14]. The three-dimensional fibrin scaffold of PRF continuously releases growth factors[15] that promote local tissue vascularization and regeneration during wound healing[16]. Moreover, PRF plays a crucial role in wound healing as an excellent anti-inflammatory and antibacterial agent[17,18].
In addition, Miron et al[19] reviewed the effects of PRF on wound healing and highlighted its positive effects on the management of soft tissue. Meanwhile, Cui et al[20] reported that the PRF membrane without a tight flap closure could achieve excellent soft tissue regeneration. In the present case, bio-guide membrane and PRF membrane were utilized to double cover the bone substitute materials without a tight flap closure for mechanical barrier and soft tissue regeneration. The patient denied any swelling and pain after the surgery, which might be attributed to the anti-inflammatory and antibacterial activity of PRF. Furthermore, at the follow-up on day 15, the vascularization of soft tissue was visible, and excellent gingival contour was obtained when the definitive restoration was placed. On top of that, the 1-year follow-up revealed harmony and stability of the gingival contour.
Bone regeneration and soft tissue management pose challenges for dental implant treatment in congenitally missing maxillary lateral incisors. In the present case, the labial bone plate was displaced but remained connected to the base bone, ensuring blood supply. A titanium plate was used to fix the labial bone plate and maintain the osteogenic space. Meanwhile, the PRF supplied growth factors and leukocytes for bone and soft tissue regeneration. This procedure reduced the surgical complexity besides demonstrating fewer adverse reactions and outstanding esthetic outcomes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dentistry, oral surgery and medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Grawish ME, Madi M, Nappe C, Şentürk MF S-Editor: Li X L-Editor: Wang TQ P-Editor: Li X
| 1. | Grunder U, Gracis S, Capelli M. Influence of the 3-D bone-to-implant relationship on esthetics. Int J Periodontics Restorative Dent. 2005;25:113-119. [PubMed] |
| 2. | Kolk A, Handschel J, Drescher W, Rothamel D, Kloss F, Blessmann M, Heiland M, Wolff KD, Smeets R. Current trends and future perspectives of bone substitute materials - from space holders to innovative biomaterials. J Craniomaxillofac Surg. 2012;40:706-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 291] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 3. | Schwartz-Arad D, Levin L. Multitier technique for bone augmentation using intraoral autogenous bone blocks. Implant Dent. 2007;16:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Schwartz-Arad D, Levin L. Symphysis revisited: clinical and histologic evaluation of newly formed bone and reharvesting potential of previously used symphysial donor sites for onlay bone grafting. J Periodontol. 2009;80:865-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Wang HL, Boyapati L. "PASS" principles for predictable bone regeneration. Implant Dent. 2006;15:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 374] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 6. | Dohle E, El Bagdadi K, Sader R, Choukroun J, James Kirkpatrick C, Ghanaati S. Platelet-rich fibrin-based matrices to improve angiogenesis in an in vitro co-culture model for bone tissue engineering. J Tissue Eng Regen Med. 2018;12:598-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 7. | Pitzurra L, Jansen IDC, de Vries TJ, Hoogenkamp MA, Loos BG. Effects of L-PRF and A-PRF+ on periodontal fibroblasts in in vitro wound healing experiments. J Periodontal Res. 2020;55:287-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 8. | Zita Gomes R, Paraud Freixas A, Han CH, Bechara S, Tawil I. Alveolar Ridge Reconstruction with Titanium Meshes and Simultaneous Implant Placement: A Retrospective, Multicenter Clinical Study. Biomed Res Int. 2016;2016:5126838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Rasia-dal Polo M, Poli PP, Rancitelli D, Beretta M, Maiorana C. Alveolar ridge reconstruction with titanium meshes: a systematic review of the literature. Med Oral Patol Oral Cir Bucal. 2014;19:e639-e646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Miron RJ, Zucchelli G, Pikos MA, Salama M, Lee S, Guillemette V, Fujioka-Kobayashi M, Bishara M, Zhang Y, Wang HL, Chandad F, Nacopoulos C, Simonpieri A, Aalam AA, Felice P, Sammartino G, Ghanaati S, Hernandez MA, Choukroun J. Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clin Oral Investig. 2017;21:1913-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 292] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 11. | Waasdorp J, Feldman S. Bone regeneration around immediate implants utilizing a dense polytetrafluoroethylene membrane without primary closure: a report of 3 cases. J Oral Implantol. 2013;39:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Durmuşlar MC, Balli U, Dede FÖ, Misir AF, Bariş E, Kürkçü M, Kahraman SA. Histological Evaluation of the Effect of Concentrated Growth Factor on Bone Healing. J Craniofac Surg. 2016;27:1494-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Strauss FJ, Stähli A, Gruber R. The use of platelet-rich fibrin to enhance the outcomes of implant therapy: A systematic review. Clin Oral Implants Res. 2018;29 Suppl 18:6-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 14. | Cortese A, Pantaleo G, Amato M, Howard CM, Pedicini L, Claudio PP. Platelet-Rich Fibrin (PRF) in Implants Dentistry in Combination with New Bone Regenerative Flapless Technique: Evolution of the Technique and Final Results. Open Med (Wars). 2017;12:24-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Kobayashi E, Flückiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, Miron RJ. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig. 2016;20:2353-2360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 435] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 16. | Bielecki T, Dohan Ehrenfest DM, Everts PA, Wiczkowski A. The role of leukocytes from L-PRP/L-PRF in wound healing and immune defense: new perspectives. Curr Pharm Biotechnol. 2012;13:1153-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 17. | Feng M, Wang Y, Zhang P, Zhao Q, Yu S, Shen K, Miron RJ, Zhang Y. Antibacterial effects of platelet-rich fibrin produced by horizontal centrifugation. Int J Oral Sci. 2020;12:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 18. | Zhang J, Yin C, Zhao Q, Zhao Z, Wang J, Miron RJ, Zhang Y. Anti-inflammation effects of injectable platelet-rich fibrin via macrophages and dendritic cells. J Biomed Mater Res A. 2020;108:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Miron RJ, Fujioka-Kobayashi M, Bishara M, Zhang Y, Hernandez M, Choukroun J. Platelet-Rich Fibrin and Soft Tissue Wound Healing: A Systematic Review. Tissue Eng Part B Rev. 2017;23:83-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 273] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 20. | Cui A, Zhou J, Mudalal M, Wang Y, Wang J, Gong M, Zhou Y. Soft tissue regeneration around immediate implant placement utilizing a platelet-rich fibrin membrane and without tightly flap closure: Two case reports. Medicine (Baltimore). 2020;99:e22507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |