Published online Feb 26, 2022. doi: 10.12998/wjcc.v10.i6.1998
Peer-review started: August 29, 2021
First decision: November 16, 2021
Revised: November 25, 2021
Accepted: January 11, 2022
Article in press: January 11, 2022
Published online: February 26, 2022
Processing time: 178 Days and 0.7 Hours
Mutations that occur in the ABCB4 gene, which encodes multidrug-resistant protein 3, underlie the occurrence of progressive familial intrahepatic cholestasis type 3 (PFIC3). Clinical signs of intrahepatic cholestasis due to gene mutations typically first appear during infancy or childhood. Reports of PFIC3 occurring in adults are rare.
This is a case study of a 32-year-old infertile female Chinese patient with a 15-year history of recurrent abnormal liver function. Her primary clinical signs were elevated levels of alkaline phosphatase and γ-glutamyl transpeptidase. Other possible reasons for liver dysfunction were eliminated in this patient, resulting in a diagnosis of PFIC3. The diagnosis was confirmed using gene detection and histological analyses. Assessments using genetic sequencing analysis indicated the presence of two novel heterozygous mutations in the ABCB4 gene, namely, a 2950C>T; p.A984V mutation (exon 24) and a 667A>G; p.I223V mutation (exon 7). After receiving ursodeoxycholic acid (UDCA) treatment, the patient's liver function indices improved, and she successfully became pregnant by in vitro fertilization. However, the patient developed intrahepatic cholestasis of pregnancy in the first trimester. Fortunately, treatment with UDCA was safe and effective.
These novel ABCB4 heterozygous mutations have a variety of clinical phenotypes. Continued follow-up is essential for a comprehensive understanding of PFIC3.
Core Tip: This is the first case report of an adult patient with progressive familial intrahepatic cholestasis type 3 (PFIC3) and infertility. Gene detection was central to making a definitive diagnosis. The novel ABCB4 heterozygous mutations observed exhibited a variety of clinical phenotypes. A genetic predisposition to infertility may also be present in this patient and requires further research. The discovery of these new mutations has enriched the information on the clinical features of PFIC3 and contributed to a more comprehensive understanding of ABCB4 disease.
- Citation: Liu TF, He JJ, Wang L, Zhang LY. Novel ABCB4 mutations in an infertile female with progressive familial intrahepatic cholestasis type 3: A case report. World J Clin Cases 2022; 10(6): 1998-2006
- URL: https://www.wjgnet.com/2307-8960/full/v10/i6/1998.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i6.1998
Progressive familial intrahepatic cholestasis (PFIC) comprises several rare, hereditary autosomal-recessive hepatic diseases that occur predominantly in neonates and infants. Intrahepatic cholestasis is the primary clinical sign observed in patients with PFIC[1]. As the disease progresses, patients develop liver fibrosis, which progresses to cirrhosis and eventually hepatic failure[2]. Based on the specific mutations in the gene, six types of PFIC have been defined, among which classical types 1, 2 and 3 are more common[3]. In terms of biochemical and histological characteristics, there are significant differences between PFIC3 and other types (Table 1). In particular, PFIC3 presents with elevated serum γ-glutamyl transpeptidase (GGT)[4]. We found few reports of cases of PFIC3 in our search of the published literature, which included the last ten years. In particular, fertility in adult PFIC3 patients has not been reported.
| PFIC1 | PFIC2 | PFIC3 | |
| Gene | ATP8B1 | ABCB11 | ABCB4 |
| Protein | FIC1 | BSEP | MDR3 |
| Serum ALT | + | ++ | + |
| Serum GGT | Normal | Normal | Elevated |
| Serum TBA | ++ | +++ | + |
| Liver histology | Mild cholestasis, mild lobular fibrosis | Cholestasis, portal fibrosis, giant cell hepatitis, hepatocellular necrosis | Bile ductular proliferation, inflammatory infiltrate, and biliary fibrosis |
A 32-year-old female patient had experienced abnormal serum liver enzyme levels for 15 years.
Fifteen years previously, the patient was diagnosed with abnormal serum liver enzyme levels during the course of a high school physical examination. As she did not exhibit any symptoms of overall liver dysfunction, no action was taken at that time. Four years later, liver function index abnormalities were observed during an occupational health examination. The patient was admitted to a local hospital with no apparent cause of her abnormal serum enzyme levels. The patient was treated with some liver-protective drugs, but her liver function indices did not improve. The patient was not experiencing any adverse effects at that time and did not actively have her liver function indices monitored in subsequent years. One month prior to her admission to our hospital, the patient visited a maternal and child health hospital due to infertility, but routine examination on admission revealed significantly abnormal liver function indices. For further diagnosis and treatment, she was admitted to the Department of Hepatology at our hospital.
Thirteen years previously, the patient had undergone splenectomy due to a ruptured spleen caused by a car accident and recovered well.
During her ten years of marriage, the patient experienced continued infertility and did not take any infertility medications or oral contraceptives. One year previously, salpingography at a maternal and child health hospital showed partial obstruction of the fallopian tube, and the natural conception rate was low. The fertility tests of the patient’s spouse were normal. Therefore, in vitro fertilization (IVF)-assisted reproduction was recommended. However, due to financial reasons, the patient did not receive IVF.
The patient indicated that she did not have a history of alcohol intake or the use of any hepatotoxic drugs. Her father had undergone surgery years previously to treat intrahepatic bile duct stones and recovered well from the surgery. Her mother, who died from a brain hemorrhage three years earlier, did not have liver disease in her medical history. Her brother’s liver function was normal.
The patient’s vital signs were all stable, and physical examination revealed no noteworthy positive signs.
Analysis of her laboratory data (November 3, 2020) revealed the following results: total bilirubin, 28.1 μmol/L; direct bilirubin, 16.1 μmol/L; alanine aminotransferase (ALT), 151 U/L; aspartate aminotransferase (AST), 119 U/L; alkaline phosphatase (ALP), 226 U/L; GGT, 1025 U/L; total bile acid (TBA), 17.2 μmol/L; and albumin, 41.8 g/L (Table 2). Several markers for hepatitis viruses (hepatitis A, B, C, and E) were determined to be negative. The analyses for antibodies to cytomegalovirus antibodies and Epstein-Barr virus DNA were also negative. All autoimmune antibodies, including antimitochondrial and antinuclear lupus-related antibodies and Sjogren's syndrome-related antibodies were negative. We also assessed serum copper levels as well as ferritin and ceruloplasmin, and the levels were within normal ranges. Routine blood analysis, coagulation function, thyroid function, and several additional laboratory test results were found to be unremarkable.
| Admission | Discharge | Before pregnancy | 13th week of pregnancy | 21th week of pregnancy | 30th week of pregnancy | |
| ALT (7-41 U/L) | 151 | 18 | 12 | 85 | 28 | 21 |
| AST (13-35U/L) | 119 | 48 | 38 | 109 | 59 | 53 |
| TBil (3.0-21.0 μmol/L) | 28.1 | 14.1 | 21.1 | 27.4 | 21.5 | 33.2 |
| DBil (0.1-6.8 μmol/L) | 16.1 | 9.3 | 12.0 | 15.3 | 11.3 | 20.8 |
| GGT (7-45U/L) | 1025 | 459 | 301 | 470 | 199 | 148 |
| ALP (35-100 U/L) | 226 | 126 | 147 | 223 | 133 | 251 |
| TBA (0.0-10.0 μmol/L) | 17.2 | 19.9 | 15.3 | 40.5 | 52.1 | 81.8 |
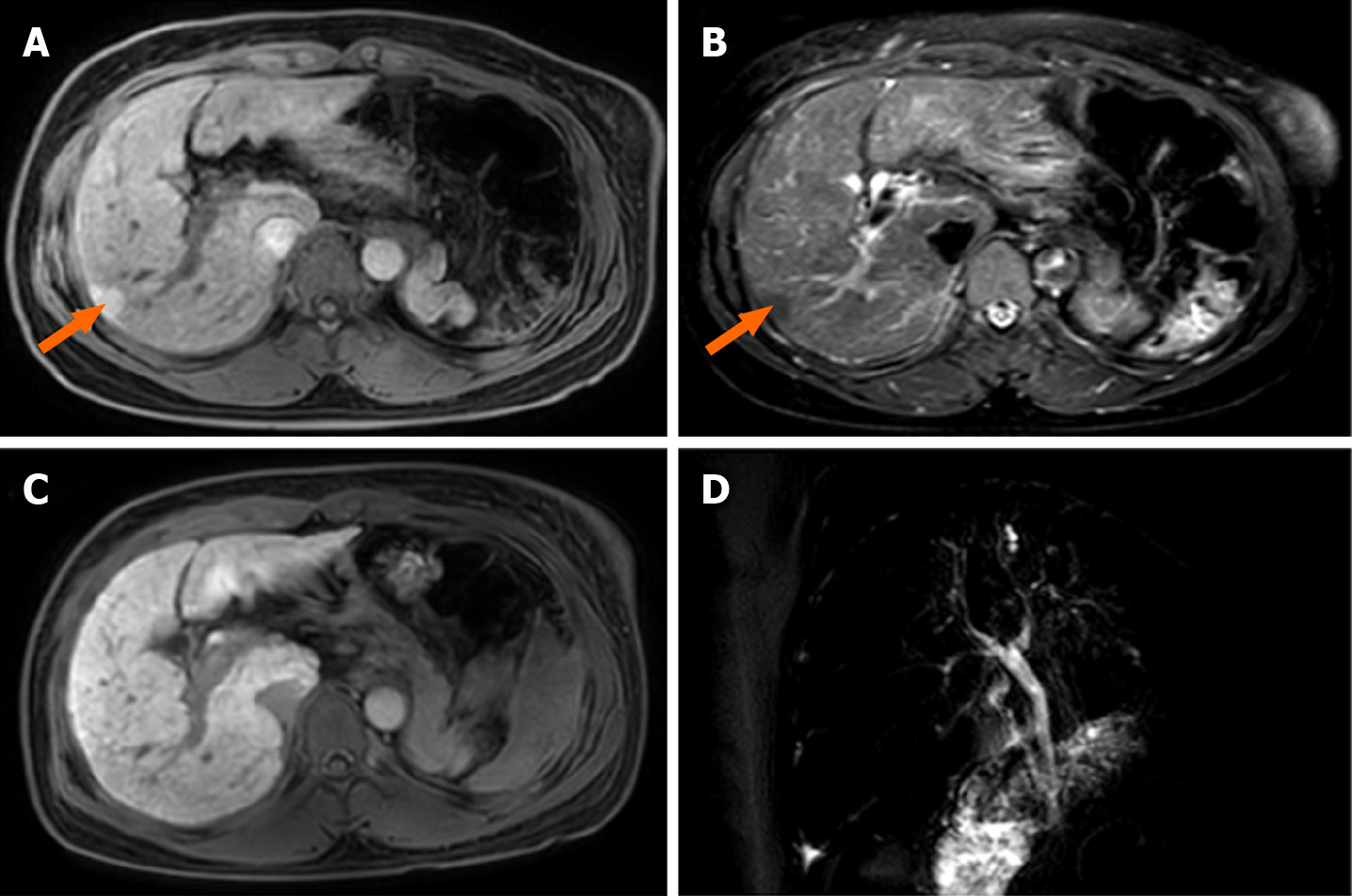
Abdominal magnetic resonance imaging (MRI) revealed an increased hepatic interstitium and multiple patchy abnormal signal shadows in hepatic segments VII and VIII. The presence of nodule regeneration was considered after enhanced examination using gadolinium-ethoxybenzyl-diethylenetriamine penta-acetic acid, which is a hepatocellular-specific contrast agent. No obstructions associated with the extrahepatic and intrahepatic bile ducts were observed following detailed examination using magnetic resonance cholangiopancreatography (Figure 1). Cardiac ultrasound and chest radiography showed no abnormalities.
We examined the sclera of the patient’s eyes for Kayser-Fleischer rings, but no rings were present. The transient elastography assessment for liver fibrosis was 14.6 kPa.
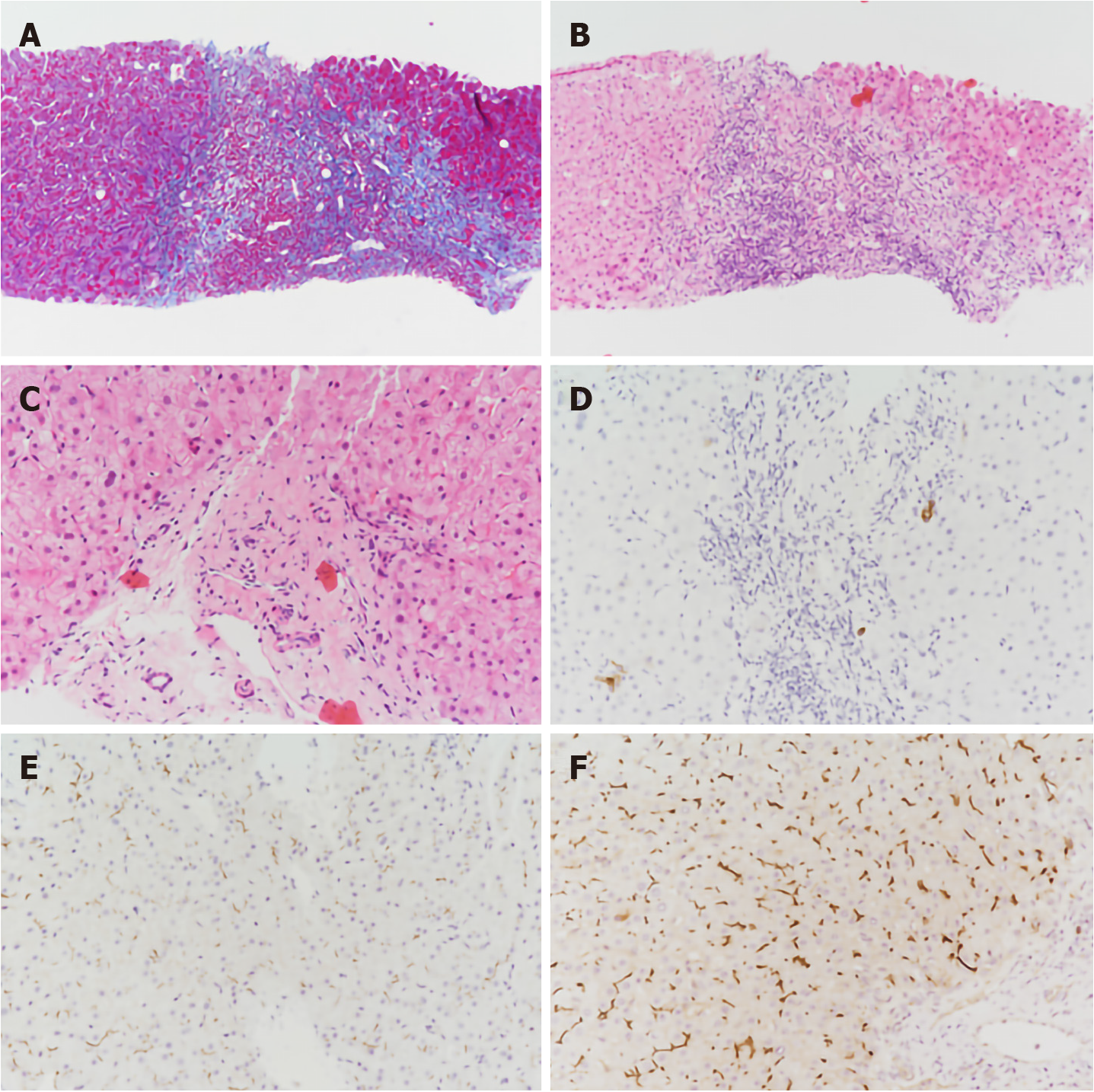
With the consent of the patient, we performed an ultrasound-guided liver biopsy. Pathological examination revealed cholestatic liver fibrosis (modified Scheuer score S2). An analysis of histological samples revealed a significant decrease in multidrug-resistant protein 3 (MDR3) protein staining compared to the levels of staining observed in samples from healthy subjects (Figure 2).
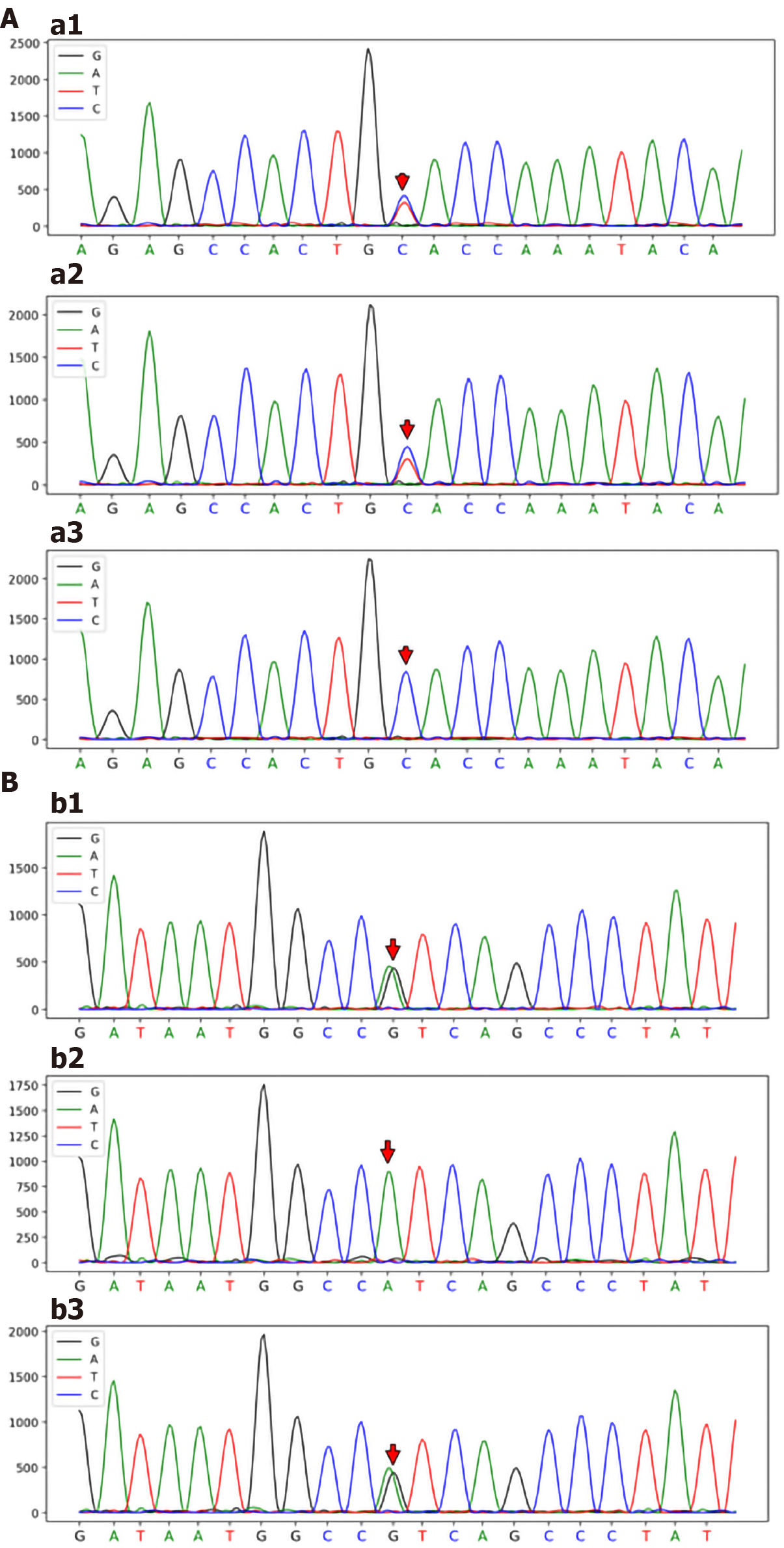
Genomic DNA was purified from peripheral blood samples. Based on Illumina NovaSeq® 6000 instruments and the xGen® sequencing Exome Research Panel, high-throughput sequencing was used to detect genomic mutations. The genetic testing results revealed two novel heterozygous mutations in the ABCB4 gene: a 2950C>T; p.A984V mutation (exon 24) and a 667A>G; p.I223V mutation (exon 7) (Figure 3).
According to the above case data, the patient’s liver function, particularly the increase in serum GGT, was abnormal for many years. Immunohistochemical analysis of liver tissue showed that MDR3 staining was significantly decreased, and gene detection showed that there were two heterozygous mutations in the ABCB4 gene. The final diagnosis of the presented case was PFIC3.
The patient received treatment with ursodeoxycholic acid (UDCA) 750 mg/d.
When the patient was discharged, the GGT decreased significantly, and the other liver function indices were close to the normal values (Table 2). After consulting with family members and saving money, the patient went to the maternal and child health care hospital again, successfully became pregnant via IVF, and stopped UDCA treatment on her own. Skin pruritus occurred at the 13th week of pregnancy, and serological examination showed that her liver function was significantly abnormal, in particular, TBA was higher than the initial value. The diagnosis was PFIC3 combined with intrahepatic cholestasis of pregnancy (ICP), and UDCA therapy was recommended. Her symptoms of itching were subsequently alleviated, and all liver function indices except TBA were improved. Fortunately, fetal indicators were normal. The liver function test results during the course of the disease are shown in Table 2.
PFIC3 occurs rarely and is primarily a sporadic disease[5], and the incidence of PFIC3 in the general population has not been reported definitively. A previous assessment of disease occurrence demonstrated that PFIC3 incidence might be 1 in 500000 people[6]. Patients exhibiting PFIC3 commonly develop cholestasis later in childhood and up to adolescence. Individuals with PFIC3 often exhibit recurring episodes of pruritus, jaundice, pale clay-like stools, hepatosplenomegaly, and gastrointestinal bleeding, which can progress to cirrhosis and liver failure before the onset of adulthood[7,8]. Gastrointestinal bleeding that is associated with cirrhosis or the occurrence of portal hypertension is often the first presenting sign of the disorder in older children or young adults[9].
In this case study, we analyzed a 32-year-old female Chinese patient who was asymptomatic. We used laboratory, MRI, and histological examinations to exclude other possible etiologies, including primary biliary cholangitis, Alagille syndrome, primary sclerosing cholangitis, Wilson's disease, and drug-induced liver injury. Subsequently, we highly suspected that this patient might have PFIC3 based on her high level of GGT. Therefore, we carried out a gene mutation analysis that revealed two novel heterozygous mutations in ABCB4, namely, a 2950C>T; p.A984V mutation and a 667A>G; p.I223V mutation. Based on immunostaining for MDR3 in liver samples and genetic testing, the patient was diagnosed with PFIC3.
PFIC3 is caused by a mutation in the ABCB4 gene, which encodes MDR3[10]. MDR3 is classified as a p-glycoprotein (pGp) and has been shown to be expressed in hepatocyte canalicular membranes[11]. The MDR3 protein transfers phospholipids from hepatocytes into the bile ducts[12]. Normally, phospholipids combine with bile salts that subsequently form microparticles. This process results in increased hydrophilicity and reduces the descaling effects produced by bile salts. These actions protect bile duct cells from toxic damage that can be induced by bile salts. A major function of phospholipids in the liver is to neutralize the detergent-like effects produced by hydrophobic bile salts[6,13]. Thus, defects in the MDR3 protein can result in damage to the biliary epithelium and bile canaliculi, which ultimately can produce cholestasis. It should be noted that the primary defect that occurs with MDR3 deficiency does not lead to the retention of bile acids in hepatocytes, so cholestasis is not a direct result of the disorder[14]. Symptoms, including cholestasis, develop as a result of the damage caused by the eventual cholangiopathy. Even patients who exhibit complete MDR3 deficiency may not present clinical symptoms for several years[15]. When MDR3 function is only partially lost, patients commonly exhibit slow disease progression[16]. Therefore, MDR3 deficiencies can result in a range of disease manifestations and ages at presentation.
Nearly 300 ABCB4 variants that cause disease have been reported, of which approximately 50 are associated with PFIC3[7]. The age at which the patient first exhibits signs of cholestatic disease, severity of the liver disorder and response to treatment have been shown to be correlated with the different mutations that occur in the ABCB4 gene[16]. Patients with homozygous mutations tend to exhibit progressive intrahepatic cholestasis, which usually leads to liver failure in early childhood and requires liver transplantation[17]. On the other hand, the age of disease onset in patients with heterozygous mutations is relatively high, and clinical symptoms are mild.
New evidence demonstrates a spectrum of diseases resulting from heterozygous mutations in ABCB4, ranging from the severe form seen in PFIC3 to milder, intermittent forms such as low-phospholipid-associated cholelithiasis syndrome (LPAC), ICP and adult-onset biliary fibrosis or cirrhosis[18-21]. Previous studies have shown that reduced or absent transport of phosphatidylcholine is indeed associated with intrahepatic sludge or stone formation[22]. The father of the patient had a genetic mutation in ABCB4, including a 2950C>T; p.A984V mutation, and his preexisting intrahepatic bile duct stone may have been a phenotypic form of LPAC[23]. Although the majority of cases of ICP present in the third trimester and are usually thought to be multifactorial in etiology, the patient's early pregnancy presentation may have been attributed to an underlying genetic susceptibility[24]. ABCB4 gene defects are one of the major causes of biliary fibrosis, and Bernardo et al[25] reported that PFIC3-associated biliary fibrosis can be partially reversed after UDCA treatment. However, the coexistence of ABCB4 variants and infertility has not been reported in previously published literature. While not proof of causality, the mutations identified in our patient certainly suggest a possible susceptibility for her rare and unique presentation.
Genetic sequencing of the ABCB4 gene in our patient revealed two novel heterozygous mutations, namely, a 2950C>T; p.A984V mutation and a 667A>G; p.I223V mutation. These two mutations were not included in the Human Gene Mutation Database or the ClinVar database. The effects on function resulting from the two mutations were evaluated using Polymorphism Phenotyping v2, Sorting Intolerant from Tolerant, and MutationTaster[26-28]. The two novel mutations were predicted to have uncertain significance. Based on the familial identification, the locations of these two mutations were determined to be on different chromosomes, which resulted in a compound heterozygous mutation that might have resulted in a partial loss of function for MDR3. It is due to this compound heterozygous mutation that this patient has a rare and unique clinical phenotype.
UDCA is typically used as the initial treatment for PFIC3[29]. Some studies have shown that a dose of 10-30 mg/kg/day can successfully treat PFIC3 and resolve the presence of cholestasis in patients[2]. UDCA has been shown to be effective in two-thirds of PFIC3 patients. Korkut et al[30] reported that UDCA treatment was effective in improving conception in women who had intrahepatic cholestasis and were infertile. Here, the patient successfully became pregnant after UDCA treatment following a single IVF treatment, and UDCA treatment may have had some potential beneficial effects. The patient responded well to UDCA therapy. After UDCA treatment, the serum GGT in this patient decreased significantly, and the other liver function indices basically returned to normal. However, after the combination of ICP, UDCA improved the symptoms of pruritus and liver function, but TBA showed an upward trend. The increase in TBA may harm the fetus, so it is necessary to closely monitor the fetal index and deliver early if necessary. The remaining one-third of patients may require additional intervention due to the degree of disease progression and inadequate symptom relief. When other treatments are unsuccessful, liver transplantation has been shown to be effective in PFIC3 patients[31]. However, long-term follow-up to monitor the patient’s liver function is necessary.
We were able to definitively diagnose PFIC3 in a 32-year-old female Chinese patient based on her clinical symptoms, pathological examination, and gene detection. Successful gene detection was essential to the diagnosis. This case illustrates the heterogeneity of genetic mutations. These novel ABCB4 heterozygous mutations have a variety of clinical phenotypes that respond well to UDCA therapy. A genetic predisposition to infertility may also be present in this patient, and this requires further research. The discovery of these new mutations has enriched the information on the clinical features of PFIC3 and contributed to a more comprehensive understanding of ABCB4 disease.
The authors thank the patient for agreeing to report her case and for providing a detailed medical history.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Shao Y, Shariati MBH S-Editor: Ma YJ L-Editor: Webster JR P-Editor: Ma YJ
| 1. | Bull LN, Thompson RJ. Progressive Familial Intrahepatic Cholestasis. Clin Liver Dis. 2018;22:657-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 2. | Gunaydin M, Bozkurter Cil AT. Progressive familial intrahepatic cholestasis: diagnosis, management, and treatment. Hepat Med. 2018;10:95-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Amirneni S, Haep N, Gad MA, Soto-Gutierrez A, Squires JE, Florentino RM. Molecular overview of progressive familial intrahepatic cholestasis. World J Gastroenterol. 2020;26:7470-7484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (5)] |
| 4. | Srivastava A. Progressive familial intrahepatic cholestasis. J Clin Exp Hepatol. 2014;4:25-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 181] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 5. | Gaur K, Sakhuja P. Progressive familial intrahepatic cholestasis: A comprehensive review of a challenging liver disease. Indian J Pathol Microbiol. 2017;60:2-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Jacquemin E, De Vree JM, Cresteil D, Sokal EM, Sturm E, Dumont M, Scheffer GL, Paul M, Burdelski M, Bosma PJ, Bernard O, Hadchouel M, Elferink RP. The wide spectrum of multidrug resistance 3 deficiency: from neonatal cholestasis to cirrhosis of adulthood. Gastroenterology. 2001;120:1448-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 323] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 7. | Delaunay JL, Durand-Schneider AM, Dossier C, Falguières T, Gautherot J, Davit-Spraul A, Aït-Slimane T, Housset C, Jacquemin E, Maurice M. A functional classification of ABCB4 variations causing progressive familial intrahepatic cholestasis type 3. Hepatology. 2016;63:1620-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Chen HL, Wu SH, Hsu SH, Liou BY, Chen HL, Chang MH. Jaundice revisited: recent advances in the diagnosis and treatment of inherited cholestatic liver diseases. J Biomed Sci. 2018;25:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 9. | Jacquemin E. Progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol. 2012;36 Suppl 1:S26-S35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 10. | Jansen PL, Müller MM. Progressive familial intrahepatic cholestasis types 1, 2, and 3. Gut. 1998;42:766-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Davit-Spraul A, Gonzales E, Baussan C, Jacquemin E. The spectrum of liver diseases related to ABCB4 gene mutations: pathophysiology and clinical aspects. Semin Liver Dis. 2010;30:134-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 12. | Keitel V, Burdelski M, Warskulat U, Kühlkamp T, Keppler D, Häussinger D, Kubitz R. Expression and localization of hepatobiliary transport proteins in progressive familial intrahepatic cholestasis. Hepatology. 2005;41:1160-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 13. | Guyot C, Stieger B. Interaction of bile salts with rat canalicular membrane vesicles: evidence for bile salt resistant microdomains. J Hepatol. 2011;55:1368-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Oude Elferink RP, Paulusma CC. Function and pathophysiological importance of ABCB4 (MDR3 P-glycoprotein). Pflugers Arch. 2007;453:601-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 199] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 15. | Ziol M, Barbu V, Rosmorduc O, Frassati-Biaggi A, Barget N, Hermelin B, Scheffer GL, Bennouna S, Trinchet JC, Beaugrand M, Ganne-Carrié N. ABCB4 heterozygous gene mutations associated with fibrosing cholestatic liver disease in adults. Gastroenterology. 2008;135:131-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Reichert MC, Lammert F. ABCB4 Gene Aberrations in Human Liver Disease: An Evolving Spectrum. Semin Liver Dis. 2018;38:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Wu Z, Zhang S, Zhang L, Li M. Novel ABCB4 mutation in a Chinese female patient with progressive familial intrahepatic cholestasis type 3: a case report. Diagn Pathol. 2020;15:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Sticova E, Jirsa M. ABCB4 disease: Many faces of one gene deficiency. Ann Hepatol. 2020;19:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Lucena JF, Herrero JI, Quiroga J, Sangro B, Garcia-Foncillas J, Zabalegui N, Sola J, Herraiz M, Medina JF, Prieto J. A multidrug resistance 3 gene mutation causing cholelithiasis, cholestasis of pregnancy, and adulthood biliary cirrhosis. Gastroenterology. 2003;124:1037-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Vitale G, Gitto S, Vukotic R, Raimondi F, Andreone P. Familial intrahepatic cholestasis: New and wide perspectives. Dig Liver Dis. 2019;51:922-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (4)] |
| 21. | van der Woerd WL, van Mil SW, Stapelbroek JM, Klomp LW, van de Graaf SF, Houwen RH. Familial cholestasis: progressive familial intrahepatic cholestasis, benign recurrent intrahepatic cholestasis and intrahepatic cholestasis of pregnancy. Best Pract Res Clin Gastroenterol. 2010;24:541-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Erlinger S. Low phospholipid-associated cholestasis and cholelithiasis. Clin Res Hepatol Gastroenterol. 2012;36 Suppl 1:S36-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Goubault P, Brunel T, Rode A, Bancel B, Mohkam K, Mabrut JY. Low-Phospholipid Associated Cholelithiasis (LPAC) syndrome: A synthetic review. J Visc Surg. 2019;156:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Johnston RC, Stephenson ML, Nageotte MP. Novel heterozygous ABCB4 gene mutation causing recurrent first-trimester intrahepatic cholestasis of pregnancy. J Perinatol. 2014;34:711-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Frider B, Castillo A, Gordo-Gilart R, Bruno A, Amante M, Alvarez L, Mathet V. Reversal of advanced fibrosis after long-term ursodeoxycholic acid therapy in a patient with residual expression of MDR3. Ann Hepatol. 2015;14:745-751. [PubMed] |
| 26. | Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet. 2013;Chapter 7:Unit7.20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1357] [Cited by in RCA: 2154] [Article Influence: 179.5] [Reference Citation Analysis (0)] |
| 27. | Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4922] [Cited by in RCA: 5187] [Article Influence: 324.2] [Reference Citation Analysis (0)] |
| 28. | Schwarz JM, Rödelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods. 2010;7:575-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2146] [Cited by in RCA: 2363] [Article Influence: 157.5] [Reference Citation Analysis (0)] |
| 29. | Stapelbroek JM, van Erpecum KJ, Klomp LW, Houwen RH. Liver disease associated with canalicular transport defects: current and future therapies. J Hepatol. 2010;52:258-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 30. | Korkut E, Kisacik B, Akcan Y, Belenli O, Bicik Z, Yucel O. Two successive pregnancies after ursodeoxycholic acid therapy in a previously infertile woman with antimitochondrial antibody-negative primary biliary cirrhosis. Fertil Steril. 2005;83:761-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Englert C, Grabhorn E, Richter A, Rogiers X, Burdelski M, Ganschow R. Liver transplantation in children with progressive familial intrahepatic cholestasis. Transplantation. 2007;84:1361-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |