Published online Feb 26, 2022. doi: 10.12998/wjcc.v10.i6.1973
Peer-review started: August 25, 2021
First decision: October 27, 2021
Revised: November 2, 2021
Accepted: January 11, 2022
Article in press: January 11, 2022
Published online: February 26, 2022
Processing time: 182 Days and 3.8 Hours
Intrapancreatic accessory spleen (IPAS) is an uncommon condition, with the majority of cases presenting as solid lesions. Thus, this condition is frequently misdiagnosed as pancreatic solid neoplasm. Moreover, splenic cavernous hemangioma is a rare disorder, whereas lesions with a cystic appearance arising from IPAS have not been reported.
Herein, we present a case involving a 32-year-old male who had a complex cystic lesion in the tail of the pancreas revealed by conventional ultrasound. The lesion was misdiagnosed as a pancreatic cystadenoma because of its confusing anatomic location, as well as due to its peripheral nodular and internal septal enhancement patterns on contrast-enhanced ultrasound. After multidisciplinary discussion, the patient finally underwent laparoscopic pancreatic body and tail resections. Postoperative pathology demonstrated the lesion to be a cavernous hemangioma arising from the IPAS.
Cavernous hemangioma in the intrapancreatic accessory spleen may mimic pancreatic cystadenoma, which is a condition with the potential to be malignant. Imaging follow-ups or surgical interventions may be helpful for the exclusion of malignant risks in complicated cystic lesions, especially those with parietal and septal enhancements.
Core Tip: Intrapancreatic accessory spleen (IPAS) is an uncommon condition; however, overlapping imaging manifestations of IPAS and pancreatic tumors may lead to unnecessary surgery. Cystic splenic cavernous hemangioma is a rare disorder, whereas lesions with a cystic appearance arising from IPAS have not been reported. Herein, we report a cavernous hemangioma in the IPAS that was misdiagnosed as being a pancreatic cystadenoma via contrast-enhanced modalities. The diagnosis of cystic lesions in IPAS can be challenging. Imaging follow-ups or surgical interventions may be needed for the possible malignancy risk of a complicated cystic lesion, especially those with parietal and septal enhancements.
- Citation: Huang JY, Yang R, Li JW, Lu Q, Luo Y. Cavernous hemangioma of an intrapancreatic accessory spleen mimicking a pancreatic tumor: A case report. World J Clin Cases 2022; 10(6): 1973-1980
- URL: https://www.wjgnet.com/2307-8960/full/v10/i6/1973.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i6.1973
An intrapancreatic accessory spleen (IPAS) is an uncommon condition, with a prevalence ranging from 1.1%-3.4% in individuals[1,2]. An IPAS is typically asymptomatic and has an innocuous nature. However, overlapping imaging manifestations of an IPAS and primary pancreatic tumors may lead to unnecessary surgery[3]. A typical IPAS demonstrates a solid lesion with a round, oval or triangular shape, which is similar to the spleen on both precontrast and contrast-enhanced images. Therefore, this disorder is frequently confused with adenocarcinomas, neuroendocrine tumors or other solid pancreatic entities. When compared with a solid IPAS, cystic lesions arising from an IPAS are rare but necessitate a differential diagnosis with pancreatic cystic neoplasms, especially those possessing the potential to be malignant. Moreover, when considering the high likelihood of false-negative results, biopsy of cystic pancreatic lesions is seldom performed, and surgery is ultimately performed in most patients.
Herein, we report such a case involving a patient who underwent laparoscopic pancreatic body and tail resections because of an indeterminate pancreatic cystic lesion. Postoperative pathology confirmed this lesion as being a cavernous hemangioma arising from an IPAS. Furthermore, the clinical and imaging characteristics of IPAS and pancreatic cystic neoplasms (according to the previous literature) were also reviewed (Table 1).
| Pancreatic cystic neoplasms | ||||||
| IPAS | Pseudocyst | SCA | MCA | SPN | IPMN | |
| Clinical features[1,17,18] | ||||||
| Age (mean: year) | 40 to 65 | At any age | 60 | 40 to 50 | 30 | 65 |
| Gender | Slightly higher in males | Males > females | Older females | Females > males | Young females | Males > females |
| Incidence | 11%–17% of AS | 5%-40% after pancreatitis | 16% of PCN | 29% of PCN | 2% and 3% of PCN | 20%-50% of PCN |
| Benign/malignant | Benign | Benign | Benign | Low malignant potential | Low malignant potential | Malignant potential |
| Anatomic location | Tail > head/body | 1/3 near the head | Head > body/tail | Body/tail > head | Body/tail > head | Arising from the pancreatic ducts |
| Size (mean: cm) | ≤ 2 | Depending on the duration of disease | 5-8 | 7-10 | 6 | 0.8 |
| Potential mimickers | NET and PDAC | MCA | MCA and IPMN | MCA: IPMN and MCAC | MCA: IPMN and MCAC | SCA: MCA and MCAC |
| Radiological diagnosis | ||||||
| Ultrasound[7,17,19-21] | ||||||
| Baseline US | Hypoechoic lesion with well-defined border | Transonic: net separation: irregular internal outline: fluid-containing lesion | Small transonic lesions with thin septa inside | Unilocular or septated cystic lesions with thickened walls and well-defined margins | Encapsulated mixed mass (solid and cystic) | Lesions developed inside the main/branch pancreatic ducts: parietal nodules and septa can be seen in the cysts |
| Doppler US | Blood supply may from the splenic vessels | No obvious blood flow encompass or inside the lesion | No obvious blood flow encompass or inside the lesion | No obvious blood flow encompass or inside the lesion | Blood flow signal around the tumor | No obvious blood flow encompass or inside the lesion |
| CEUS[19,21] | Inhomogeneous hyperenhancement followed by homogeneous hyperenhancement | Iso- or hyperenhancement of the cystic wall: without definite washout | Isoenhancement of the cystic walls and septa: without definite washout | Iso-enhancement of the cystic walls and nodules: without definite washout | Rim hyperenhancement in the capsule:centripetal hyperenhancement followed by mild washout in the solid part: no enhancement in the cystic components | Iso-enhancement in the cystic wall and nodules |
| CECT[18,21-23] | Inhomogeneous hyperenhancement followed by homogeneous hyperenhancement | Round or oval fluid collection with a thin: hardly perceptible wall or enhancing thick wall | Well-defined: polycystic or honeycomb lesions showing enhancing internal septa and cyst walls | Well-circumscribed round/oval macrocystic lesions with enhancement of the walls | Hypo-attenuating on pancreatic phase followed by homogeneous gradual enhancement to iso-attenuating on the hepatic venous phase | Dilated main/side pancreatic ducts: nodules arising from the ducts manifest hyperattenuating at contrast-enhanced CT |
| CEMRI[22,24] | ||||||
| T1-W | Inhomogeneous hypointensity | Blood products and necrotic components commonly present intrinsically increased t1 signal intensity: the thickend wall shows a rim hyperintensity | High intensity fluid in the cysts | Homogeneous low t1 signal intensity | Low signal intensity: SPN with hemorrhage presents t1 hyperintensity | Loss of t1 signal and delayed uptake of contrast material |
| T2-W | Homogeneous hyperintensity | The hyperintensity in tissues surrounding the pseudocyst represents the inflammation on t2 fat-suppressed images | Honeycomb pattern (microcysts) or macrocysts manifest signal intensity of simple fluid | Homogeneous high t2 signal intensity | Predominantly solid show mildly increased t2 signal intensity: cystic-dominated present t2 signal intensity closer to that of fluid | Papillary excrescences or nodules in the walls of the dilated ducts present hypointense on t2-weighted images |
| Management | Usually require no treatment | Serial imaging follow-up | Follow-up or resection depending on the size of the tumor | Surgical resection | Surgical resection | Recommended to be surgically resected |
A 32-year-old male was referred to our hospital because of a suspicious lesion neighboring the hilum of the spleen, which was detected via conventional grayscale ultrasound in a local community hospital. The patient did not complain of obvious discomfort.
The patient had a history of chronic hepatitis B.
The patient did not complain of abdominal pain or any remarkable discomfort during the physical examination.
In addition to a slightly increased albumin-globulin ratio (2.96) and glutamine transpeptidase level (63 IU/L), no abnormal laboratory test results, including those of related tumor markers, were found.
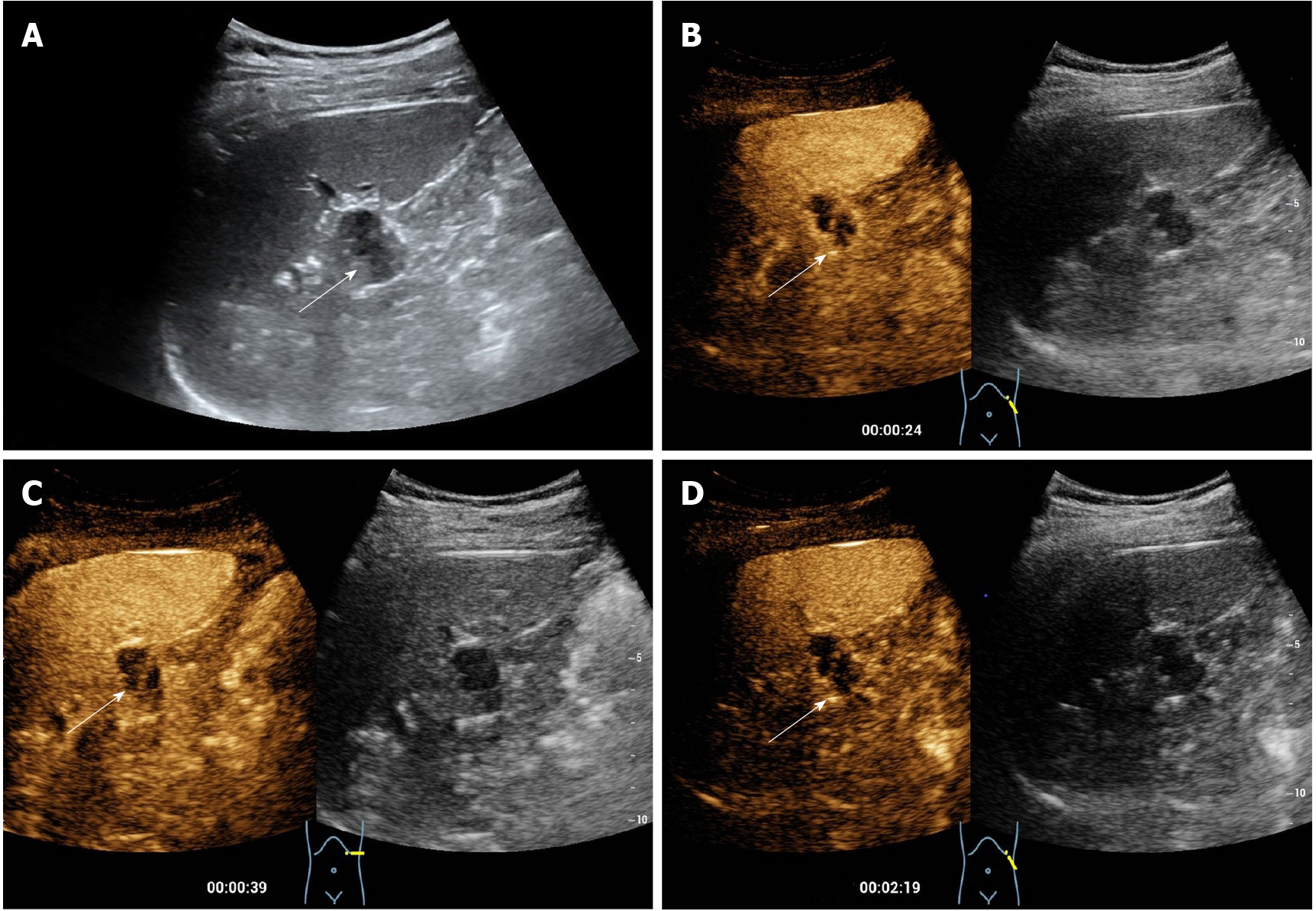
The patient underwent contrast-enhanced ultrasound (CEUS) in our department. Before the CEUS, a baseline ultrasound illustrated a complicated cystic nodule measuring 2 cm, with a well-defined border in the tail of the pancreas without salient blood supply on color Doppler ultrasound (Figure 1). For the CEUS, a bolus injection of the US contrast agent SonoVue (Bracco, Milan, Italy) was administered through the antecubital vein, followed by a flush of 5 mL of 0.9% normal saline. The lesion demonstrated peripheral nodular and internal septal isoenhancement in the arterial phase, followed by slight hyperenhancement of the enhanced area in the venous phase. The predominant cystic area of the lesion did not show any enhancement in either phase. According to the aforementioned enhancing pattern in the CEUS, the lesion was suspected to be a pancreatic cystadenoma via CEUS (Figure 1).
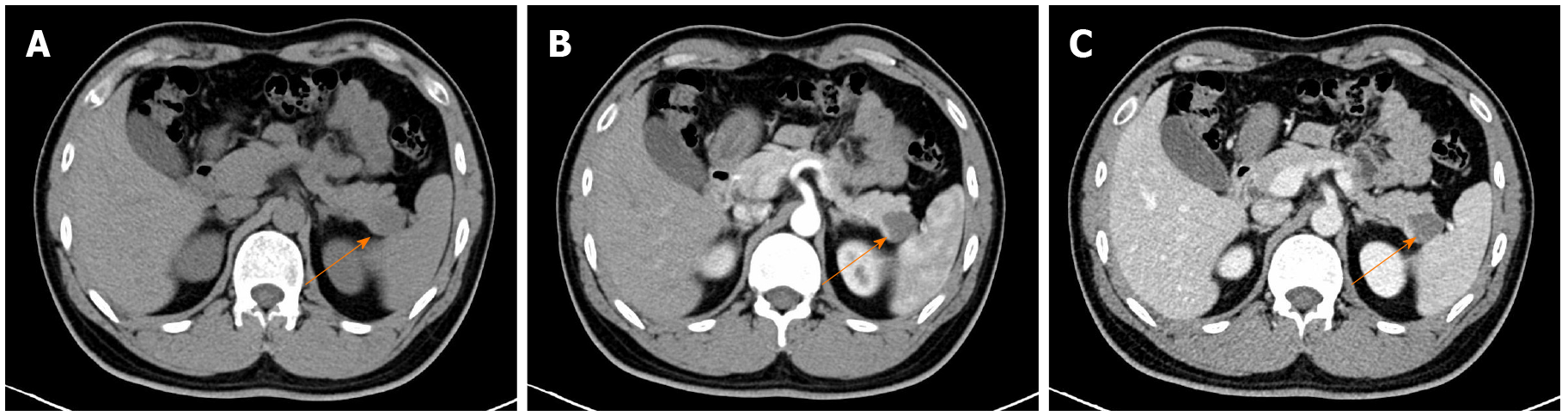
Contrast-enhanced computed tomography (CECT) was performed to further examine the lesion. On the unenhanced CT, a nodule with a diameter of 2.2 cm and slightly low density was identified in the tail of the pancreas. Septa were observed, whereas no significant enhancement was presented within the lesion (Figure 2). The nodule was diagnosed as being a pancreatic cystic lesion via the CECT. Moreover, no salient abnormalities were found in the liver, kidney, spleen or biliary system via imaging evaluations.
The lesion was misdiagnosed as pancreatic cystadenoma by CEUS and CECT.
After multidisciplinary discussion and communication with the patient, as well as with his family, laparoscopic pancreatic body and tail resections were performed.
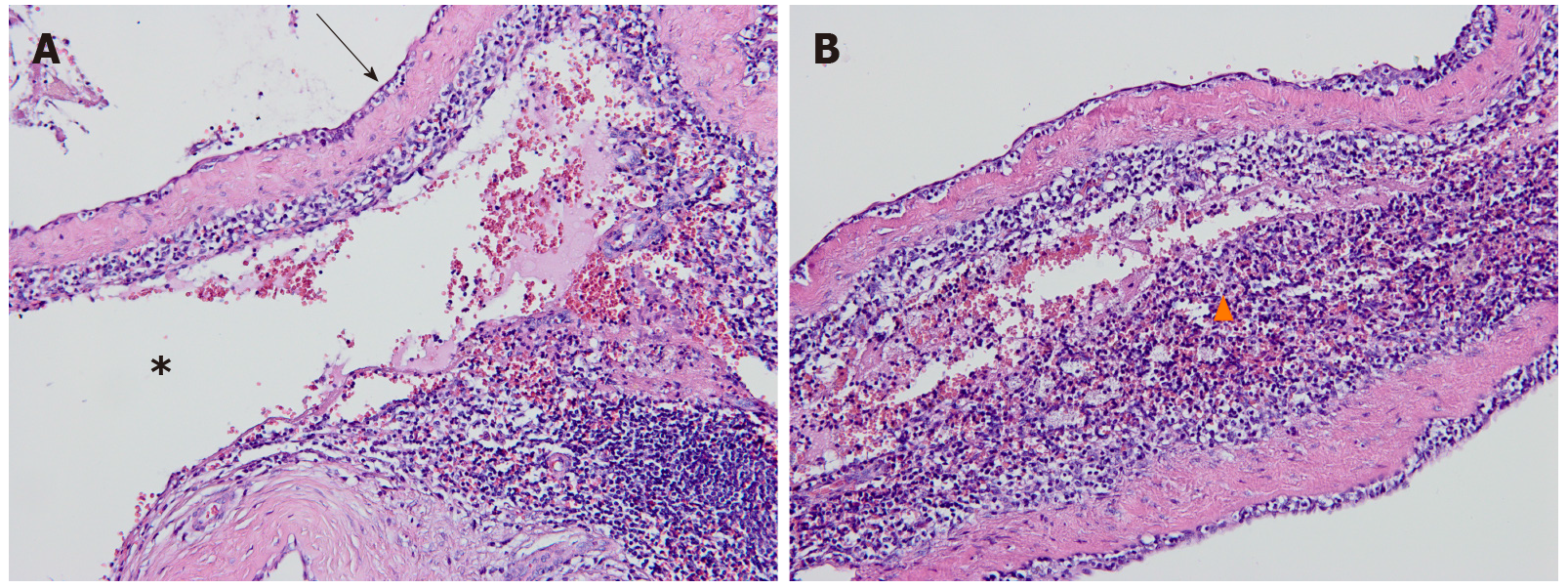
Postoperative pathology demonstrated that the lesion was a splenic cavernous hemangioma in the pancreas (Figure 3). After an uneventful postoperative course, the patient was discharged on postoperative day 5. No obvious abnormality was found in a follow-up abdominal US one month later (Timeline of diagnosis and treatment of the pancreatic lesion is presented in Supplementary Figure 1).
Intrapancreatic accessory spleen is a rare congenital condition, compared with an accessory spleen located at the hilum of the spleen[2,4]. Due to its innocuous nature and infrequent induction of symptoms, IPAS seldom requires therapy unless they cause symptoms as a result of the compression, torsion or spontaneous rupture of a hemorrhage[5,6].
Typical IPAS presents as a solid lesion and demonstrates similar manifestations to the spleen on both precontrast and contrast-enhanced ultrasound[7,8]. However, cystic neoplasm development in IPASs is rare. Sporadic cases of epidermoid cysts in IPASs (known as ECIPASs) have been reported[6,9-11]. The walls of ECIPASs are irregularly thickened and thicker than those of mucinous cystic neoplasms (MCNs) and intraductal papillary mucinous neoplasms (IPMNs)[9]. Moreover, the evident contrast enhancement of the partially thickened wall of ECIPAS (which is similar to that of the spleen) makes it possible to distinguish ECIPASs from MCNs or IPMNs.
The differential diagnosis was even more considerable in our case. The cystic cavernous hemangioma in the IPAS (known as CHIPAS) presented peripheral nodular and internal septal enhancements, which are frequently observed in pancreatic mucinous cystadenomas (MCAs). Furthermore, the majority of MCAs are located in the tail of the pancreas, where IPASs are also frequently discovered[12]. Therefore, this increases the difficulty of an accurate diagnosis. However, the ancillary features of a fibrous pseudocapsule or calcified contents inside of the MCNs have also been reported[13]. Another pancreatic cystic lesion that warrants vigilant discrimination from the CHIPAS is an IPMN. An IPMN in the main duct possesses a high risk of malignancy, with 38%–68% being confirmed as high-grade dysplasia or pancreatic cancer in postoperative specimens[14]. Fortunately, CEUS is sensitive in being demonstrated in the dilated main pancreatic duct and the polycystic lesion connecting to the pancreatic duct or in developing within the duct in cases of IPMNs[15].
To our knowledge, there is only one case report of solid cavernous hemangioma detected in both the spleen and the IPAS[16]. In this case, the CHIPAS was accurately identified by the investigators because of a similar enhancement pattern of the pancreatic lesion and the splenic lesions on CECT and contrast-enhanced magnetic resonance imaging. An accurate diagnosis was more difficult, as in our patient, because there was no lesion in the spleen for comparison. Moreover, a splenic hemangioma typically shows a hyperechoic and solid appearance. The atypical cystic appearance in our patient increased the difficulty of making an accurate diagnosis.
Herein, we presented on an extremely rare case of a cystic cavernous hemangioma arising from an IPAS. Contrast-enhanced ultrasound is sensitive in demonstrating the enhancements of the septa and the parietal nodule. However, an accurate diagnosis of cystic cavernous hemangioma arising from an IPAS via imaging tools is challenging. Imaging follow-ups or surgical interventions may be needed, due to the possible malignancy risk of a complicated cystic lesion with parietal and septal enhancements.
Cavernous hemangioma in the intrapancreatic accessory spleen may mimic pancreatic cystadenoma, which is a condition with the potential for malignancy. Imaging follow-ups or surgical interventions may be helpful for the exclusion of malignant risks in complicated cystic lesions, especially those with parietal and septal enhancements.
The authors would like to express their gratitude to the patient and his family.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Khuroo S, Zharikov YO S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Li BQ, Xu XQ, Guo JC. Intrapancreatic accessory spleen: a diagnostic dilemma. HPB (Oxford). 2018;20:1004-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Halpert B, Gyorkey F. Lesions observed in accessory spleens of 311 patients. Am J Clin Pathol. 1959;32:165-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 181] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 3. | Sica GT, Reed MF. Case 27: intrapancreatic accessory spleen. Radiology. 2000;217:134-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Jang KM, Kim SH, Lee SJ, Park MJ, Lee MH, Choi D. Differentiation of an intrapancreatic accessory spleen from a small (<3-cm) solid pancreatic tumor: value of diffusion-weighted MR imaging. Radiology. 2013;266:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Obuchi T, Takagane A, Sato K, Yonezawa H, Funato O, Kobayashi M. Single-incision laparoscopic excision of symptomatic accessory spleen in the pelvis: An initial report. J Minim Access Surg. 2017;13:321-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Davidson ED, Campbell WG, Hersh T. Epidermoid splenic cyst occurring in an intrapancreatic accessory spleen. Dig Dis Sci. 1980;25:964-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Kim SH, Lee JM, Han JK, Lee JY, Kim KW, Cho KC, Choi BI. Intrapancreatic accessory spleen: findings on MR Imaging, CT, US and scintigraphy, and the pathologic analysis. Korean J Radiol. 2008;9:162-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Kim SH, Lee JM, Lee JY, Han JK, Choi BI. Contrast-enhanced sonography of intrapancreatic accessory spleen in six patients. AJR Am J Roentgenol. 2007;188:422-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Hwang HS, Lee SS, Kim SC, Seo DW, Kim J. Intrapancreatic accessory spleen: clinicopathologic analysis of 12 cases. Pancreas. 2011;40:956-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Yamanishi H, Kumagi T, Yokota T, Koizumi M, Azemoto N, Watanabe J, Mizuno Y, Sugita A, Abe M, Ikeda Y, Matsuura B, Hiasa Y, Onji M. Epithelial cyst arising in an intrapancreatic accessory spleen: a diagnostic dilemma. Intern Med. 2011;50:1947-1952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Kato T, Matsuo Y, Ueda G, Aoyama Y, Omi K, Hayashi Y, Imafuji H, Saito K, Tsuboi K, Morimoto M, Ogawa R, Takahashi H, Kato H, Yoshida M, Naitoh I, Hayashi K, Takahashi S, Takiguchi S. Epithelial cyst arising in an intrapancreatic accessory spleen: a case report of robotic surgery and review of minimally invasive treatment. BMC Surg. 2020;20:263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Crippa S, Salvia R, Warshaw AL, Domínguez I, Bassi C, Falconi M, Thayer SP, Zamboni G, Lauwers GY, Mino-Kenudson M, Capelli P, Pederzoli P, Castillo CF. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. Ann Surg. 2008;247:571-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 274] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 13. | De Robertis R, D'Onofrio M, Crosara S, Dal Corso F, Barbi E, Canestrini S, Mucelli RP. Contrast-enhanced ultrasound of pancreatic tumours. Australas J Ultrasound Med. 2014;17:96-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Stark A, Donahue TR, Reber HA, Hines OJ. Pancreatic Cyst Disease: A Review. JAMA. 2016;315:1882-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 177] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 15. | Wang Y, Wang Y, Fan Z, Shan J, Yan K. The Value of Contrast-Enhanced Ultrasound Classification in Diagnosis of Pancreatic Cystic Lesions. Biomed Res Int. 2019;2019:5698140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Makino I, Tajima H, Kitagawa H, Nakagawara H, Ohta T. A rare case of hemangiomatosis of the spleen and intrapancreatic accessory spleen. Abdom Imaging. 2014;39:1169-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Choi JY, Kim MJ, Lee JY, Lim JS, Chung JJ, Kim KW, Yoo HS. Typical and atypical manifestations of serous cystadenoma of the pancreas: imaging findings with pathologic correlation. AJR Am J Roentgenol. 2009;193:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Garcea G, Ong SL, Rajesh A, Neal CP, Pollard CA, Berry DP, Dennison AR. Cystic lesions of the pancreas. A diagnostic and management dilemma. Pancreatology. 2008;8:236-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Vasile TA, Socaciu M, Stan Iuga R, Seicean A, Iancu C, al Hajjar N, Zaharie T, Badea R. Added value of intravenous contrast-enhanced ultrasound for characterization of cystic pancreatic masses: a prospective study on 37 patients. Med Ultrason. 2012;14:108-114. [PubMed] |
| 20. | Misra AP, Misra R, Kumar A. Giant cavernous haemangioma of the wandering spleen. Indian J Surg. 2013;75:54-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Yamashita Y, Ueda K, Itonaga M, Yoshida T, Maeda H, Maekita T, Iguchi M, Tamai H, Ichinose M, Kato J. Usefulness of contrast-enhanced endoscopic sonography for discriminating mural nodules from mucous clots in intraductal papillary mucinous neoplasms: a single-center prospective study. J Ultrasound Med. 2013;32:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Lee JE, Choi SY, Min JH, Yi BH, Lee MH, Kim SS, Hwang JA, Kim JH. Determining Malignant Potential of Intraductal Papillary Mucinous Neoplasm of the Pancreas: CT versus MRI by Using Revised 2017 International Consensus Guidelines. Radiology. 2019;293:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 23. | Tang JY, Chen J, Pan C, Yin MZ, Zhu M. Diffuse cavernous hemangioma of the spleen with Kasabach-Merritt syndrome misdiagnosed as idiopathic thrombocytopenia in a child. World J Pediatr. 2008;4:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Kang BK, Kim JH, Byun JH, Lee SS, Kim HJ, Kim SY, Lee MG. Diffusion-weighted MRI: usefulness for differentiating intrapancreatic accessory spleen and small hypervascular neuroendocrine tumor of the pancreas. Acta Radiol. 2014;55:1157-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |