Published online Feb 26, 2022. doi: 10.12998/wjcc.v10.i6.1937
Peer-review started: August 4, 2021
First decision: October 16, 2021
Revised: October 26, 2021
Accepted: January 11, 2022
Article in press: January 11, 2022
Published online: February 26, 2022
Processing time: 203 Days and 10 Hours
Vascular complications of transradial percutaneous coronary intervention (PCI) are rare and usually occur at the access site below the elbow. Life-threatening vascular complications during transradial PCI therapy, such as vessel perforation and dissection in the brachiocephalic, subclavian, internal mammary, and thyrocervical arteries, are rarely reported. Subclavian artery bleeding is a potentially serious complication of vascular interventional procedures leading to tracheal obstruction, hemothorax, respiratory failure, hemorrhagic shock, and death if not diagnosed early and treated promptly.
A male patient with typical angina pectoris underwent coronary angiography and stent implantation. During the procedure, the patient felt pharyngeal pain and tightness, which we mistook for myocardial ischemia. After PCI, swelling in the right neck and supraclavicular area was observed. The patient experienced dyspnea, emergency endotracheal intubation was performed, and then a sudden drop in blood pressure was observed. Ultrasound and contrast-enhanced computed tomography scans demonstrated a cervical hematoma severely compressing the trachea due to subclavian artery bleeding. Brachiocephalic angiography revealed a vascular injury site at the root of the right subclavian artery at the intersection of the right common carotid artery. A covered stent was deployed to the right subclavian artery with successful sealing of the perforation, and a bare stent was implanted in the junction of the right common carotid and brachiocephalic arteries to prevent obstruction of blood flow to the brain.
Subclavian artery bleeding is a lifethreatening complication of PCI. Early prevention, rapid recognition, and prompt treatment may improve the prognosis.
Core Tip: Subclavian artery bleeding is a rare and serious complication of transradial percutaneous coronary intervention leading to tracheal obstruction, hemothorax, respiratory failure, hemorrhagic shock, and death if not diagnosed early and treated promptly. Bleeding at the root of the subclavian artery might manifest as pharyngeal pain and cervical hematoma, which requires the prompt decision to perform emergency endotracheal intubation. Computed tomography scans should be performed as early as possible for patients with suspected hematoma. Endovascular treatment with covered stents appears to be less time consuming and more effective, especially for large, life-threatening perforations, with great success rates and immediate control of bleeding.
- Citation: Shi F, Zhang Y, Sun LX, Long S. Life-threatening subclavian artery bleeding following percutaneous coronary intervention with stent implantation: A case report and review of literature. World J Clin Cases 2022; 10(6): 1937-1945
- URL: https://www.wjgnet.com/2307-8960/full/v10/i6/1937.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i6.1937
Percutaneous coronary intervention (PCI) is an extensively used medical therapy for acute and chronic coronary artery disease (CAD)[1,2]. Vascular complications of transradial PCI are rare and usually occur at the access site below the elbow[3-6]. A study population consisting of 1984998 PCI procedures performed at 1292 participating sites demonstrated that the median rate of bleeding at the access site was 0.2%-0.5%[7]. Life-threatening vascular complications during transradial PCI therapy, such as vessel perforation and dissection in the brachiocephalic, subclavian, internal mammary, and thyrocervical arteries, are rarely reported and can lead to mortality due to acute respiratory and circulatory dysfunction[8-10]. The present study reports a case of subclavian artery bleeding following PCI, which caused severe cervical hematoma, tracheal compression, and unstable hemodynamics.
A 59-year-old Chinese man presented to the outpatient department of our hospital with retrosternal chest pain.
Over the prior 12 years, the patient had experienced intermittent retrosternal pain on effort with palpitations, shortness of breath, and fatigue lasting from 5 to 10 min. Because of the manifestation of typical angina pectoris, he was admitted to our institution for coronary angiography (CAG) 10 years ago. CAG showed severe stenosis in the mid-segment of the left anterior descending coronary artery (LAD), and 3 stents were implanted. Since that time, he had been treated with aspirin, clopidogrel, statins, and nitrate. However, his chest pain had recently returned and occurred more frequently at 1 to 2 times a day.
The patient had a history of hypertension for 30 years and poor blood pressure control (blood pressure ranging from 150/100 mmHg to 205/120 mmHg), even after treatment with felodipine and enalapril. Secondary hypertension was excluded after an outpatient evaluation. He suffered a cerebral hemorrhage one year ago. Due to the small size of the cerebral hemorrhage, no sequelae were noted.
He had been smoking approximately 60 cigarettes a day for 30 years. No significant family history was noted.
Physical examination demonstrated poorly controlled hypertension with a blood pressure of 180/105 mmHg. His body weight was 65 kg, and his height was 166 cm (body mass index 23.9 kg/m2). Jugular venous distention was not detected, and the thyroid gland was not enlarged. The breath sounds of the two lungs were normal, and no dry or moist rales were detected. Heart auscultation revealed a regular rhythm, normal heart sounds without murmur, and a heart rate of 63 bpm. The liver and spleen were normal, and no swelling was observed in the lower extremities.
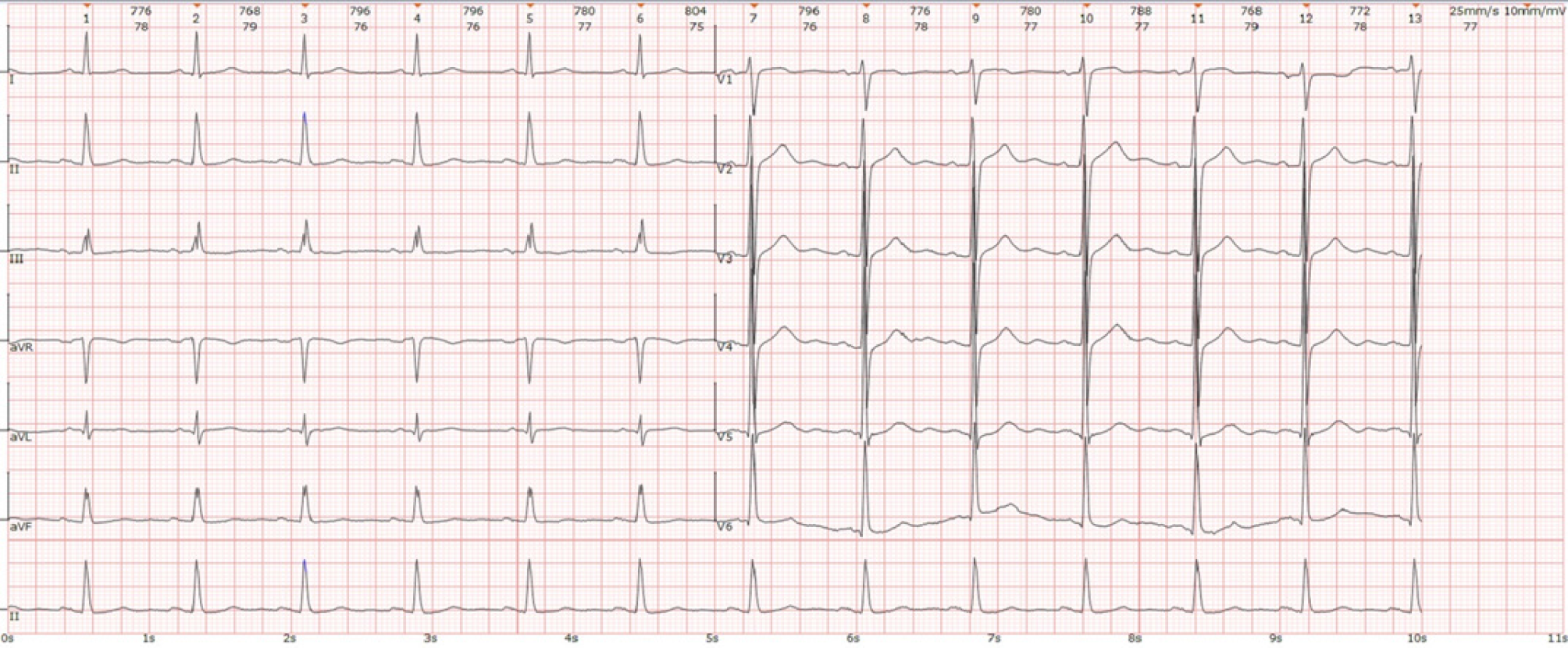
Electrocardiography indicated sinus rhythm and a high-voltage left ventricle but no significant change in the ST segment or T wave (Figure 1). Echocardiography demonstrated symmetrical left ventricular hypertrophy (wall thickness 13-14 mm, normal reference ≤ 11 mm), left atrial enlargement (42 mm, normal reference ≤ 35 mm), mild mitral regurgitation, and some reduction in left ventricular diastolic function[11]. Ultrasound of lower limb arteries revealed a rough intima and spotty calcified plaques without significant stenosis. All these observations suggested hypertensive heart disease and peripheral vascular atherosclerosis. No other abnormalities were present in other examinations, including routine blood tests (platelet count 211 × 109/L, normal reference 125-350 × 109/L), coagulogram, liver and kidney function, blood electrolyte levels, blood glucose, blood lipids, thyroid gland function, myocardial damage markers, and brain natriuretic peptide, a biomarker of heart failure.
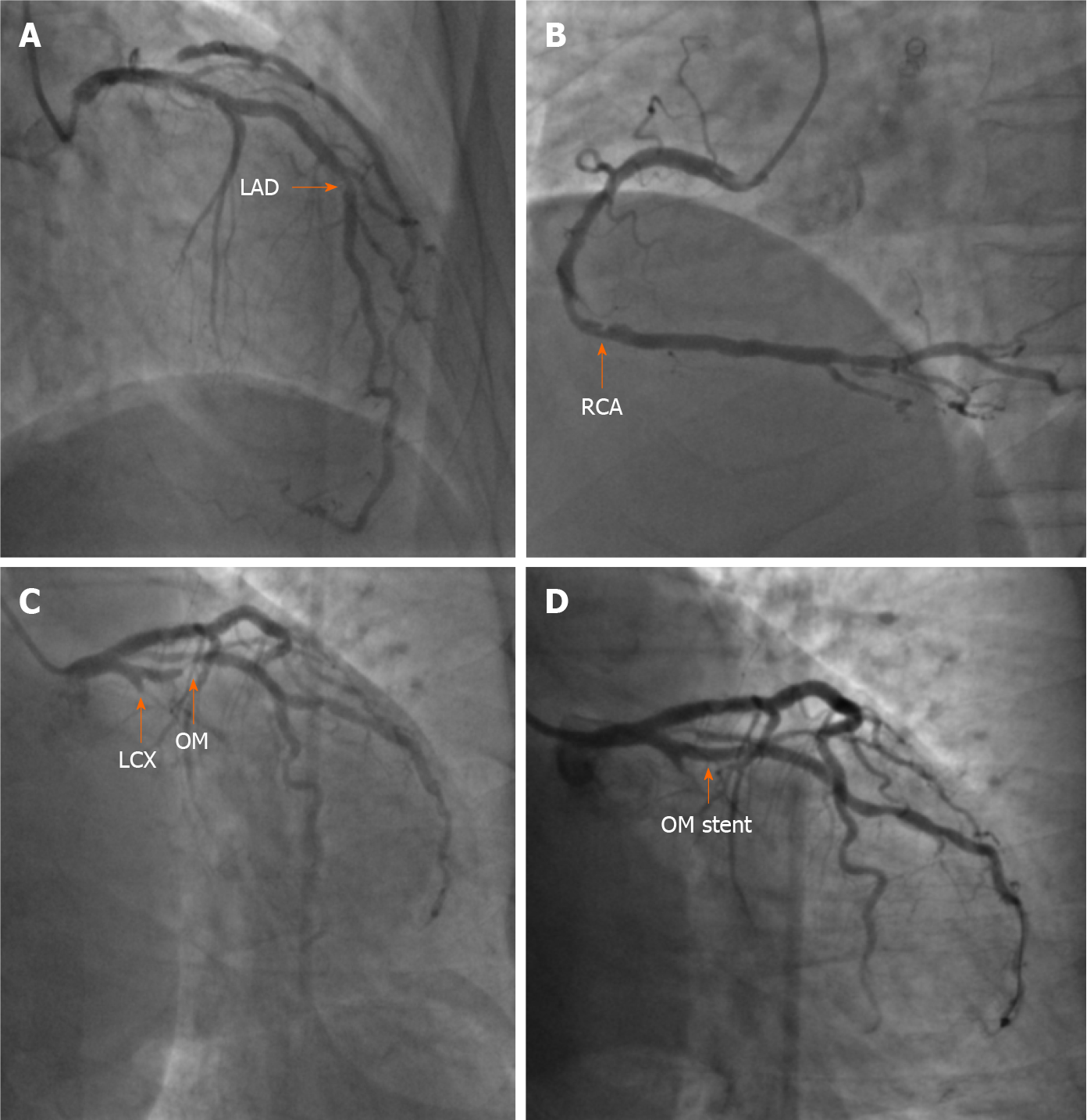
The patient was preliminarily diagnosed with CAD, unstable angina, grade 3 hypertension, and hypertensive heart disease[12-14]. He was treated with aspirin (100 mg daily), clopidogrel (75 mg daily), atorvastatin (20 mg daily), isosorbide mononitrate (20 mg twice daily), amlodipine (5 mg twice daily), and enalapril (5 mg twice daily). For the coronary anatomy evaluation, CAG was performed by puncturing the right brachial artery because the right radial pulse was absent due to the PCI 10 years prior. A 6 French (F) sheath, a 0.035 cm × 260 cm J-Tip guidewire (Cordis, Ireland), and a 5 F 100-cm Tig coronary catheter (Terumo, Japan) were used during CAG under fluoroscopic guidance. CAG showed total occlusion of the proximal left circumflex artery (LCX), subtotal occlusion at the opening of the first obtuse marginal branch (OM) and 60%-70% in-stent restenosis at the middle LAD. In addition, 60%-70% stenosis of the distal right coronary artery was observed (Figure 2). For PCI of the left coronary artery, the 5 F angiography catheter was replaced with a 6 F EBU 3.5 A LAUNCHER coronary catheter (Medtronic, United States) and a 0.014-cm × 180-cm ASAHI SION coronary guidewire (ASAHI INTECC, Japan), which were passed through the distal OM. Then, repeated attempts were made to pass through the proximal LCX using a 0.014-cm × 180-cm ASAHI SION blue coronary guidewire (ASAHI INTECC), a 0.014-cm × 190-cm Fielder XT-R coronary guidewire (ASAHI INTECC), and a 1.8 F 0.018-cm × 130-cm FINECROSS coronary microcatheter (Terumo, Japan), but these attempts failed. We then decided to address the stenosis of the OM. During balloon angioplasty with a 2.5-mm × 12-mm TREK balloon (Abbott, United States), the patient began to feel pharyngeal pain and tightness, which we mistook for myocardial ischemia. Stenosis was eliminated by implanting a 2.75-mm × 16-mm PROMUS Element Plus stent (Boston Scientific, United States) in the proximal segment of the OM (Figure 2). PCI was terminated after high-pressure balloon angioplasty using a 2.75-mm × 12-mm NC Demax noncompliant balloon (Demax Medical Technology, Beijing, China). The procedure lasted 52 min, and 6000 units of heparin was administered through the artery. The activated clotting time measured immediately after the operation was 305 s (normal reference 80-120 s).
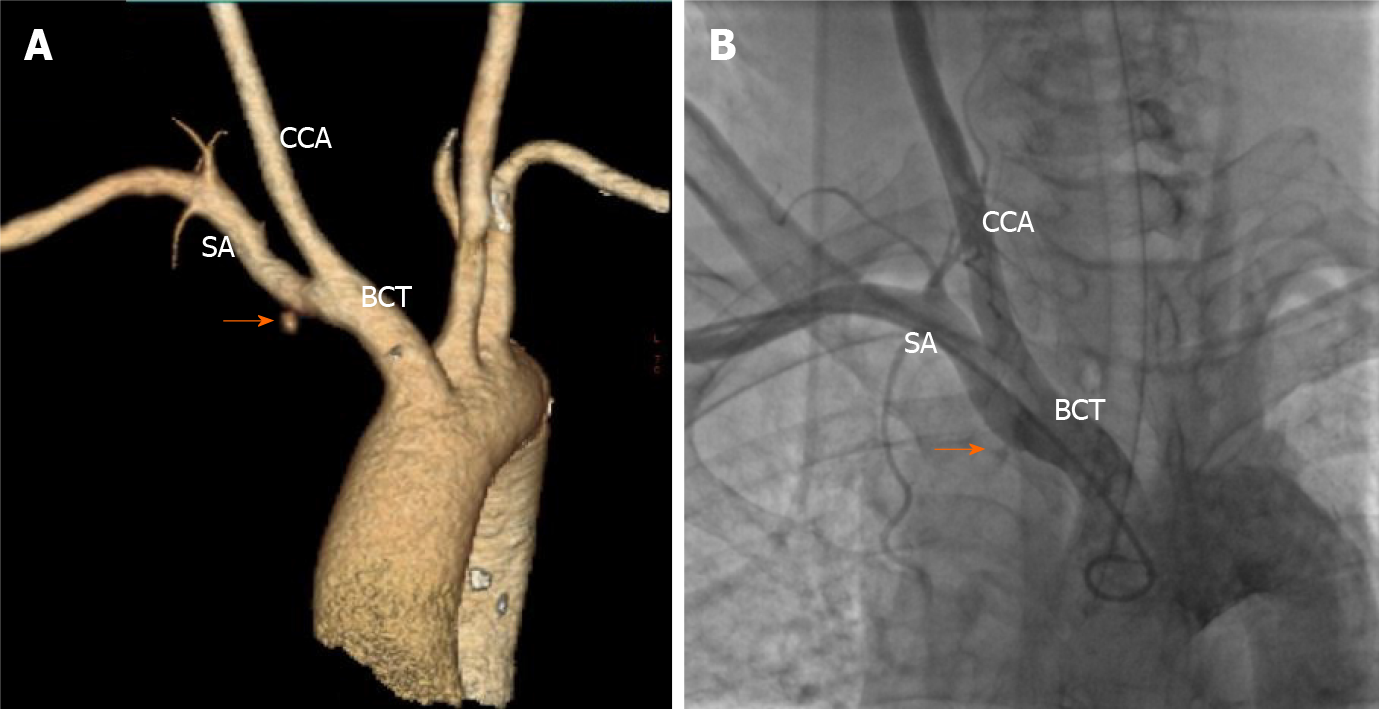
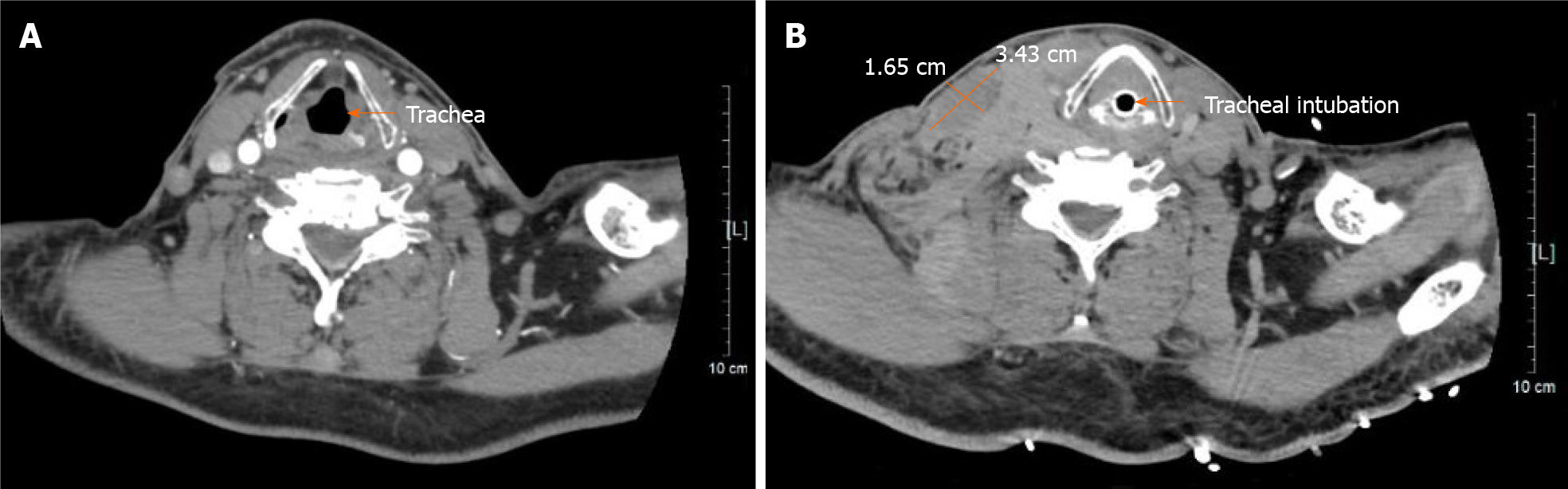
After the patient was returned to the critical care unit, swelling in the right neck and right supraclavicular area was observed and gradually increased, with bleeding spots under the skin. The patient felt dyspneic, and physical examination showed that the trachea was compressed to the left. Bedside ultrasound demonstrated a poorly defined hematoma behind the right internal jugular vein measuring 26.0 mm × 30.1 mm × 12.3 mm. A contrast-enhanced computed tomography (CT) scan performed immediately thereafter also showed contrast extravasation surrounding the proximal subclavian artery (Figure 3) and a cervical hematoma compressing the trachea (Figure 4), but no hemothorax, subclavian artery rupture or bleeding was diagnosed.
The final diagnosis was CAD, unstable angina, grade 3 hypertension, hypertensive heart disease, and subclavian artery bleeding.
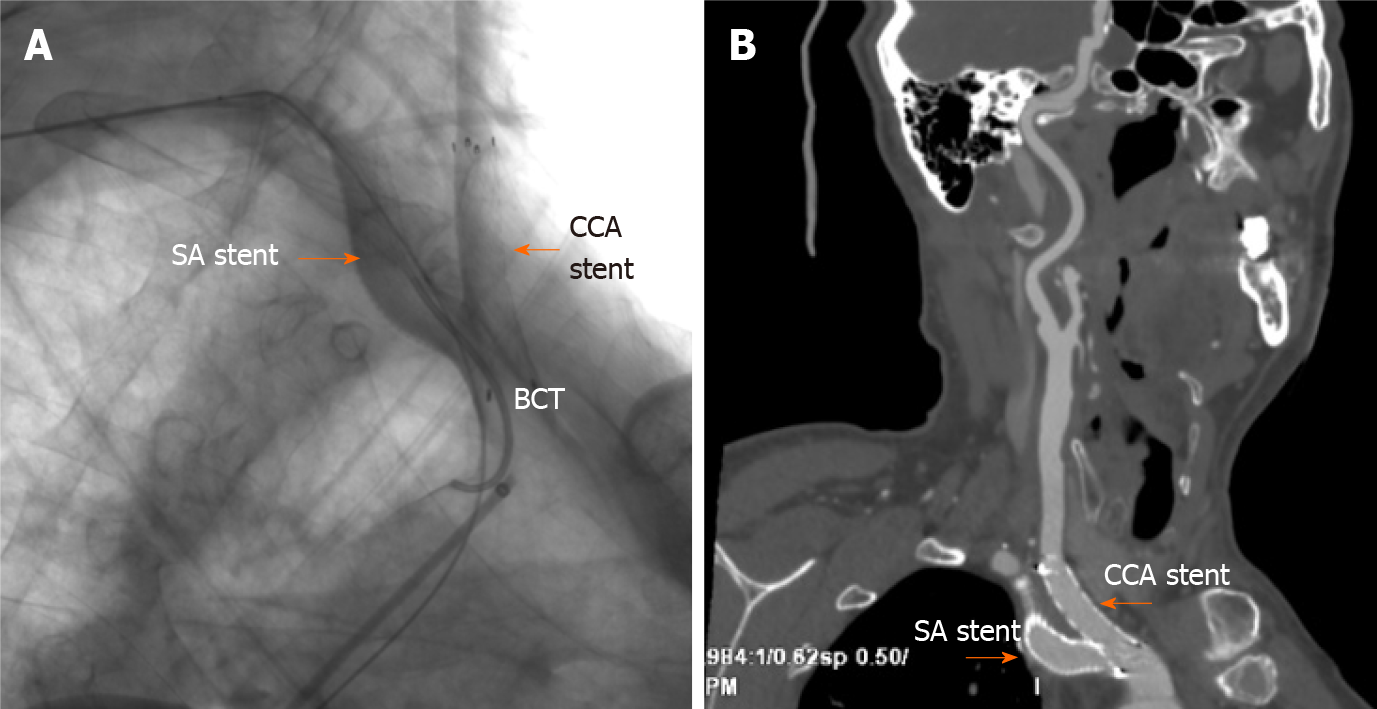
Emergency endotracheal intubation was successfully performed to avoid asphyxia. However, during rescue intubation, the patient’s blood pressure dropped sharply from 175/89 mmHg to 88/52 mmHg and recovered to 128/66 mmHg by intravenous fluid hydration and vasopressor support with norepinephrine and dopamine. A concerning finding on the CT scan was that the trachea was tightly affixed to the tube, which would complicate later intubation. Hypotension was thought to be due to hemorrhage and aggravated by sedation, which was supported by subsequent routine blood monitoring showing a reduction in hemoglobin from 138 g/L to 91 g/L (normal reference 130-175 g/L). The carotid sinus baroreflex might have also been a cause of exacerbated hypotension based on the location of the hematoma. Subclavian artery injury occurred at the site where it was not compressable. Obtaining adequate proximal exposure of the subclavian artery is an intricate process, and antiplatelet agents and heparin may complicate hemostasis during open surgical repair; thus, endovascular intervention was suggested. Brachiocephalic angiography from the right brachial access revealed a vascular injury site at the root of the right subclavian artery at the intersection of the right common carotid artery (Figure 3). An 11-mm × 50-mm covered stent was deployed to the right subclavian artery with successful sealing of the perforation via a femoral artery approach. Since the proximal segment of the covered stent was located at the brachiocephalic artery, a 10-mm × 40-mm bare stent was implanted in the junction of the right common carotid and brachiocephalic arteries to prevent obstruction of blood flow to the brain (Figure 5). Digital subtraction angiography after stent graft deployment showed that the rupture was repaired, and no contrast extravasation was observed.
The patient was moved to the intensive care unit for ventilator support and close hemodynamic monitoring. To avoid fatal tracheal compression, endotracheal intubation and ventilator care were maintained for 4 d while the cervical hematoma was gradually absorbed without discontinuation of aspirin and clopidogrel due to the coronary stent. The patient was discharged from the hospital on Day 10 after PCI. After discharge, he had good compliance with medication and smoking cessation. At the 1.5-mo follow-up, the patient had no complaints, and contrast-enhanced CT showed that the cervical hematoma was completely absorbed and that the two stent grafts in the right subclavian artery and common carotid artery were in a satisfactory position and unobstructed (Figure 5).
Transradial PCI is recommended as the preferred method by various guidelines due to its significant reduction in vascular bleeding complications[15-18]. However, if arterial injury from the transradial approach occurs at the access site, especially where it cannot be compressed, the injury could be fatal. Subclavian artery bleeding is a potentially serious complication of vascular interventional procedures leading to tracheal obstruction, hemothorax, respiratory failure, hemorrhagic shock, and death if not diagnosed early and treated promptly[19]. The total number of PCIs performed in our center is 1500 to 2000 per year, and the number of transradial PCIs performed by each clinician is approximately 200 per year. This is the first case of subclavian artery bleeding in our center in the past 5 years (Table 1).
| Date | Events |
| June 1, 2011 | Angina pectoris was reported |
| June 25, 2011 | CAG showed severe stenosis in the left anterior descending artery, and 3 stents were implanted |
| February 11, 2019 | Cerebral hemorrhage was identified |
| December 11, 2020; January 11, 2021 | Recurrent chest pain was reported; Unstable angina was diagnosed |
| January 15, 2021 | CAG revealed severe coronary artery stenosis, and a stent was implanted; After PCI, subclavian artery bleeding was diagnosed; Emergency endotracheal intubation and covered stent implantation were performed |
| January 19, 2021; February 15, 2021 | The endotracheal tube was removed; The patient was discharged |
| March 1, 2021 | At the follow-up, the patient had no complaints, and a CT scan showed the stent in the subclavian artery and an unobstructed common carotid artery |
Some of the reported predictors for vascular injury associated with interventional procedures are divided into potentially modifiable and nonmodifiable factors. Potentially modifiable factors include a larger sheath size, excessive guidewire manipulation, manual inflation, an oversized balloon, procedure time, anticoagulation or thrombolytics, and high intraoperative blood pressure. Nonmodifiable factors include diffuse vascular calcification, vascular tortuosity, vascular stenosis, female sex, advanced age, chronic kidney disease, a past history of hypertension and/or diabetes, prior stroke, connective tissue disease, and long-term steroid therapy[20-24]. In this case, the guidewire and coronary catheter were inserted under continuous fluoroscopy, and the guidewire was not observed to penetrate the blood vessel. We speculated that calcified plaques and even stenosis may have been present in the subclavian artery, as the presence of calcified plaques in the lower limb arteries and diffuse coronary artery stenosis suggested that the patient had a high possibility of systemic atherosclerosis. Subclavian artery hemorrhage might have been caused by plaque damage during the interventional procedure and aggravated by antithrombotic agents and hypertension. Another cause may have been spontaneous bleeding, and aneurysms cannot be excluded.
The patient felt pharyngeal pain and tightness during balloon angioplasty, which we mistook for myocardial ischemia. We ignored the fact that these symptoms were not relieved after stent implantation, which would be inconsistent with intraoperative myocardial ischemia. This case suggested that bleeding at the root of the subclavian artery might manifest as pharyngeal pain and cervical hematoma. The patient experienced dyspnea as the cervical hematoma progressed, compressing the trachea, which needed to be differentiated from a contrast-induced allergic reaction and required a prompt decision to proceed with emergency endotracheal intubation[25].
The management of this vascular bleeding complication includes open surgical repair and multiple percutaneous interventions, including repeatedly prolonged balloon tamponade, covered stent grafts, collagen plugs, and localized thrombin injection[26-29]. Some clinicians have used balloon tamponade for a few minutes as a treatment for vascular bleeding, but this method is thought to be unreliable, as bleeding may resume later, especially in the great vessels. Although multiple therapeutic modalities have been applied to seal vascular injury, endovascular treatment with covered stents appears to be less time-consuming and more effective, especially for large, life-threatening perforations, with high success rates of immediate control of bleeding[30,31].
Preventing vascular bleeding complications of PCI is also important. Careful assessment of the individual risk should guide the choice of strategy during different stages of interventional procedures. Avoiding excessive manipulation, controlling intraoperative blood pressure and the activated clotting time, and timely adjustment or termination of the procedure can reduce the occurrence of vascular bleeding complications. Reasons to stop a PCI attempt include a high radiation dose (> 5 Gy air kerma dose), large contrast volume administration (> 3.7 × the estimated creatinine clearance), exhaustion of crossing options, or patient or physician fatigue[32].
Subclavian artery bleeding is a lifethreatening complication of PCI. Bleeding at the root of the subclavian artery might manifest as pharyngeal pain and cervical hematoma and requires a prompt decision to proceed with emergency endotracheal intubation. Rapid recognition and prompt treatment with covered stents may significantly improve the prognosis for these patients. Careful assessment of the individual risk and standardized and appropriate interventional procedures can reduce the occurrence of vascular bleeding complications.
The authors thank the patient and his family for their participation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cimen SG, Govindarajan KK S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, Smith PK. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 Appropriate Use Criteria for Coronary Revascularization in Patients With Stable Ischemic Heart Disease: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;69:2212-2241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 482] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 2. | Bhatt DL. Percutaneous Coronary Intervention in 2018. JAMA. 2018;319:2127-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 3. | Choi S, Joh JH, Choe JW. Fatal vascular complications during transradial percutaneous coronary intervention: A case report. Medicine (Baltimore). 2020;99:e21205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Tatli E, Buturak A, Cakar A, Vatan BM, Degirmencioglu A, Agac TM, Kilic H, Gunduz H, Akdemir R. Unusual Vascular Complications Associated with Transradial Coronary Procedures Among 10,324 Patients: Case Based Experience and Treatment Options. J Interv Cardiol. 2015;28:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Kandan SR, Johnson TW. Management of percutaneous coronary intervention complications. Heart. 2019;105:75-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Sandoval Y, Bell MR, Gulati R. Transradial Artery Access Complications. Circ Cardiovasc Interv. 2019;12:e007386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 7. | Hess CN, Rao SV, McCoy LA, Neely ML, Singh M, Spertus JA, Krone RJ, Weaver WD, Peterson ED. Identification of hospital outliers in bleeding complications after percutaneous coronary intervention. Circ Cardiovasc Qual Outcomes. 2015;8:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Luo X, Shi W, Zhang X, Yang X, Wang W, Zeng C, Wang H. Multiple-site bleeding at pleural adhesions and massive hemothorax following percutaneous coronary intervention with stent implantation: A case report. Exp Ther Med. 2018;15:2351-2355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Giannini F, Candilio L, Mitomo S, Ruparelia N, Chieffo A, Baldetti L, Ponticelli F, Latib A, Colombo A. A Practical Approach to the Management of Complications During Percutaneous Coronary Intervention. JACC Cardiovasc Interv. 2018;11:1797-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 10. | Farooqi F, Alexander J, Sarma A. Rare vascular perforation complicating radial approach to percutaneous coronary angioplasty. BMJ Case Rep. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Quiñones MA, Douglas PS, Foster E, Gorcsan J 3rd, Lewis JF, Pearlman AS, Rychik J, Salcedo EE, Seward JB, Stevenson JG, Thys DM, Weitz HH, Zoghbi WA, Creager MA, Winters WL Jr, Elnicki M, Hirshfeld JW Jr, Lorell BH, Rodgers GP, Tracy CM; American Society of Echocardiography; Society of Cardiovascular Anesthesiologists; Society of Pediatric Echocardiography. ACC/AHA clinical competence statement on echocardiography: a report of the American College of Cardiology/American Heart Association/American College of Physicians-American Society of Internal Medicine Task Force on clinical competence. J Am Soc Echocardiogr. 2003;16:379-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Jellinger PS, Handelsman Y, Rosenblit PD, Bloomgarden ZT, Fonseca VA, Garber AJ, Grunberger G, Guerin CK, Bell DSH, Mechanick JI, Pessah-Pollack R, Wyne K, Smith D, Brinton EA, Fazio S, Davidson M. American association of clinical endocrinologists and American college of endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23:1-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 674] [Article Influence: 112.3] [Reference Citation Analysis (0)] |
| 13. | Touyz RM, Dominiczak AF. Hypertension Guidelines: Is It Time to Reappraise Blood Pressure Thresholds and Targets? Hypertension. 2016;67:688-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Slivnick J, Lampert BC. Hypertension and Heart Failure. Heart Fail Clin. 2019;15:531-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 149] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 15. | Mason PJ, Shah B, Tamis-Holland JE, Bittl JA, Cohen MG, Safirstein J, Drachman DE, Valle JA, Rhodes D, Gilchrist IC; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; and Council on Genomic and Precision Medicine. An Update on Radial Artery Access and Best Practices for Transradial Coronary Angiography and Intervention in Acute Coronary Syndrome: A Scientific Statement From the American Heart Association. Circ Cardiovasc Interv. 2018;11:e000035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 354] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 16. | Kolkailah AA, Alreshq RS, Muhammed AM, Zahran ME, Anas El-Wegoud M, Nabhan AF. Transradial vs transfemoral approach for diagnostic coronary angiography and percutaneous coronary intervention in people with coronary artery disease. Cochrane Database Syst Rev. 2018;4:CD012318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 17. | Goel S, Pasam RT, Raheja H, Gotesman J, Gidwani U, Ahuja KR, Reed G, Puri R, Khatri JK, Kapadia SR. Left main percutaneous coronary intervention-Radial vs femoral access: A systematic analysis. Catheter Cardiovasc Interv. 2020;95:E201-E213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Chugh Y, Bavishi C, Mojadidi MK, Elgendy IY, Faillace RT, Brilakis ES, Tamis-Holland J, Mamas M, Chugh SK. Safety of transradial access compared to transfemoral access with hemostatic devices (vessel plugs and suture devices) after percutaneous coronary interventions: A systematic review and meta-analysis. Catheter Cardiovasc Interv. 2020;96:285-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Yang W, Qiao S, Liu R, Hu F, Qin X, Dou K, Gao L, Liu H, Wu Y, Zhang J, Qiu H, Chen J, Yang Y. [Clinical features and outcome of eight patients with mediastinal and neck hematoma after transradial cardiac catheterization approach]. Zhonghua Xin Xue Guan Bing Za Zhi. 2014;42:406-412. [PubMed] |
| 20. | Awan MU, Omar B, Qureshi G, Awan GM. Successful Treatment of Iatrogenic External Iliac Artery Perforation With Covered Stent: Case Report and Review of the Literature. Cardiol Res. 2017;8:246-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Kim M, Chu A, Khan Y, Malik S. Predicting and preventing vascular complications following percutaneous coronary intervention in women. Expert Rev Cardiovasc Ther. 2015;13:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Perl L, Bental T, Vaknin-Assa H, Assali A, Codner P, Talmor-Barkan Y, Greenberg G, Samara A, Witberg G, Orvin K, Kornowski R. Independent Impact of Peripheral Artery Disease on Percutaneous Coronary Intervention. J Am Heart Assoc. 2020;9:e017655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Natsuaki M, Morimoto T, Yamaji K, Watanabe H, Yoshikawa Y, Shiomi H, Nakagawa Y, Furukawa Y, Kadota K, Ando K, Akasaka T, Hanaoka KI, Kozuma K, Tanabe K, Morino Y, Muramatsu T, Kimura T; CREDO‐Kyoto PCI/CABG Registry Cohort 2, RESET, and NEXT trial investigators. Prediction of Thrombotic and Bleeding Events After Percutaneous Coronary Intervention: CREDO-Kyoto Thrombotic and Bleeding Risk Scores. J Am Heart Assoc. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 154] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 24. | Brener SJ, Kirtane AJ, Rinaldi MJ, Stuckey TD, Witzenbichler B, Weisz G, Neumann FJ, Metzger DC, Henry TD, Cox DA, Duffy PL, Mazzaferri EL Jr, Gurbel PA, Brodie BR, Mehran R, McAndrew T, Stone GW. Prediction of Ischemic and Bleeding Events Using the Dual Antiplatelet Therapy Score in an Unrestricted Percutaneous Coronary Intervention Population. Circ Cardiovasc Interv. 2018;11:e006853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Wu YW, Leow KS, Zhu Y, Tan CH. Prevention and Management of Adverse Reactions Induced by Iodinated Contrast Media. Ann Acad Med Singap. 2016;45:157-164. [PubMed] |
| 26. | Evans C, Chaplin T, Zelt D. Management of Major Vascular Injuries: Neck, Extremities, and Other Things that Bleed. Emerg Med Clin North Am. 2018;36:181-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Salazar GM, Walker TG. Evaluation and management of acute vascular trauma. Tech Vasc Interv Radiol. 2009;12:102-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Burzotta F, Mariani L, Trani C, Coluccia V, Brancati MF, Porto I, Leone AM, Niccoli G, Tommasino A, Tinelli G, Mazzari MA, Mongiardo R, Snider F, Schiavoni G, Crea F. Management and timing of access-site vascular complications occurring after trans-radial percutaneous coronary procedures. Int J Cardiol. 2013;167:1973-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Balasundaram P, Sebastian LJD, Jain N, Prabhakar A, Garg A, Gaikwad S. Management of Arterial Pseudoaneurysms of the Neck in a Pediatric Population: An Endovascular Case Series and Review of Literature. World Neurosurg. 2019;125:e273-e281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Tian X, Liu JL, Jia W, Jiang P, Cheng ZY, Zhang YX, Li JY, Tian CY. Comparison of traditional vascular reconstruction with covered stent in the treatment of subclavian artery injury. Chin J Traumatol. 2020;23:25-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Ruffino MA, Fronda M, Varello S, Discalzi A, Mancini A, Muratore P, Rossato D, Bergamasco L, Righi D, Fonio P. Emergency management of iatrogenic arterial injuries with a low-profile balloon-expandable stent-graft: Preliminary results. Medicine (Baltimore). 2020;99:e19655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Brilakis ES, Mashayekhi K, Tsuchikane E, Abi Rafeh N, Alaswad K, Araya M, Avran A, Azzalini L, Babunashvili AM, Bayani B, Bhindi R, Boudou N, Boukhris M, Božinović NŽ, Bryniarski L, Bufe A, Buller CE, Burke MN, Büttner HJ, Cardoso P, Carlino M, Christiansen EH, Colombo A, Croce K, Damas de Los Santos F, De Martini T, Dens J, Di Mario C, Dou K, Egred M, ElGuindy AM, Escaned J, Furkalo S, Gagnor A, Galassi AR, Garbo R, Ge J, Goel PK, Goktekin O, Grancini L, Grantham JA, Hanratty C, Harb S, Harding SA, Henriques JPS, Hill JM, Jaffer FA, Jang Y, Jussila R, Kalnins A, Kalyanasundaram A, Kandzari DE, Kao HL, Karmpaliotis D, Kassem HH, Knaapen P, Kornowski R, Krestyaninov O, Kumar AVG, Laanmets P, Lamelas P, Lee SW, Lefevre T, Li Y, Lim ST, Lo S, Lombardi W, McEntegart M, Munawar M, Navarro Lecaro JA, Ngo HM, Nicholson W, Olivecrona GK, Padilla L, Postu M, Quadros A, Quesada FH, Prakasa Rao VS, Reifart N, Saghatelyan M, Santiago R, Sianos G, Smith E, C Spratt J, Stone GW, Strange JW, Tammam K, Ungi I, Vo M, Vu VH, Walsh S, Werner GS, Wollmuth JR, Wu EB, Wyman RM, Xu B, Yamane M, Ybarra LF, Yeh RW, Zhang Q, Rinfret S. Guiding Principles for Chronic Total Occlusion Percutaneous Coronary Intervention. Circulation. 2019;140:420-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 272] [Article Influence: 45.3] [Reference Citation Analysis (0)] |