Published online Feb 26, 2022. doi: 10.12998/wjcc.v10.i6.1922
Peer-review started: August 4, 2021
First decision: November 6, 2021
Revised: November 20, 2021
Accepted: January 19, 2022
Article in press: January 19, 2022
Published online: February 26, 2022
Processing time: 203 Days and 1.4 Hours
Proliferative myositis is a rare benign tumor that is typically self-limiting and does not become malignant. It can be cured by simple resection without reported recurrence. Due to its rapid growth, hard structure and ill-defined borders, it can however be mistaken for malignant tumors such as sarcomas.
We investigate the case of a 64-year-old male with proliferative myositis of the abdominal wall, who was preoperatively administered a needle aspiration biopsy and given a simple excision and patch repair. We then compared it with other similar cases to determine the effectiveness of this treatment method.
Resection with follow-up observation has shown to be an effective treatment method for proliferative myositis. To avoid unnecessarily extended or destructive resection, a thorough and conclusive diagnosis is crucial, which requires adequate imaging and pathological knowledge.
Core Tip: Proliferative myositis is a rare and self-limiting benign tumor. Although preoperative imaging can identify certain characteristics, it is still difficult to achieve a conclusive diagnosis. Most cases are not diagnosed until after surgical resection. A needle biopsy is helpful in the diagnosis of proliferative myositis, and surgery involving local excision is sufficient. When the resected mass is located in the abdominal wall, local defects can be considered for patch repair. The key to treatment lies in avoiding misdiagnosis as a malignant tumor, resulting in excessive extended resection and corresponding trauma.
- Citation: Xing RW, Nie HQ, Zhou XF, Zhang FF, Mou YH. Left abdominal wall proliferative myositis resection and patch repair: A case report. World J Clin Cases 2022; 10(6): 1922-1928
- URL: https://www.wjgnet.com/2307-8960/full/v10/i6/1922.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i6.1922
Proliferative myositis (PM) is a rare fibroblastic proliferative disease. It mainly occurs in adults, although cases have also been reported in children[1]. The pathogenesis of the disease is unknown and may be related to previous trauma of the corresponding part of the body. The clinical manifestations are local, rapidly growing masses, most of which have no clinical symptoms but can be painful in some cases. Imaging usually shows unclear capsular masses with fuzzy boundaries and infiltrative growth. The cut surface of surgical specimens reveals fish flesh, which can easily be misdiagnosed as sarcoma or another malignant tumor[2]. This may lead to unnecessary resection or radical surgery to treat these lesions.
PM is, however, a benign lesion with the potential for spontaneous contraction or complete regression. If it can be identified early, no treatment or only a simple resection is required. It is difficult to make a definitive diagnosis prior to surgery. Preoperative B-ultrasound, computed tomography (CT) scans, magnetic resonance imaging, and other imaging examinations as well as needle biopsies are helpful in the evaluation of the lesions, with surgical resection being the choice of treatment for many patients[3,4]. The most common sites of PM are the muscles of the head and neck, the chest wall, the scapula, and the limbs[4,5]. After a literature review, case reports of PM were retrieved from the PubMed and Web of Science databases using the search terms “proliferative myositis” and “abdominal wall,” from 2000 to 2020. There were no matching case reports. Here, we examine a case of a large PM in the left anterior abdominal wall, where patch repair was employed following its resection.
The patient, a 64-year-old male, was admitted to our hospital with a mass on the left anterior abdominal wall that had been present for more than 2 years.
The mass was painless, but it was gradually becoming larger, especially in the preceding 2 mo.
The patient had no previous medical history.
The patient denied family history.
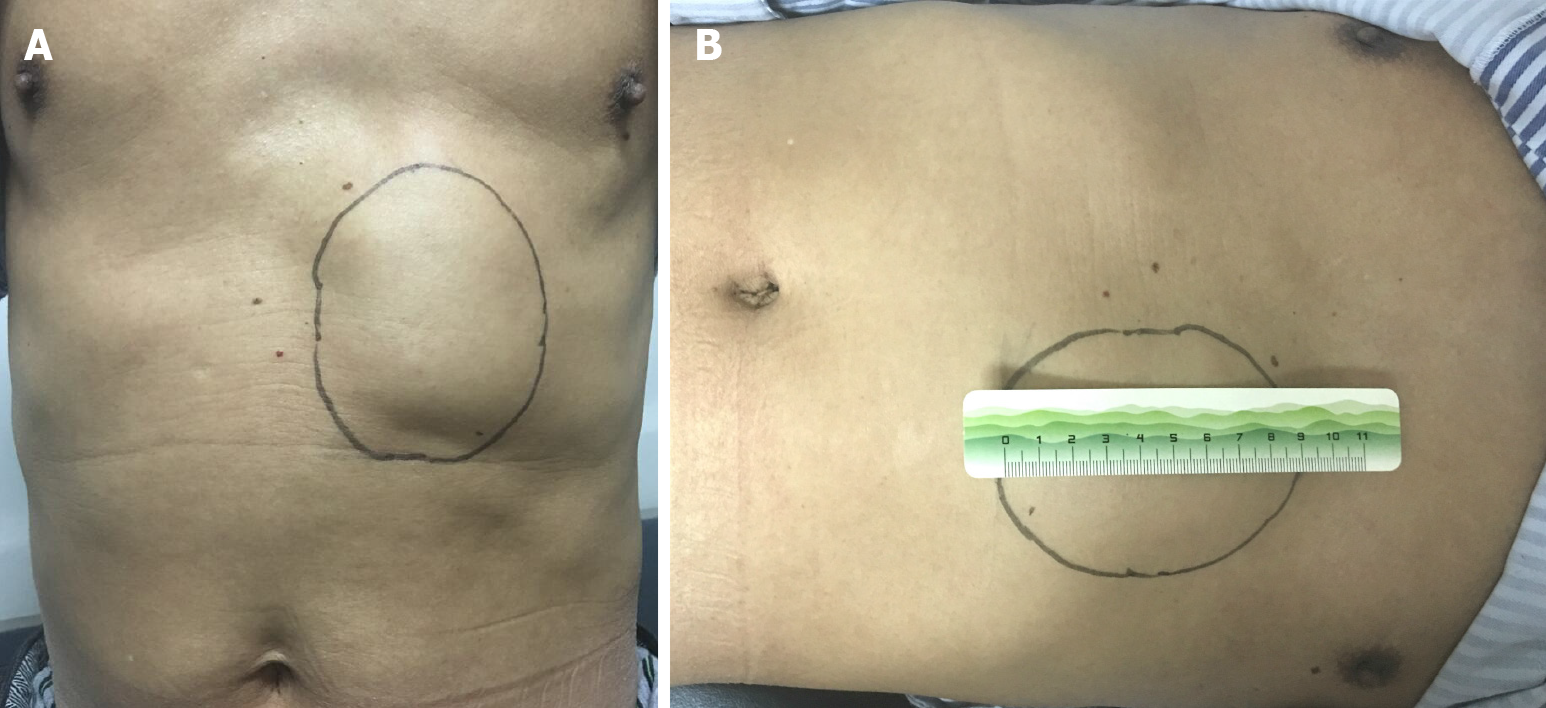
Physical examination revealed a 70 mm × 90 mm mass that was palpated at the costal margin of the quaternary rib region of the left abdominal wall (Figure 1). The skin above the mass was raised, without pigmentation or abnormal temperature. The mass was hard in texture, had a smooth surface, had no tenderness, poor mobility, and formed an unclear boundary with the surrounding tissues.
Results of the laboratory examination (routine blood) were: white blood cell = 8.4 × 109/L, nitrogen = 64.1%, C-reactive protein = 4.6 mg/L.
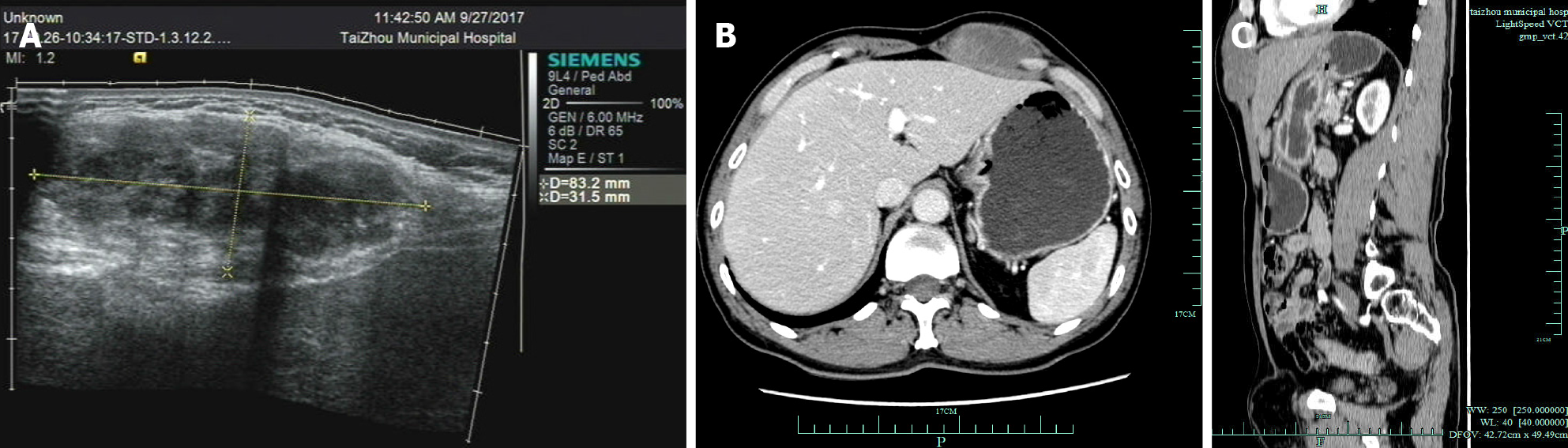
B-ultrasound examination revealed an 83 mm × 32 mm × 50 mm hypoechoic mass that was seen in the seasonal ribs of the left anterior abdominal wall, with an unclear boundary and uneven internal echo (Figure 2A). Several strong echoes with echo shadows were seen, and the color Doppler flow imaging revealed no definite blood flow. Abdominal, contrast-enhanced CT scans showed a mass in the left anterior abdominal wall: 56 mm (transverse diameter) × 30 mm (width). The CT scan values were 40 Hu, heterogeneous post enhancement, and 72-84 Hu, and the possibility of malignancy could not be excluded (Figure 2B and C).
The puncture biopsy pathology showed that it was a spindle cell tumor within the left abdominal wall and was considered benign or borderline.
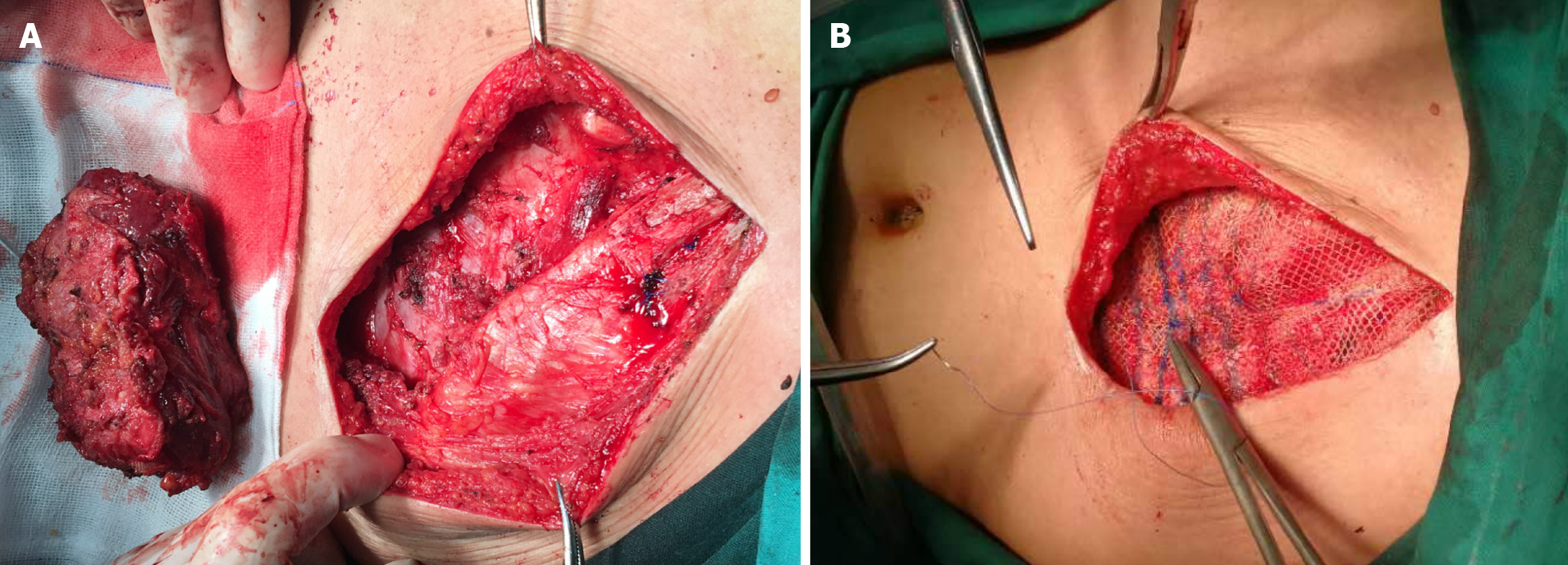
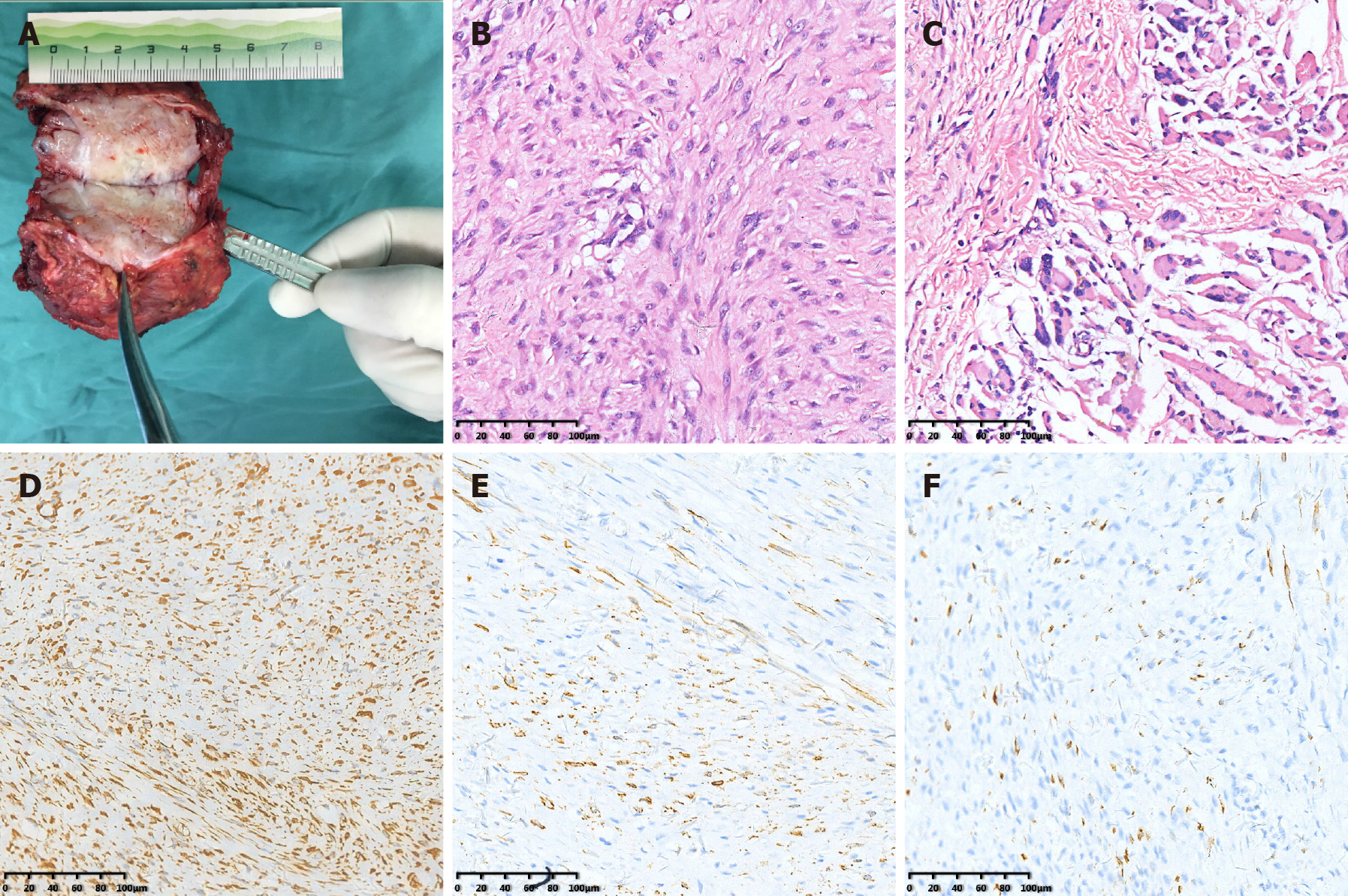
Subsequently, the tumor was resected together with a small amount of muscle and myometrial tissue that infiltrated the peripheral part. Considering the absence of the muscle layer of the abdominal wall under the costal margin of the left-upper abdomen, patch repair and reinforcement was conducted to avoid an abdominal wall hernia (Figure 3). The gross pathological examination showed that the transverse section of the mass was gray-white and light yellow (Figure 4A). Microscopically, the tumor was composed of spindle cells and focally expressed ganglion-like cells (Figure 4B), which have a checkerboard-like structure (Figure 4C). Combining the morphological and immunohistochemical pathology resulted in it being considered PM. The results of the immunohistochemistry were as follows (Figure 4D-F): Vimentin (+), Smooth muscle actin (+), Desmin (partial +), S100 (partial +), cytokeratin (wide) (-), Ki67 (< 1% +), CD34 (-), CD68 (-/+), CD99 (-), and B-cell lymphoma-2 (-).
A follow-up was done with the patient after 31 mo. The incision healed well, with no local recurrence of the mass and no occurrence of an abdominal hernia.
PM was first described by Kern[6] in 1960. It is a rare benign tumor with unknown pathogenesis. While local trauma may be an important trigger[7], other theories include ischemia, paracrine myopathy, etc[8]. It mostly presents clinically as a mass that grows rapidly, may be accompanied by pain, and typically forms a sarcoma-like infiltrative border. It therefore results in a high misdiagnosis rate clinically, with malignant tumors often suggested as the initial diagnosis[2-5,9].
We summarized two articles on a total of 66 patients (Table 1), of which 33 PM patients were reported in earlier literature by Enzinger and Dulcey[5] and 33 from Trerattanavong et al[9], all from 2000 to 2018 retrieved from the PubMed and Web of Science databases. In these 66 cases, it can be seen that there are slightly more males than females, adults over 45-years-old account for the majority, the location of the mass is relatively common in the skeletal muscles of the upper extremities, the size of the mass is mostly less than 3 cm, and surgical resection was chosen in early-stage cases. Eleven earlier cases in the Enzinger and Dulcey[5] report underwent extended resection because they were misdiagnosed as rhabdomyosarcoma. After the year 2000, the advancement of techniques such as needle biopsy allowed patients to choose observation for follow-up; 11 of the 13 cases resolved spontaneously and 2 cases showed no obvious change No recurrence was reported in any case after elective resection.
| Enzinger and Dulcey[5], 1967 (33 cases) | Trerattanavong et al[9], 2019 (33 case review) | |
| Sex | ||
| Male | 19 | 19 |
| Female | 14 | 14 |
| Age (yr) | ||
| 0-18 | 0 | 2 |
| 18-45 | 7 | 4 |
| 45 + | 26 | 27 |
| Location | ||
| Head and neck | 4 | 8 |
| Trunk | 4 | 8 |
| Extremity | 25 | 17 |
| LD (cm) | ||
| 1 cm < LD ≤ 3 cm | 12 | 14 |
| 3 cm < LD ≤ 5 cm | 10 | 3 |
| 5 cm < LD | 5 | 8 |
| NA | 6 | 7 |
| Treatment | ||
| Excision | 30 | 16 |
| Wait and see | 3 | 13 |
| NA | 4 | |
| Outcome | ||
| Excision-recurrence | 0 | 0 |
| No recurrence | 27/30 | 9/16 |
| NA | 3/30 | 7/16 |
| Wait and see-spontaneous resolution | 11/13 | |
| No further growth | 2/13 | |
| NA | 3 | |
| NA | 4 |
Imaging is an essential step following appropriate medical history assessment and physical examination. B-scans show a mass that appears as a strip-like hypoechoic pattern between the inter- and intraphases of hyperechogenicity. Blood flow signals can be seen within, with relatively normal muscle fascicles. A transverse section shows the characteristic “glans dorsum or schitic soil like pattern”[10]. CT scans show a poorly demarcated intramuscular lesion that appears isointense or hypointense compared to surrounding muscle. Homogeneous or heterogeneous enhancement may be demonstrated after the injection of a contrast medium, but enhancement may not be detectable in some cases[11]. Magnetic resonance images with a low or isointense T1 signal compared with surrounding muscle tissue show a T2-weighted magnetic resonance that appears as a hyperintense soft tissue mass[3,11,12].
In most cases, PM is still difficult to clearly identify in laboratory tests and imaging studies, and a fine-needle aspiration biopsy may be helpful for further diagnosis[13,14]. Trerattanavong et al[9] reviewed 33 cases of PM where a definitive diagnosis was based on the occurrence of spindle-shaped fibrocytes admixed with giant ganglion-like cells on biopsy. Malhotra et al[15] considered the cytological diagnosis of PM based on the presence of a polymorphic population consisting of three cellular components: nonlesional skeletal muscle fibers, lesional myofibroblasts, and a ganglion cell-like component. The aspirate is similar to nodular fasciitis. However, PM differs from nodular fasciitis in two ways: proliferating spindle cells are located in the skeletal muscle tissue and giant ganglion-like cells are more abundant[10]. At the same time, PM also had strong similarity to striated myoblasts, but no cytoplasmic cross striations were identified and the nuclear chromatin was fine with a smooth nuclear membrane. These features help exclude rhabdomyosarcoma as a diagnosis[15].
PM is a benign lesion, and some cases experience spontaneous regression[4,5,15,16]. For example, in one case “the local mass had disappeared after a 2 mo follow-up, which was considered PM as result of a fine needle aspiration biopsy of the neck mass”[15]. If a diagnosis can be clearly established preoperatively, close observation and follow-ups are therefore permitted. If the mass causes compression on peripheral organs or causes a large psychological burden affecting the daily life of the patient, surgical resection may be considered[4,7]. Radical resection can, however, be avoided if there are no reports of recurrence or malignant transformation following the initial resection[4,5,8].
During surgery, PM generally appears as a poorly circumscribed mass that forms grey white striated fascicles or scar-like masses between muscle fascicles or as a wedge-shaped, pointed insertion between fascicles, with the base lying within the fascia. The resected specimen can have a fish flesh-like aspect, microscopically resembling proliferative fasciitis except that the lesion mainly involves the striated muscle fascicles and muscle interstitium rather than the muscle fibers themselves. Cross-sections may therefore show a checkerboard-like structure dominated by increased active fibroblasts and giant basophilic cells. These cellular and peculiar structural findings may be of value in the diagnosis of PM.
In our patient, preoperative enhanced CT scans suggested malignant lesions, while preoperative puncture pathology suggested benign or borderline possibilities. Therefore, further surgical treatment was provided. At the same time, the literature indicated that the most commonly affected sites were the muscles of the head and neck, chest wall, scapula and extremities, with only resection being performed. However, the case of the 64-year-old male was different because the mass was larger and the partial excision of the muscle layer of the abdominal wall was accompanied by local weakness. Further patch repair was therefore done to improve the strength of the abdominal wall and to avoid the formation of an abdominal wall hernia. There has been no local recurrence or abdominal wall hernia after more than 2 years of postoperative follow-ups.
PM is a rare and self-limiting benign tumor. Although preoperative imaging can identify certain characteristics, it is still difficult to achieve a conclusive diagnosis. Most cases are not diagnosed until after surgical resection. A needle biopsy is helpful in the diagnosis of PM, and surgery involving local excision is sufficient. When the resected mass is located in the abdominal wall, problem areas can be considered for patch repair. The key to treatment lies in avoiding misdiagnosis as a malignant tumor, resulting in excessive resection and related trauma.
We thank all the authors helping with the writing and publication of this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Meglio LD S-Editor: Liu JH L-Editor: Filipodia P-Editor: Liu JH
| 1. | Acharya RV. Proliferative Myositis: A Rare Pseudosarcoma in Children. J Clinic Case Reports. 2015;5:487. [DOI] [Full Text] |
| 2. | Binesh F, Sobhanardekani M, Zabihi S, Behniafard N. Proliferating Myositis: An Inflammatory Lesion often Misdiagnosed as A Malignant Tumor. Rom J Intern Med. 2016;54:243-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Jarraya M, Parva P, Stone M, Klein MJ, Guermazi A. Atypical proliferative myositis: original MR description with pathologic correlation: case report. Skeletal Radiol. 2014;43:1155-1159. [PubMed] |
| 4. | Gan S, Xie D, Dai H, Zhang Z, Di X, Li R, Guo L, Sun Y. Proliferative myositis and nodular fasciitis: a retrospective study with clinicopathologic and radiologic correlation. Int J Clin Exp Pathol. 2019;12:4319-4328. [PubMed] |
| 5. | Enzinger FM, Dulcey F. Proliferative myositis. Report of thirty-three cases. Cancer. 1967;20:2213-2223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Kern WH. Proliferative myositis; a pseudosarcomatous reaction to injury: a report of seven cases. Arch Pathol. 1960;69:209-216. [PubMed] |
| 7. | McHugh N, Tevlin R, Beggan C, Ryan DJ, Larkin J, Moloney F, Bennett MW, Kelly J. Proliferative myositis of the latissimus dorsi presenting in a 20-year-old male athlete. Ir Med J. 2017;110:605. [PubMed] |
| 8. | Fauser C, Nährig J, Niedermeyer HP, Arnold W. Proliferative myositis: a rare pseudomalignant tumor of the head and neck. Arch Otolaryngol Head Neck Surg. 2008;134:437-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Trerattanavong P, Suriyonplengsaeng C, Waisayarat J. Proliferative Myositis: A Comprehensive Review of 33 Case Reports. Rama Med J. 2019;42:60-70. [DOI] [Full Text] |
| 10. | Dion B, Coulier B, Van den Broeck S. The Typical "Cracked Dry Mud" Ultrasound Pattern of Proliferative Myositis. J Belg Soc Radiol. 2019;103:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Yiğit H, Turgut AT, Koşar P, Astarci HM, Koşar U. Proliferative myositis presenting with a checkerboard-like pattern on CT. Diagn Interv Radiol. 2009;15:139-142. [PubMed] |
| 12. | Pagonidis K, Raissaki M, Gourtsoyiannis N. Proliferative myositis: value of imaging. J Comput Assist Tomogr. 2005;29:108-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Wong NL. Fine needle aspiration cytology of pseudosarcomatous reactive proliferative lesions of soft tissue. Acta Cytol. 2002;46:1049-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Klapsinou E, Despoina P, Dimitra D. Cytologic findings and potential pitfalls in proliferative myositis and myositis ossificans diagnosed by fine needle aspiration cytology: report of four cases and review of the literature. Diagn Cytopathol. 2012;40:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Malhotra KP, Husain N, Shukla S, Jain P, Sonkar AA. Pseudosarcomatous proliferative myositis of the sternocleidomastoid: a case report. Diagn Cytopathol. 2014;42:1096-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Colombo JR, Dagher W, Wein RO. Benign proliferative myositis of the sternohyoid muscle: review and case report. Am J Otolaryngol. 2015;36:87-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |