Published online Feb 26, 2022. doi: 10.12998/wjcc.v10.i6.1914
Peer-review started: July 28, 2021
First decision: October 26, 2021
Revised: November 4, 2021
Accepted: January 14, 2022
Article in press: January 14, 2022
Published online: February 26, 2022
Processing time: 209 Days and 23.9 Hours
Resection of deep intracranial tumors requires significant brain retraction, which frequently causes brain damage. In particular, tumor in the trigone of the lateral ventricular presents a surgical challenge due to its inaccessible location and intricate adjacent relationships with essential structures such as the optic radiation (OR) fibers. New brain retraction systems have been developed to minimize retraction-associated injury. To date, there is little evidence supporting the superiority of any retraction system in preserving the white matter tract integrity. This report illustrates the initial surgical excision in two patients using a new retraction system termed the cerebral corridor creator (CCC) and demonstrates its advantage in protecting OR fibers.
We report two patients with nonspecific symptoms, who had trigone ventricular lesions that involved the neighboring OR identified on preoperative diffusion tensor imaging (DTI). Both patients underwent successful surgical excision using the CCC. Total tumor removal was achieved without additional neurological deficit. DTI showed that the OR fibers were preserved along the surgical field. Preoperative symptoms were alleviated immediately after surgery. Clinical outcomes were improved according to the Glasgow-Outcome-Scale and Activity-of-Daily-Living Scale assessments.
In the two cases, the CCC was a safe and useful tool for creating access to the deep trigonal area while preserving the white matter tract integrity. The CCC is thus a promising alternative brain retractor.
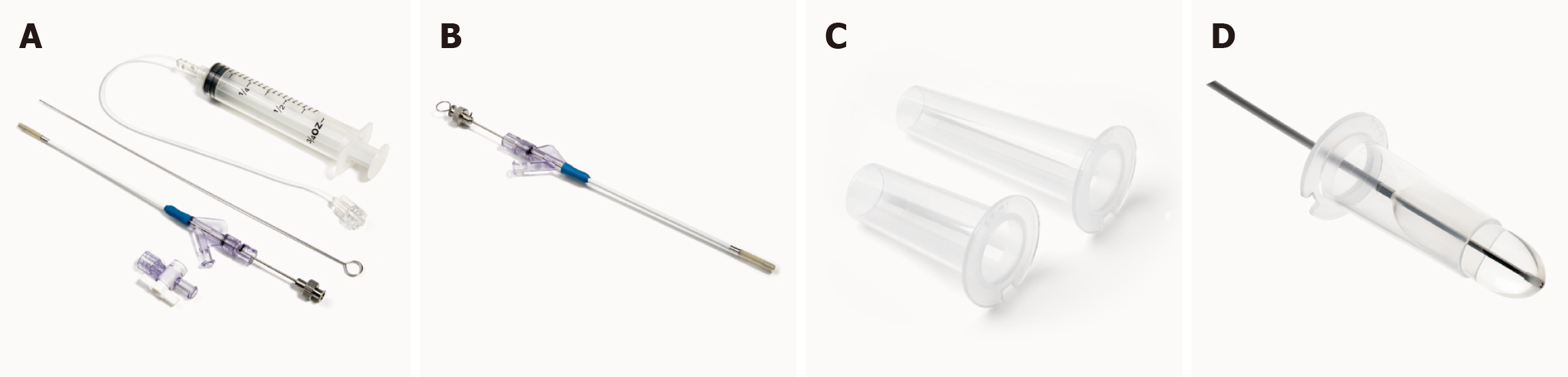
Core Tip: The cerebral corridor creator (CCC) is a specially designed surgical tool set containing a balloon catheter and a transparent tubular retractor. The balloon is made of natural latex and can gently open up the brain tissue with minimum fluctuations in intracranial pressure and creates a surgical corridor by gradually inflating and deflating, thereby reducing damage to the incised cortical tissue and deep white matter tracts. The transparent tubular retractor provides a clear view of the trigonal area and surrounding brain tissue, helps maintain the corridor, protects brain tissue from surgical instrument, and avoids brain tissue collapse during surgery. The CCC provides an innovative and minimally invasive surgical corridor especially for small to medium and deep-seated brain lesions.
- Citation: Liu XW, Lu WR, Zhang TY, Hou XS, Fa ZQ, Zhang SZ. Cerebral corridor creator for resection of trigone ventricular tumors: Two case reports. World J Clin Cases 2022; 10(6): 1914-1921
- URL: https://www.wjgnet.com/2307-8960/full/v10/i6/1914.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i6.1914
The trigonal area of the lateral ventricle is the predilection site of intraventricular tumors, particularly primary meningiomas[1,2]. Due to the fluid cavity, tumors in this area are usually asymptomatic at the early stage and are considerably large upon diagnosis[3]. To date, complete resection is recommended for the majority of brain tumors. However, the surgical procedures can be challenging, especially for deep brain tumors[4,5]. The lateral ventricular trigone is situated deep within the cerebral hemisphere and is in close proximity to essential neural structures, such as the optic radiation (OR)[6]. The OR spans from the lateral geniculate body to the calcarine cortex and forms the apical wall of the lateral ventricular trigonal area. A common complication is postoperative visual field defect (VFD) due to OR damage[7]. Hence, one big challenge for the neurosurgery community is to create corridors to reach deep-seated tumors and achieve complete tumor resection without causing VFDs.
Conventional neurosurgery operations require brain spatulas to create a corridor for exposure. The brain spatulas are asymmetrically pressed on soft brain tissue, which frequently cause brain damage, especially when multiple brain spatulas are used in continuous retraction[8]. Additionally, brain spatulas may cause ischemia in local normal white matter due to contact pressure[9]. A longer retraction time causes greater risk of white matter injury. Minimally invasive surgery has been proposed to minimize operation-induced brain damage and is associated with lower surgical-related morbidity[10-12].
Minimally invasive techniques, such as tubular retractor systems, are effective and safe for the resection of deep-seated lesions and have shown satisfactory outcomes. Tubular retractors exert even pressure to the surrounding white matter tracts rather than focused retraction pressure, thus decreasing the likelihood of severe brain damage[10,13]. The cerebral corridor creator (CCC) (Shineyard Medical Corp., Shenzhen, Guangdong Province, China) is a tailored tubular retractor which represents an innovative minimally invasive method to create a surgical corridor (Figure 1). The CCC can be used for the removal of deep-seated tumors, blood clots associated with hemorrhages, and foreign matter. Diffusion tensor imaging (DTI) and intra-operative ultrasound enable identification of the best route to the trigonal region with minimal damage to white matter tracts compared with conventional surgical techniques, potentially diminishing morbidity.
We here report the successful resection of lateral ventricular trigonal tumors in two patients using the CCC. We presented our experience of surgical techniques and peri-operative management using this new brain retraction tool.
Case 1: Acute vomiting, headache, and dizziness for 3 d.
Case 2: Headache for 2 mo.
Case 1: The patient was a 63-year-old healthy woman with acute vomiting, and she had headache and dizziness for 3 d. A computed tomography scan at a local hospital revealed an intracranial mass lesion, and she was referred to our institution.
Case 2: The patient was a 53-year-old woman patient who had suffered from headaches for 2 m.
Case 1: The patient had no past medical problems.
Case 2: The patient had a 2-year history of stage IV colon adenocarcinoma, and all tumors were surgically removed.
In both patients, physical examination of the nervous system showed no obvious abnormalities, including visual deficits.
Laboratory evaluation was performed before surgery, and no abnormalities were found in both patients.
Preoperative magnetic resonance imaging (MRI) and DTI were performed with a Philips 3.0 T scanner using a standard radio-frequency head coil. Pre-processing was performed using FMRIB Software Library v5.0 (http://www.fmrib.ox.ac.uk/fsl, created by the Analysis Group, FMRIB, Oxford, United Kingdom), as previously reported[14]. Then, diffusion metrics were calculated to obtain each diffusion tensor model and fractional anisotropy results. Following these steps, tract-graphic reconstruction was performed by using Diffusion Toolkit and Track-Vis software (http://www.trackvis.org/dtk/)[15]. The regions of interest of the OR were the lateral geniculate body and the calcarine cortex[16,17]. The reconstructed tracts were also overlaid onto T1-weighted images. Based on preoperative DTI images, surgical trajectories were planned to avoid crossing the OR in the lateral ventricular trigone.
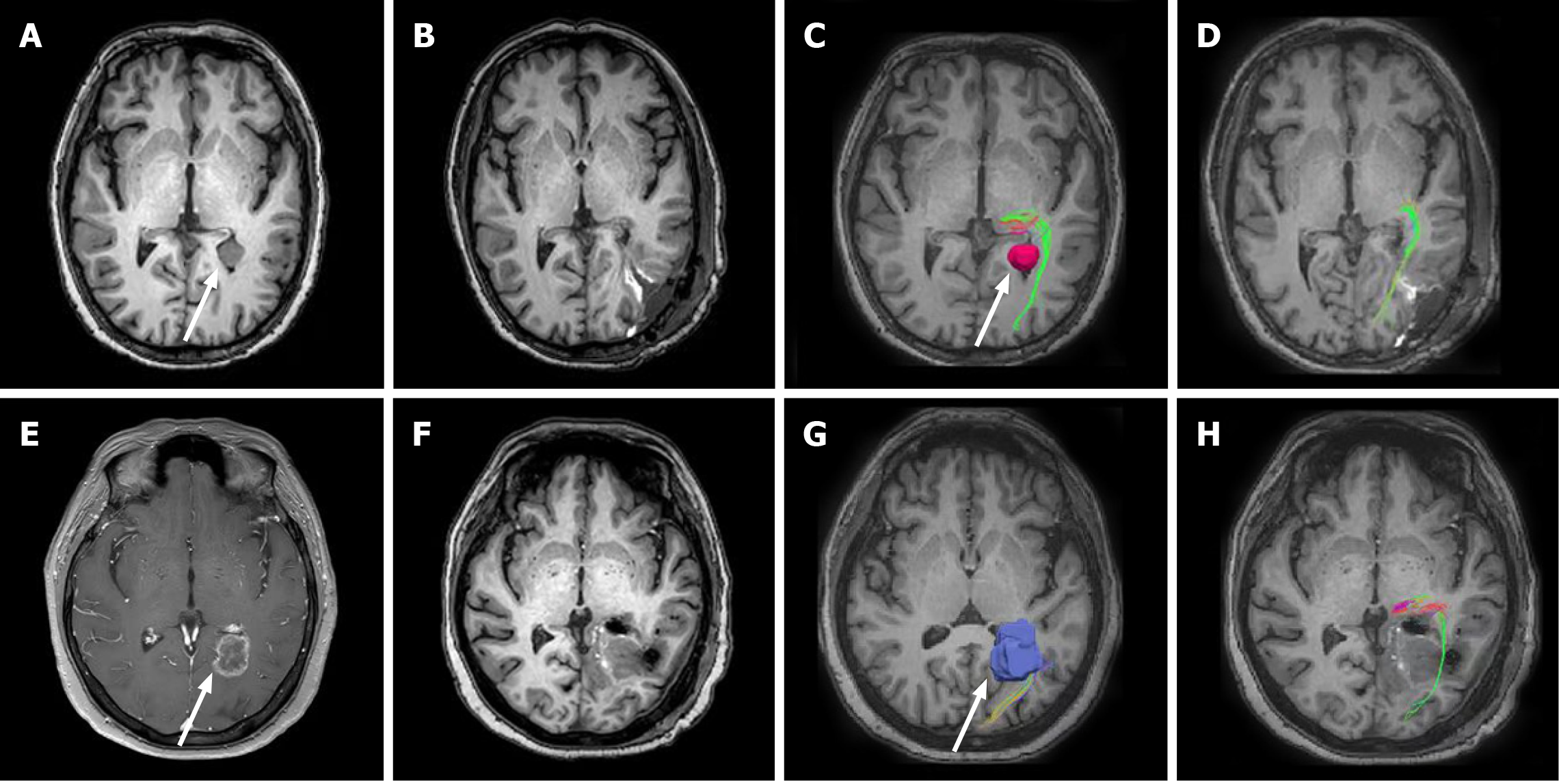
Case 1: MRI revealed a well-defined lesion in the left lateral ventricular trigone with maximum diameter of 16 mm. A benign meningioma was initially suspected (Figure 2A). DTI depicted the close relations between the OR (green) and the lesion (pink, arrow head in Figure 2C). The OR under the tumor had shifted slightly.
Case 2: A heterogeneously ring-enhancing lesion (21 mm × 18 mm) in the left lateral ventricular trigone was found on the brain MRI (Figure 2E). The DTI-reconstructed OR tract was wrapped around the lesion and on its path through and intersecting the tumor (Figure 2G).
Meningothelial meningiomas.
Metastatic adenocarcinoma with immunohistochemistry positive staining of cytokeratin, CEA, SATB-2, and Ki-67 (80%).
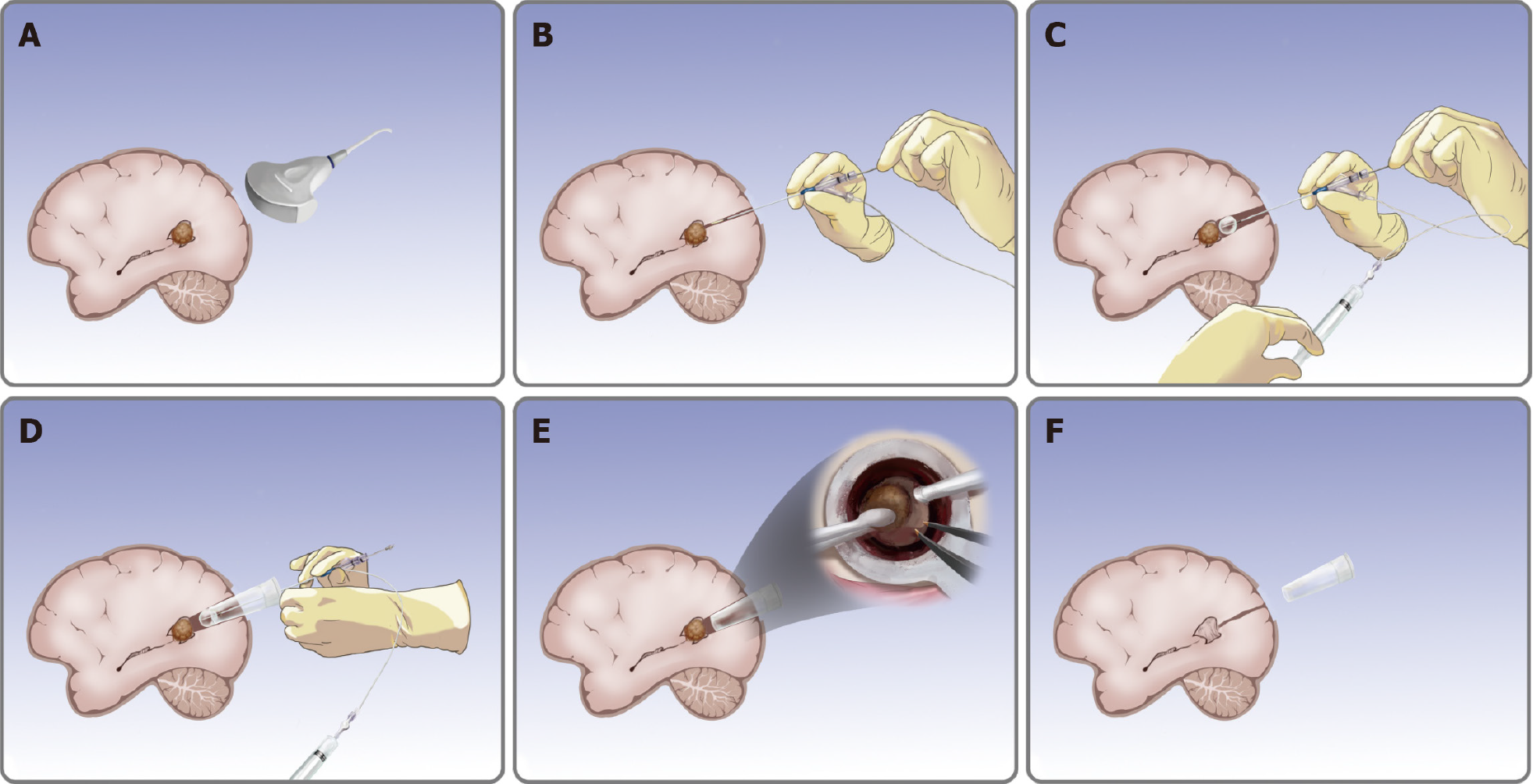
Operations were performed under general endotracheal anesthesia. The patient was placed in the prone position with the head fixed toward the opposite side of the tumor. First, a curvilinear skin incision was made, followed by a temporoparietal-occipital mini-craniotomy. The intra-operative ultrasound (ALOKA a7, Japan) was used to localize the lesion, to determine the entry point and to confirm that the entry route was parallel to the major white matter fibers over a non-eloquent gyrus (Figure 3A). The balloon catheter was used to gently puncture the lateral ventricular trigone based on the preoperative images (Figure 3B). After placement of the balloon catheter, saline was injected into the balloon to create an operative corridor from the cortical to the white matter by intermittent dilatation (Figure 3C).
When an adequate corridor was created, the outer tubular retractor was slowly pushed into the well-expanded corridor, and the inner balloon catheter was removed (Figure 3D). When the inner balloon was properly filled and contracted during the pushing process, the tubular plate was easier to insert. The tumor was resected using standard bimanual microsurgical methods under a microscope (Carl Zeiss Shanghai Co. Ltd., China) through the tubular retractor (Figure 3E).
The diameter (15 mm) of the retractor allows satisfactory visualization with sufficient lighting and a three-dimensional view. The direction of the tubular retractor can be gently adjusted to reach the margin areas of the tumor. Routine brain tumor resection techniques, including suction, tissue-biting, and bipolar cautery were used to remove the tumor until the intra-operative gross total resection was achieved. Following tumor resection, meticulous hemostasis was performed by standard bipolar coagulation. The tubular retractor was then gently withdrawn (Figure 3F). When withdrawing the transparent retractor, the surrounding regions were assessed for re-bleeding. The dura, skull, and skin were closed following the standard procedure.
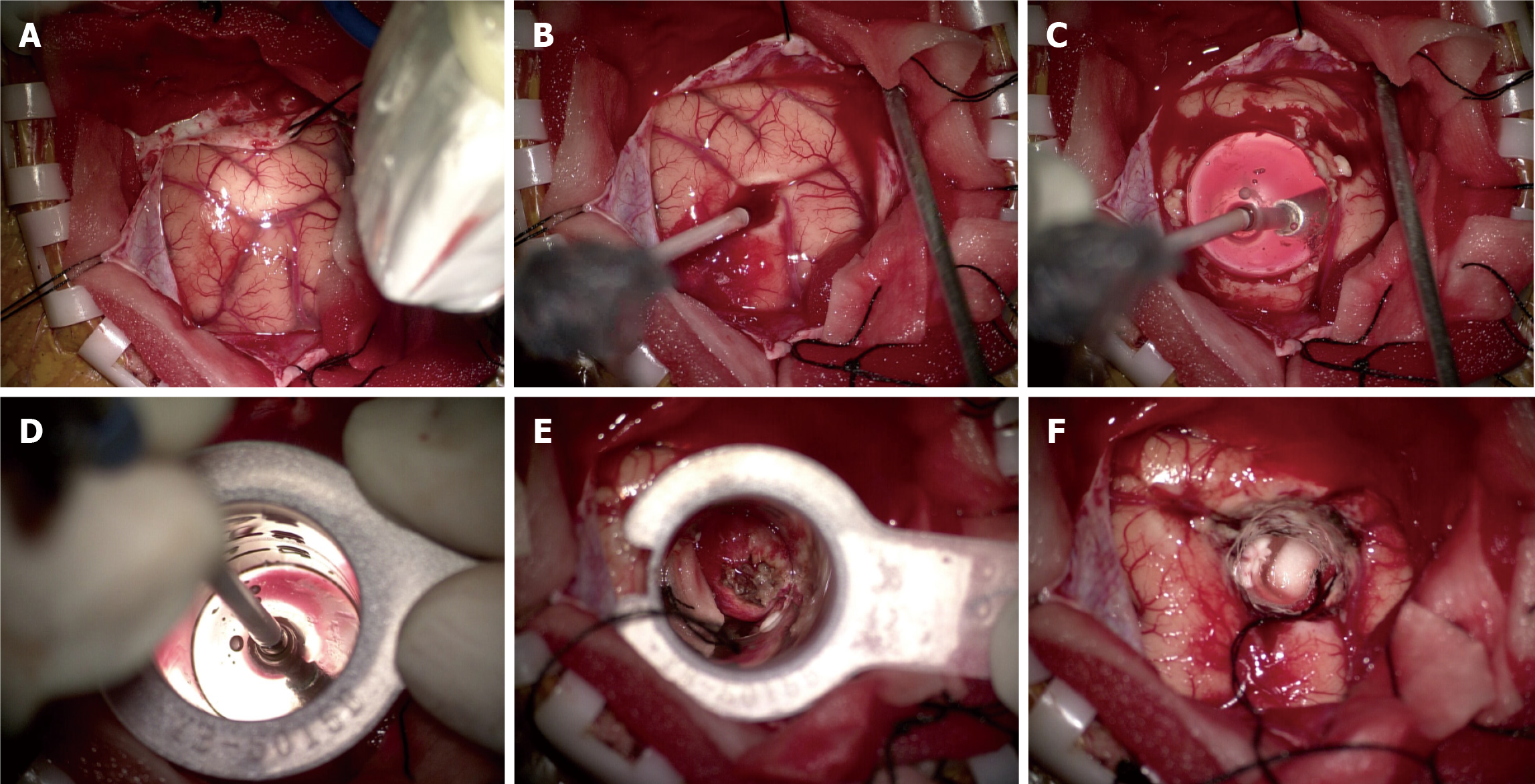
Minimally invasive surgery with the CCC was performed in this patient. As mentioned earlier, resection was performed using the CCC following routine procedure (Figure 4A-F). The pale-white tumor was easily visualized through the tubular retractor, and the bottom part of the lesion was adherent to the choroidal vessels (Figure 4E and F). The tumor could not be sucked out, bipolar coagulation was used to cut off the blood supply on the surface of the tumor, and then the tumor was removed.
The patient underwent surgery using the CCC. The procedure was performed in the same manner as in case 1. After placing the tubular retractor, the lesion was not found in the center of vision field. As the surrounding structures were visible through the transparent tubular wall, we adjusted the angle of the tubular retractor for lesion resection. The lesion was firm with moderate blood supply. After cauterizing the surrounding blood vessels, the lesion was excised.
Headache and dizziness resolved after surgery. The patient was discharged at day 6 post-operation without additional deficits. The postoperative MRI scan confirmed no residual tumor except for a small amount of hematocele in the operative area (Figure 2B). Postoperative DTI showed the reconstructed OR (green) from the lateral geniculate body with no distinguishable loss of fibers (Figure 2D). The intact OR could be seen clearly spanning from the surgical field to the occipital lobe.
A neurosurgeon carried out a follow-up on the post-operation outcomes based on the activity-of-daily-living (ADL) scale and the Glasgow Outcome Scale (GOS). Follow-up findings showed that the patient’s ADL score was 85 and GOS grade 4 before discharge. At 1 mo, the ADL score and GOS grade improved to normal levels. At 3-mo follow-up, she was back to normal life. To date, the patient has been observed without further treatment.
The patient’s headache resolved immediately after surgery. The 7-d postoperative MRI confirmed the total removal of the tumor and barely showed any sign of the surgical corridor (Figure 2F). Postoperative DTI showed that the OR fibers along the surgical corridor were successfully preserved (Figure 2H).
The patient recovered without additional neurological deficit according to follow-up neurologic examinations. She was discharged on day 7 after surgery with an ADL score of 90 and GOS grade 4. She further underwent standard chemotherapy with Capecitabine (1000 mg twice a day) for 2 wk. One month after the operation, she resumed all previous activities with ADL score and GOS grade back to normal levels. The patient will be followed up with imaging to evaluate long-term outcome.
Deep-seated brain lesions (e.g., trigone ventricular tumors) require significant retraction to maintain the surgical corridor open. Traditional retractor systems such as brain spatulas frequently cause severe damage to adjacent normal brain tissue[18]. Exposure of deep-seated brain lesions by brain spatulas has been conducted for decades, although significant retraction-associated complications often occur[9].
Novel brain retraction systems have been developed to minimize brain damage. Based on an experimental study, Shahbabian et al[19] reported that a balloon catheter could access the subcortical lesions with minimal disruption of white matter fibers, which was easier and safer than blunt dissection with a metallic instrument. Kelly et al[12] proposed the concept of tubular retractor systems with stereotactic craniotomy in the 1980s. For the next 30 years, tubular retractors have been considered an effective technique and revolutionized minimally invasive surgery. Various commercial tubular retractor systems have been developed in recent years, including BrainPath (NICO, Indianapolis, IN, United States), VBAS (ViewSite Brain Access System, Vycor Medical, Boca Raton, FL, United States), and METRx (Minimal Exposure Tubular Retractor System, Medtronic, Memphis, TN, United States)[10,13,20-23]. The CCC extends the advantages of existing retractor systems by adding a balloon into the device. The balloon gradually dilatates the brain tissue, whereby white fiber tracts are separated, and the dilation process protected by increasing the balloon diameter.
The CCC can be combined with intra-operative ultrasound and DTI to locate the optimal site of incision toward achieving a more precise and less damaging technique. The combination of techniques minimizes the injury during scalp incision and craniotomy, and reduces durotomy size. It has many advantages. For example, it reduces blood loss and operation time, obviates the need for prolonged intensive care unit stay, and reduces the risk of complications such as postoperative pain and postoperative wound infection. The cost of healthcare is reduced, and the patient’s outcome is improved.
Although previous studies have reported that tubular retractor systems provide optimal visualization and decrease the incidence of postoperative complications, no direct evidence has been reported on the preservation of fiber tracts[18]. In previous studies, fiber tracts with fewer functions were selected as invasion paths. However, insignificance may be mapped to an area simply because of our current lack of anatomical knowledge. Therefore, in the present two cases, we identified the OR fibers in the lateral ventricular trigone using DTI in order to achieve an objective evaluation of the integrity of the white matter fibers. DTI is used for pre-operative evaluation and treatment guidance[20].
By identifying the spatial relationship between tumor and the exact course of the OR, we can significantly improve the preservation of the OR and reduce the risk of postoperative VFD. Furthermore, DTI can predict the functional outcome by quantitatively assessing the integrity of white matter fibers. Our study demonstrated that the CCC can provide satisfactory protection to the surrounding white matter tracts as indicated by DTI.
Visualization of tumors can be achieved using a microscope or an endoscope. We found that the microscope is preferable to the endoscope in most circumstances. The microscope-assisted approach has several advantages. First, its wider and 3D field of view maintains higher quality visualization. Second and more importantly, the microscope shows the area outside the surgical cavity, whereas the endoscope decreases the scale of movement and results in a narrow work space. Moreover, microscopes facilitate the handling of intra-operative emergencies in a wider operation space. We believe that firmer tumors such as meningiomas and certain gliomas are easier to remove using a microscope. In contrast, soft lesions, such as hematoma, can be removed by suction with the aid of endoscope. Consequently, in our opinion, the firmness and presumed histology of the lesion are two important factors when selecting a suitable viewing device.
In this study, we described a novel brain retractor, the CCC, which creates a satisfactory surgical corridor with minimal brain injury. We also depicted the feasibility and advantages of combining the CCC with imaging techniques (intra-operative ultrasound and DTI). Resection in the two patients with lateral ventricle trigonometric tumors was presented as examples. However, evaluation of the CCC in other deep intracranial locations is warranted. Large multi-institutional, randomized clinical trials are needed for clinical implementation of the CCC. Based on our initial experience, patients with other deep intracranial tumors may also benefit from the CCC approach.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Marickar F S-Editor: Wang LYT L-Editor: A P-Editor: Wang LYT
| 1. | Nakashima T, Hatano N, Kanamori F, Muraoka S, Kawabata T, Takasu S, Watanabe T, Kojima T, Nagatani T, Seki Y. Tumor Volume Decrease via Feeder Occlusion for Treating a Large, Firm Trigone Meningioma. NMC Case Rep J. 2018;5:9-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Zanini MA, Faleiros AT, Almeida CR, Clara CA, Gabarra RC. Trigone ventricular meningiomas: surgical approaches. Arq Neuropsiquiatr. 2011;69:670-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Cikla U, Swanson KI, Tumturk A, Keser N, Uluc K, Cohen-Gadol A, Baskaya MK. Microsurgical resection of tumors of the lateral and third ventricles: operative corridors for difficult-to-reach lesions. J Neurooncol. 2016;130:331-340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Lin Z, Zhang X, Shen S, Gao Z, Guan C, Liu T, Guo D, Qi X, Ren X, Jiang Z. Postoperative delayed trapped temporal horn in patients with lateral ventricular trigone meningioma: Risk factors, surgical management, and literature review. Eur J Surg Oncol. 2020;46:2324-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Liu M, Wei Y, Liu Y, Zhu S, Li X. Intraventricular meninigiomas: a report of 25 cases. Neurosurg Rev. 2006;29:36-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Kawashima M, Li X, Rhoton AL Jr, Ulm AJ, Oka H, Fujii K. Surgical approaches to the atrium of the lateral ventricle: microsurgical anatomy. Surg Neurol. 2006;65:436-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Sampath R, Katira K, Shi R, Vannemreddy P, Patil S, Nanda A. Radio-anatomic measurements and statistical generation of a safe surgical corridor to enter the ventricular trigone while avoiding injury to the optic radiations. J Neurol Surg A Cent Eur Neurosurg. 2014;75:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Yokoh A, Sugita K, Kobayashi S. Intermittent vs continuous brain retraction. An experimental study. J Neurosurg. 1983;58:918-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Zagzoog N, Reddy KK. Modern Brain Retractors and Surgical Brain Injury: A Review. World Neurosurg. 2020;142:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Eichberg DG, Di L, Shah AH, Luther EM, Jackson C, Marenco-Hillembrand L, Chaichana KL, Ivan ME, Starke RM, Komotar RJ. Minimally invasive resection of intracranial lesions using tubular retractors: a large, multi-surgeon, multi-institutional series. J Neurooncol. 2020;149:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 11. | Jamshidi AO, Beer-Furlan A, Hardesty DA, Ditzel Filho LFS, Prevedello LM, Prevedello DM. Management of large intraventricular meningiomas with minimally invasive port technique: a three-case series. Neurosurg Rev. 2021;44:2369-2377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Kelly PJ, Goerss SJ, Kall BA. The stereotaxic retractor in computer-assisted stereotaxic microsurgery. Technical note. J Neurosurg. 1988;69:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Okasha M, Ineson G, Pesic-Smith J, Surash S. Transcortical Approach to Deep-Seated Intraventricular and Intra-axial Tumors Using a Tubular Retractor System: A Technical Note and Review of the Literature. J Neurol Surg A Cent Eur Neurosurg. 2021;82:270-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 14. | Yang M, Li Y, Li J, Yao D, Liao W, Chen H. Beyond the Arcuate Fasciculus: Damage to Ventral and Dorsal Language Pathways in Aphasia. Brain Topogr. 2017;30:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Wang R, Benner T, Sorensen AG, Wedeen VJ. Diffusion Toolkit: a software package for diffusion imaging data processing and tractography. Proc Intl Soc Mag Reson Med. 2007;15:3720. |
| 16. | Bertani GA, Bertulli L, Scola E, Di Cristofori A, Zavanone M, Triulzi F, Rampini PM, Carrabba GG. Optic Radiation Diffusion Tensor Imaging Tractography: An Alternative and Simple Technique for the Accurate Detection of Meyer's Loop. World Neurosurg. 2018;117:e42-e56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Zhang Y, Wan SH, Wu GJ, Zhang XL. Magnetic resonance diffusion tensor imaging and diffusion tensor tractography of human visual pathway. Int J Ophthalmol. 2012;5:452-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Bander ED, Jones SH, Kovanlikaya I, Schwartz TH. Utility of tubular retractors to minimize surgical brain injury in the removal of deep intraparenchymal lesions: a quantitative analysis of FLAIR hyperintensity and apparent diffusion coefficient maps. J Neurosurg. 2016;124:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Shahbabian S, Keller JT, Gould HJ 3rd, Dunsker SB, Mayfield FH. A new technique for making cortical incisions with minimal damage to cerebral tissue. Surg Neurol. 1983;20:310-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Day JD. Transsulcal Parafascicular Surgery Using Brain Path® for Subcortical Lesions. Neurosurgery. 2017;64:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Echeverry N, Mansour S, MacKinnon G, Jaraki J, Shapiro S, Snelling B. Intracranial Tubular Retractor Systems: A Comparison and Review of the Literature of the BrainPath, Vycor, and METRx Tubular Retractors in the Management of Deep Brain Lesions. World Neurosurg. 2020;143:134-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Eichberg DG, Buttrick SS, Sharaf JM, Snelling BM, Shah AH, Ivan ME, Komotar RJ. Use of Tubular Retractor for Resection of Colloid Cysts: Single Surgeon Experience and Review of the Literature. Oper Neurosurg (Hagerstown). 2019;16:571-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Eichberg DG, Di L, Shah AH, Ivan ME, Komotar RJ, Starke RM. Use of Tubular Retractors for Minimally Invasive Resection of Deep-Seated Cavernomas. Oper Neurosurg (Hagerstown). 2020;18:629-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |