Published online Feb 26, 2022. doi: 10.12998/wjcc.v10.i6.1909
Peer-review started: July 28, 2021
First decision: October 25, 2021
Revised: October 26, 2021
Accepted: January 14, 2022
Article in press: January 14, 2022
Published online: February 26, 2022
Processing time: 210 Days and 13.7 Hours
Maturity-onset diabetes of the young 3 (MODY3), caused by mutations in the HNF1A gene, is the most common subtype of MODY. The diagnosis of MODY3 is critical because a low dose of sulfonylurea agents can achieve glucose control.
We describe a patient with MODY3 involving a novel splicing mutation, in whom low-dose gliclazide was sufficient to control clinically significant hyperglycemia. Sanger sequencing identified a splicing HNF1A mutation in 12q24 NM_000545.5 Intron5 c.1108-1G>A. Glycemic control has been maintained without insulin therapy for 28 mo after the diagnosis of diabetes.
This case report highlights a novel HNF1A gene mutation in MODY3 that is responsive to sulfonylurea therapy.
Core Tip: We describe a patient with maturity-onset diabetes of the young 3 caused by a splicing mutation in intron 5 at position 24 of chromosome 12, where the base sequence was replaced from G to A, and the protein encoded was changed accordingly. Excellent blood glucose control can be achieved by using low-dose sulfonylureas.
- Citation: Xu Q, Kan CX, Hou NN, Sun XD. Novel HNF1A gene mutation in maturity-onset diabetes of the young: A case report. World J Clin Cases 2022; 10(6): 1909-1913
- URL: https://www.wjgnet.com/2307-8960/full/v10/i6/1909.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i6.1909
Maturity-onset diabetes of the young (MODY) is the most common single-gene type of diabetes with an early age of onset. MODY often occurs in children or adolescents, beginning with mild symptoms that continue until middle age. The genetic heterogeneity of MODY is responsible for its clinical heterogeneity[1,2]. In total, 15 MODY subtypes have been identified. The most common are hepatocyte nuclear factor 4A (MODY1), glucokinase (MODY2), and HNF1A (MODY3). In patients with MODY3, mutations in HNF1A cause changes in proteins, such as glucose transporter 2, amylin, insulin, and L-pyruvate kinase. These changes are associated with insulin secretion and glucose metabolism[3]. Patients with MODY3 have clinical characteristics of hyperglycemia, including polyuria, polydipsia, and weight loss[4].
Therefore, accurate distinction between MODY3 and other types of diabetes is an important challenge in the diagnosis of MODY[5]. Patients with MODY3 are often initially misdiagnosed with other types of diabetes because the correct MODY3 molecular genetic diagnosis approach is not performed[6,7]. Therefore, early identification of MODY3 is critical for patient treatment.
In the treatment of MODY3, the response of pancreatic β cells to sulfonylurea drugs (SUs) is an important parameter[8-10]. Here, we report a Chinese patient with MODY3 involving a new splice mutation (12q24 NM_000545.5 Intron5 c.1108-1G>A) for whom low-dose gliclazide was sufficient to control clinically significant hyperglycemia[11]. The findings indicate that genetic factors are critical for early-onset diabetes. Our report provides insights regarding genetic diagnosis during the treatment of patients with MODY3.
A 22-year-old Chinese man presented to the Affiliated Hospital of Weifang Medical University for treatment of hyperglycemia. The patient had attended follow-up for 28 mo to undergo blood glucose monitoring.
The patient completed the relevant laboratory and imaging examination on admission. His fasting blood glucose and HbA1C levels were 8.08 mmol/L and 7.2%, respectively. Urine tests were positive for glucose. Insulin and C-peptide release test results were as follows: fasting blood glucose, 7.48 mmol/L; postprandial blood glucose (PBG), 15.94 mmol/L; fasting insulin, 7.16 µIU/mL; fasting C-peptide, 2.89 ng/mL; postprandial insulin (2 h later), 22.13 µIU/mL; C-peptide (2 h later), 4.65 ng/mL. The antibodies results were as follows: Glutamic acid decarboxylase antibody, 2.74U/mL; insulin autoantibody, 0.25 U/mL; tyrosine phosphatases antibody: 0.44 U/mL; zinc transporter 8- antibody was negative.
He had no history of previous illness or diabetic ketoacidosis.
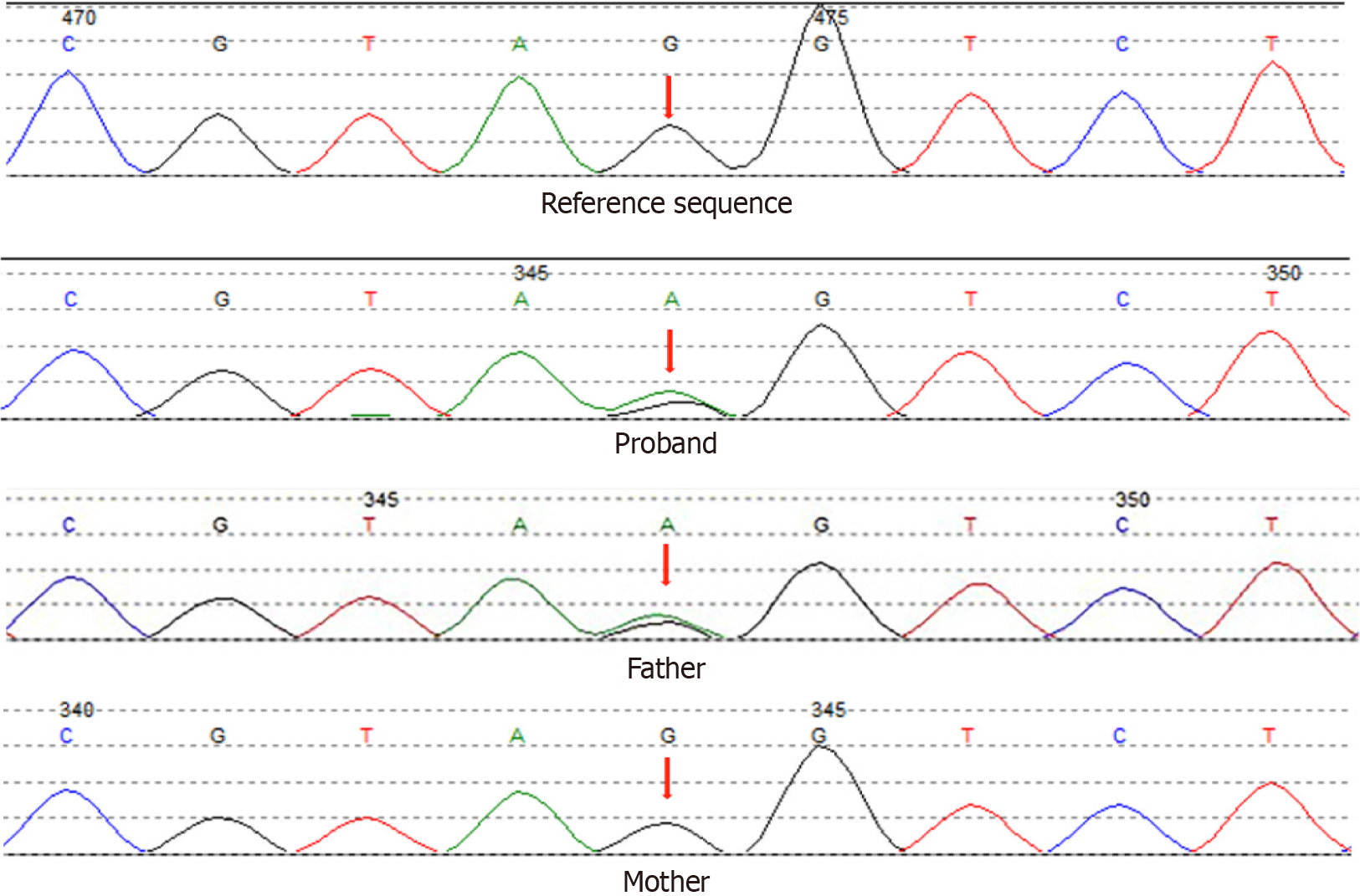
The patient’s father had been diagnosed with diabetes at the age of 45 years, and two of his aunts had been diagnosed at the ages of 48 and 52 years. Furthermore, his grandmother had been diagnosed with diabetes before her death. Gene sequencing analysis confirmed that the patient and his father had an identical mutation site. The gene mutation was not present in the patient’s mother (Figure 1).
The patient completed the relevant laboratory and imaging examination on admission.
The patient’s characteristics were as follows: Age, 25 years; age at onset, 22 years; duration, 3 years; body mass index (BMI), 24.7 kg/m2; weight, 74 kg; waist circumference, 85 cm; systolic BP, 126 mmHg; total triglycerides, 0.78 mmol/L; LDL, 1.95 mmol/L; HDL, 1.07 mmol/L; VITD-T, 21.38 ng/mL; and TG, 0.78 mmol/L.
The ankle-brachial index and somatosensory potential findings were normal. Abdominal ultrasound showed fatty liver (light-medium).
Comprehensive laboratory and imaging examinations could not exclude the presence of a unique type of diabetes. To clarify the cause of the patient’s clinical manifestations, gene sequencing was performed. Sanger sequencing identified an HNF1A splicing mutation in 12q24 NM_000545.5 Intron5 c.1108-1G>A (Figure 1). This substantially affected mRNA splicing, thus causing the coded protein to become disordered and lose its normal function. To the best of our knowledge, there is no related literature reported in the Human Gene Mutation Database; moreover, this mutation is absent from the ESP6500siv2_ALL, dpSNP147, and Thousand Human Genome (1000g2015aug_ALL) databases.
After admission, the patient was treated with insulin glargine; his blood glucose was controlled within 1 wk. Subsequently, treatment was changed to metformin (0.5 g/d) and saxagliptin (5 mg/d). Two months later, the patient’s Hb1Ac level was 5.5%. The genetic testing results supported a diagnosis of MODY3. Because MODY3 is reportedly sensitive to gliclazide, the patient’s treatment was changed to gliclazide (30 mg/d).
The patient's glycemic control has been excellent (fasting blood glucose, 6 mmol/L; Hb1Ac level, 6%). No hypoglycemia episodes or complications have occurred during the past 28 mo of monitoring.
Patients with MODY3 require precise therapy. Animal experiments show that HNF-1A gene mutation results in defective insulin secretion through affecting glucose transport, glycolysis and glucose stimulated-ATP production in B cells[12]. SUs can effectively reduce blood glucose in MODY3 patients on ATP-sensitive potassium channels, while metformin is poor effectiveness[13]. This is the main reason why gliclazide can effectively control glycose. Clinical studies have also shown that the level of blood glucose control is significantly better in patients treated with SUs than in patients treated with insulin[2,6,9,10]. An appropriate SU dose is necessary to reduce the incidence of hypoglycemia. Current recommendations for the treatment of patients with MODY3 involve the use of SUs. Shepherd et al[14] have reported that SUs is critical during MODY3 treatment. If SUs is withdrawn from the diagnosis and treatment plan, glucose deterioration is likely. Notably, our patient showed good blood glucose control when his dose of gliclazide was titrated to 30 mg/d. Diabetes treatment guidelines indicate that the common dose of gliclazide for diabetes treatment can reach 80 mg/d; patients receiving this treatment have a lower risk of hypoglycemia. A study from the United Kingdom showed that medication compliance is not the only influencing factor with respect to SU treatment success. Other important factors include lower glycosylated hemoglobin, lower BMI, and shorter diabetes course[14,15]. Our patient exhibited typical abdominal obesity and had an irregular diet, which included substantial consumption of soft drinks. He was provided with medical guidance to address this lifestyle consideration. Furthermore, unaffected family members were advised to be vigilant for signs of diabetes. To provide appropriate treatment for affected members, a molecular diagnosis of the mutation is recommended. The prevalence of MODY3 among Asian ethnic groups is considered to be low, there is insufficient information available regarding MODY3.
In this report, we have described a new splice mutation and evaluated its effects. The findings indicate that symptoms in patients with non-autoimmune diabetes should be assessed for common genetic causes to clarify their pathogenesis; the assessment results can be used to guide appropriate genetic counseling and treatment indications. Further accumulation of information regarding MODY3 can aid in the identification of disease patterns, as well as timely diagnosis and treatment.
We have described a 25-year-old patient with MODY3, which involved an HNF1A splicing mutation at 12q24. The patient has maintained excellent diabetes control with low-dose SU treatment. To the best of our knowledge, this is the first report of a new gene mutation and exceptional response to SU treatment in a patient with MODY3. Our results highlight the need for genetic diagnosis, particularly in patients with early-onset diabetes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Liang M, Mrozikiewicz-Rakowska B, Papadopoulos VP, Papazafiropoulou A S-Editor: Wang LYT L-Editor: A P-Editor: Wang LYT
| 1. | Siddiqui K, Musambil M, Nazir N. Maturity onset diabetes of the young (MODY)--history, first case reports and recent advances. Gene. 2015;555:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Bansal V, Gassenhuber J, Phillips T, Oliveira G, Harbaugh R, Villarasa N, Topol EJ, Seufferlein T, Boehm BO. Spectrum of mutations in monogenic diabetes genes identified from high-throughput DNA sequencing of 6888 individuals. BMC Med. 2017;15:213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 3. | Plengvidhya N, Tangjittipokin W, Teerawattanapong N, Narkdontri T, Yenchitsomanus PT. HNF1A mutation in a Thai patient with maturity-onset diabetes of the young: A case report. World J Diabetes. 2019;10:414-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Czyrski A, Resztak M, Hermann T. Determination of gliclazide minimum concentration in type 2 diabetes mellitus patients. Biomed Pharmacother. 2018;106:1267-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Carmody D, Lindauer KL, Naylor RN. Adolescent non-adherence reveals a genetic cause for diabetes. Diabet Med. 2015;32:e20-e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Irgens HU, Molnes J, Johansson BB, Ringdal M, Skrivarhaug T, Undlien DE, Søvik O, Joner G, Molven A, Njølstad PR. Prevalence of monogenic diabetes in the population-based Norwegian Childhood Diabetes Registry. Diabetologia. 2013;56:1512-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Gandica RG, Chung WK, Deng L, Goland R, Gallagher MP. Identifying monogenic diabetes in a pediatric cohort with presumed type 1 diabetes. Pediatr Diabetes. 2015;16:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Thirumalai A, Holing E, Brown Z, Gilliam LK. A case of hepatocyte nuclear factor-1β (TCF2) maturity onset diabetes of the young misdiagnosed as type 1 diabetes and treated unnecessarily with insulin. J Diabetes. 2013;5:462-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Johansson BB, Irgens HU, Molnes J, Sztromwasser P, Aukrust I, Juliusson PB, Søvik O, Levy S, Skrivarhaug T, Joner G, Molven A, Johansson S, Njølstad PR. Targeted next-generation sequencing reveals MODY in up to 6.5% of antibody-negative diabetes cases listed in the Norwegian Childhood Diabetes Registry. Diabetologia. 2017;60:625-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Kura RR, Kilari EK, Shaik M. Influence of aprepitant on the pharmacodynamics and pharmacokinetics of gliclazide in rats and rabbits. PeerJ. 2018;6:e4798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Liang H, Zhang Y, Li M, Yan J, Yang D, Luo S, Zheng X, Yang G, Li Z, Xu W, Groop L, Weng J. Recognition of maturity-onset diabetes of the young in China. J Diabetes Investig. 2021;12:501-509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Dukes ID, Sreenan S, Roe MW, Levisetti M, Zhou YP, Ostrega D, Bell GI, Pontoglio M, Yaniv M, Philipson L, Polonsky KS. Defective pancreatic beta-cell glycolytic signaling in hepatocyte nuclear factor-1alpha-deficient mice. J Biol Chem. 1998;273:24457-24464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 124] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Tuomi T, Honkanen EH, Isomaa B, Sarelin L, Groop LC. Improved prandial glucose control with lower risk of hypoglycemia with nateglinide than with glibenclamide in patients with maturity-onset diabetes of the young type 3. Diabetes Care. 2006;29:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Shepherd MH, Shields BM, Hudson M, Pearson ER, Hyde C, Ellard S, Hattersley AT, Patel KA; UNITED study. A UK nationwide prospective study of treatment change in MODY: genetic subtype and clinical characteristics predict optimal glycaemic control after discontinuing insulin and metformin. Diabetologia. 2018;61:2520-2527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 15. | Christensen AS, Hædersdal S, Støy J, Storgaard H, Kampmann U, Forman JL, Seghieri M, Holst JJ, Hansen T, Knop FK, Vilsbøll T. Efficacy and Safety of Glimepiride With or Without Linagliptin Treatment in Patients With HNF1A Diabetes (Maturity-Onset Diabetes of the Young Type 3): A Randomized, Double-Blinded, Placebo-Controlled, Crossover Trial (GLIMLINA). Diabetes Care. 2020;43:2025-2033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |