Published online Feb 26, 2022. doi: 10.12998/wjcc.v10.i6.1883
Peer-review started: August 5, 2021
First decision: November 6, 2021
Revised: November 12, 2021
Accepted: January 11, 2022
Article in press: January 11, 2022
Published online: February 26, 2022
Processing time: 202 Days and 6.6 Hours
Insertions in exon 19 in the epidermal growth factor receptor gene (EGFR) is a rarely seen mutation in non-small cell lung cancer. These patients have been effectively treated with sequential EGFR tyrosine kinase inhibitors (TKIs).
Here, we presented a case of non-small cell lung cancer, stage IIIB, with EGFR exon 19 insertion mutation as detected in the right lower lobe by next-generation sequencing. The patient was sequentially treated with first, second, and third-generation EGFR TKIs after the surgical operation. The overall survival of the patient was 21.3 mo. There was no dynamic analysis of drug resistance mechan
This case emphasized the importance of following the guidelines. In patients with EGFR mutations, repeated and dynamic next-generation sequencing monitoring is necessary to prescribe a personalized treatment plan.
Core Tip: We presented a case of non-small cell lung cancer carrying the rare EGFR exon 19 insertion mutation. The patient had a good and durable response to afatinib, which provided clinical evidence for the use of afatinib in these patients.
- Citation: Shan BB, Li Y, Zhao C, An XQ, Zhang QM. Efficacy of EGFR-TKI sequential therapy in patients with EGFR exon 19 insertion-positive non-small-cell lung cancer: A case report. World J Clin Cases 2022; 10(6): 1883-1888
- URL: https://www.wjgnet.com/2307-8960/full/v10/i6/1883.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i6.1883
The epidermal growth factor receptor (EGFR), a kind of receptor tyrosine kinase, plays critical roles in the initiation, promotion, and progression of malignant tumors by modulating downstream signaling pathways[1]. It has been documented that EGFR is overexpressed and mutated in several tumors, including non-small cell lung cancer (NSCLC)[2]. Apparently, EGFR serves as an important regulator of lung cancer growth, and overexpression of EGFR symbolizes the advancement of lung cancer, which is correlated with poor prognosis[3]. These characteristics suggest EGFR as a promising molecular target for tumor-specific therapy. EGFR mutations occur primarily in the EGFR tyrosine kinase (EGFR-TK) coding region, the target of EGFR tyrosine kinase inhibitors (TKIs)[4]. In NSCLC, especially lung adenocarcinoma, EGFR mutation is an important indicator for the use of EGFR TKIs. Therefore, the detection of EGFR mutation can facilitate the optimal use of these TKIs. The United States Food and Drug Administration has successively approved several EGFR TKIs as standard treatment regimens for NSCLC in first-line treatment. Different EGFR mutants showed different sensitivity to EGFR TKIs.
The most common EGFR mutations are short, in-frame deletions in exon 19 (usually 15 or 18 base pairs) and the exon 21 point mutation L858R and sensitive to the EGFR TKIs[5]. Other EGFR mutations are rare and respond differently to EGFR TKIs. Among these, EGFR exon 20 insertion mutation and T790M mutation are related to drug resistance. G719X, E709K, S768I are reported as moderate sensitive mutations[6]. The EGFR exon19 insertion mutation is also rare and accounts for only 0.11% of all lung cancer patients and 0.23% of EGFR mutation patients in the East Asian population[7]. Studies and case reports have shown that first-generation EGFR TKIs are effective in lung adenocarcinoma patients with EGFR exon 19 insertion. In contrast, second-generation afatinib has limited reports concerning this mutation[8].
Herein, we presented a NSCLC case carrying the rare EGFR exon 19 insertion mutation. The patient had a good and durable response to afatinib, which provides clinical evidence for the use of afatinib in these patients.
In this study, a 63-year-old Chinese male (45 pack year history) presented with chest pain for several days.
Patients a had history of chest pain.
Healthy.
Patient had a long history of heavy smoking.
One month later, the patient underwent right lower lobectomy, right upper lobe wedge resection, and mediastinal lymphadenectomy under general anesthesia. Postoperative pathology suggested that patient had a T4N2M0 (stage IIIB) right lung adenocarcinoma with a positive surgical margin.
Next-generation sequencing (NGS) was performed to identify the targeted mutations and identified an EGFR exon 19 insertion mutation.
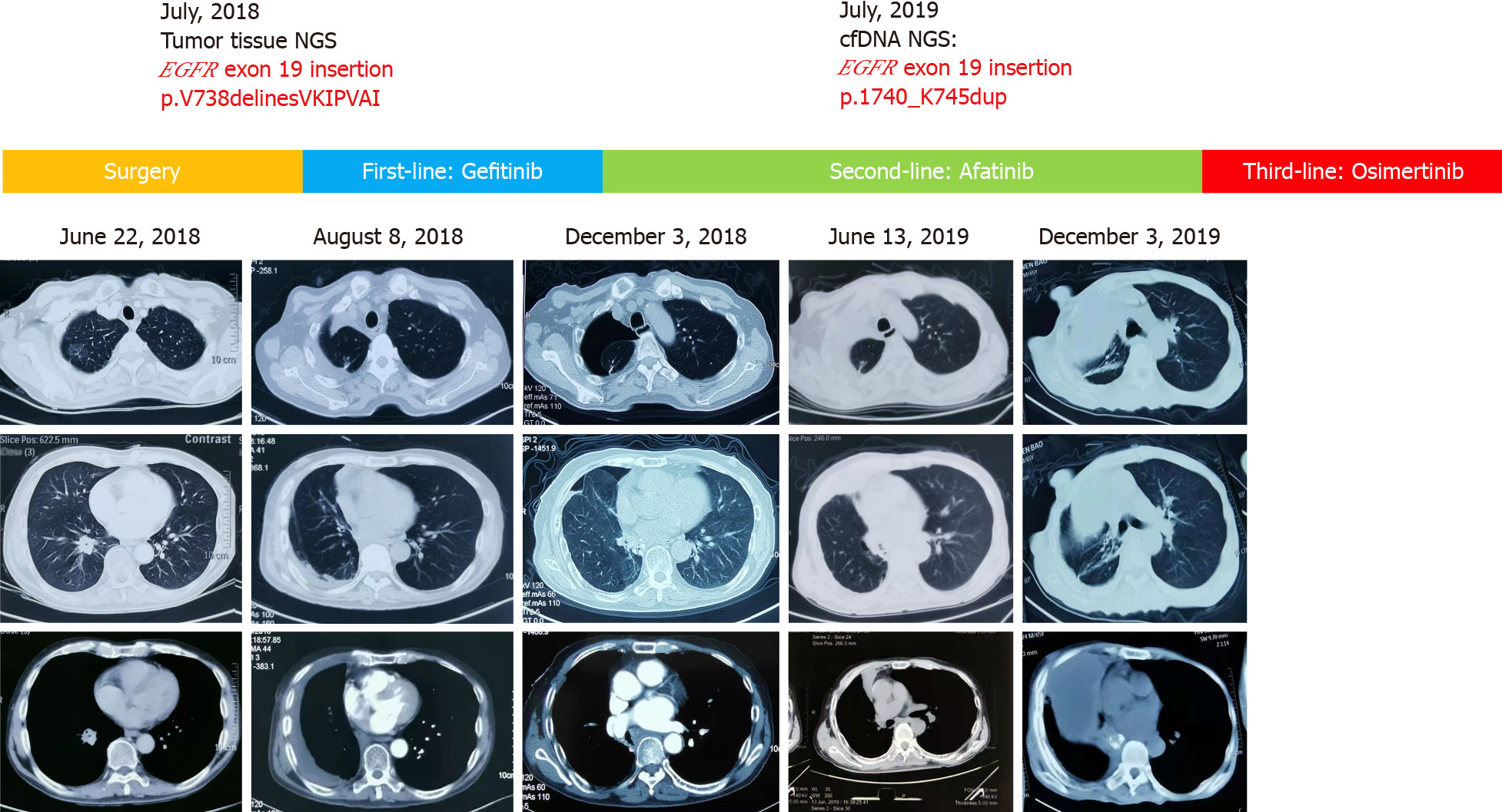
Chest computed tomography (CT) revealed a ground-glass nodule in the right upper lobe (about 2 cm × 1.5 cm), a nodular soft tissue density (about 2.02 cm × 2.7 cm) in the right lower lobe, and the presence of multiple lymph nodes in mediastinal space (Figure 1).
After 1 mo, the patient received Gefitinib, a first-generation EGFR-TKI at a dose rate of 250 mg per day. Four months later, the patient visited again with chest and back pain. The patient underwent preoperative examination, and results showed no distant metastasis, as diagnosed by magnetic resonance imaging, abdominal ultrasound, and full-body bone scan. However, chest CT showed a postoperative change in the right lung. Meanwhile, bone scan showed multiple metastasis bone lesions of the sternum and the left seventh rib (Figure 1). The patient presented with recurrent postoperative metastasis with disease-free survival of 4 mo.
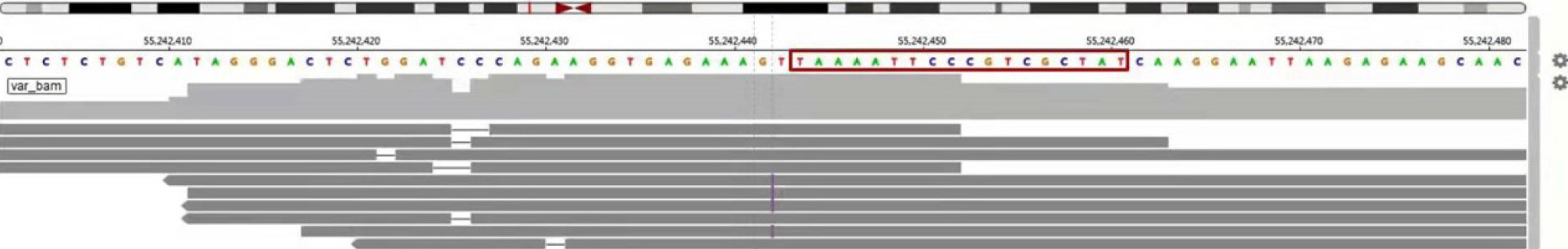
Since the diagnosis of the recurrent metastasis, afatinib 30 mg p.o. daily was started to achieve the symptomatic control of the chest pain. After 8 mo, patient showed slow progression of the right upper lobe lesion (Figure 1). Due to the elevated tumor marker, carcinoembryonic antigen, a second NGS-based genetic testing of 73 cancer-related genes was performed on the patient’s peripheral blood sample (Geneplus-Beijing Ltd., Beijing, China) to identify possible causes and potentially targeted mutations. The test revealed a somatic EGFR exon 19 insertion (NM_005228.3, c.2214_2231dupTAAAATT CCCGTCGCTAT, p.I740_K745dup) (Figure 2), which was identical to the mutation previously detected in the lung cancer samples. The NGS results suggest that afatinib was the best treatment to follow. Therefore, the patient continued to receive oral afatinib for 5 mo. Adverse reactions, such as skin rashes, nausea, vomiting, and diarrhea, were noted during the afatinib treatment course, which were treated symptomatically. Meanwhile, the patient reported the onset of acute sharp chest pain. Chest CT showed multiple bands in both lungs and a right pleural effusion (Figure 1). It is noteworthy that, initially, pleural effusion was slowly elevated without distinct clinical symptoms and pleural effusion puncture and drainage. After that, pleural effusion of the patient was augmented with chest depression, shortness of breath, and poor fluid quality, which was manifested as clinical progress. Later, the patient presented with dyspnea and cachexia, indicating clinical progression. The progression-free survival for the patient treated with afatinib was 13.4 mo. The patient, with no clinical improvement, was then switched to oral Osimertinib treatment, a third-generation EGFR-TKI. The three generations of drugs were taken orally instead of pleural effusion puncture and drainage.
The patient passed away after receiving 2 mo of treatment, with overall survival length of 21.3 mo.
The insertion mutation in exon 19 in EGFR gene is usually sensitive to targeted therapy[9]. Most patients with this mutation are females with adenocarcinoma who are non-smokers or light smokers. In patients with advanced NSCLC, first-generation EGFR TKIs are often used as the postoperative adjuvant or first-line treatment. The efficacy of TKIs fluctuates from 15.5%-24%[8]. A limited number of studies used the second-generation EGFR TKI, which achieved the best clinical results[9]. The case that we presented in this study is a male patient with a history of heavy smoking, who was treated with sequential EGFR-TKIs treatment. However, the efficacy of the first generation of TKI was only 30% and achieved 4 mo disease-free survival. The second-generation TKI afatinib treatment resulted in progression-free survival of 13.4 mo, which could be contributed to the fact that afatinib is a pan human epidermal growth factor receptor family inhibitor[9]. The last sequential use of Osimertinib treatment lasted 2 mo, and the overall survival was 21.3 mo. So far, this is the first time that Osimertinib was used to treat patient carrying EGFR19 insertion mutation. There are reports that the incidence of rare mutation T790M is low after treatment with afatinib[10].
In the course of treatment, the surgical and peripheral blood samples were sent for second-generation sequencing to evaluate the mutations in 73 genes. The sequencing analysis to determine the mutations, including point mutations, small fragment insertions and/or deletions, copy number variations, and known fusion gene variations, are related to tumor occurrence and development. The results showed insertion mutation in exon 19 of EFGR gene. The nucleotide mutation was p.i740 K745dup, which results in the insertion of certain amino acids in the protein encoded by the EGFR gene. Previously, this mutation was not recorded in Catalogue of Somatic Mutations in Cancer and Memorial Sloan Kettering databases[11]. All patients with EGFR exon 19 insertion had amino acid change causing substitution of leucine at residue 747 by proline (L747P). The amino acid sequences of EGFR exon19 insertions reported in the literature include I740_P741insPVAIKI, I740_K745insIPVAIK, I744_K745insKIPVAI, K745_E746insIPVAIK, K745_E746insVPVAIK, and K745_E746insTPVAIK. Among these, the first four forms of mutation cause the same changes in the amino acid sequence. This amino acid change finally activates the tyrosine phosphorylation by binding with ligands. Autophosphorylation promotes downstream signal transduction pathways, including mitogen-activated protein kinase, phosphatidylinositol 3 kinase, and jun N-terminal kinase pathways, which induce cell proliferation and differentiation[12].
The patient reported in the current report had lesions in both right upper and lower lobes, as demonstrated on preoperative chest CT images, and there were ground-glass nodules in both lung lobes. The postoperative pathology of both nodules was adenocarcinoma. Mediastinal lymph nodes were positive. It is unclear whether they are both primary foci or one of them metastasized from the other. The earliest diagnostic criteria for multiple primary lung cancer (MPLC) was reported by Martini[13], which focused on different tissue types. With the development of molecular pathology, the American Association of Chest Physicians revised the diagnostic criteria of MPLC. The new criteria classified the simultaneous multiple cancers located in different lobes without N2 and N3 lymph node infiltration and without systemic metastasis as MPLC. It also added molecular genetic characteristics. The histological subtype of lung adenocarcinoma is recommended to distinguish MPLC from lung metastasis[14]. There is literature showing that second-generation sequencing can be used to increase the diagnostic accuracy. There is a general consensus among several countries on the treatment of MPLC. However, the surgical treatment is considered as the first choice. Stella et al[15] suggested that surgical treatment should be performed no matter whether the multiple lesions are MPLC or pulmonary metastasis, as long as the lung function is acceptable and there is no lymph node metastasis.
In conclusion, we presented a case of lung adenocarcinoma with rare EGFR exon 19 insertion benefitting from afatinib therapy. This case provides unequivocal clinical evidence for the afatinib effectiveness in lung adenocarcinoma patients harboring EGFR exon 19 insertion and also provides evidence that these patients may benefit from EGFR TKIs sequential therapy. The treatment of this case is worth further discussing the importance of following the guidelines and initiating the standardized treatment. On the other hand, in the case of two nodules in different lobes, more molecular diagnosis is required to confirm the origin of the two nodules, which would be helpful for the selection of suitable drugs.
We acknowledge the contributions to this study from the patient and his family, the pathology department, and the radiology department.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell biology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kermenli T S-Editor: Liu JH L-Editor: Filipodia P-Editor: Liu JH
| 1. | de Marinis F, Laktionov KK, Poltoratskiy A, Egorova I, Hochmair M, Passaro A, Migliorino MR, Metro G, Gottfried M, Tsoi D, Ostoros G, Rizzato S, Mukhametshina GZ, Schumacher M, Novello S, Dziadziuszko R, Tang W, Clementi L, Cseh A, Kowalski D. Afatinib in EGFR TKI-naïve patients with locally advanced or metastatic EGFR mutation-positive non-small cell lung cancer: Interim analysis of a Phase 3b study. Lung Cancer. 2021;152:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Chen CY, Yu ZY, Chuang YS, Huang RM, Wang TC. Sulforaphane attenuates EGFR signaling in NSCLC cells. J Biomed Sci. 2015;22:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Zhen JJ, Li SQ, Wen L, Lai MY, Cai LB. Cerebrospinal fluid carcinoembryonic antigen predict prognosis in leptomeningeal metastasis from non-small cell lung cancer. Neuro-Oncology. 2019;21:52. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Gelatti ACZ, Drilon A, Santini FC. Optimizing the sequencing of tyrosine kinase inhibitors (TKIs) in epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC). Lung Cancer. 2019;137:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 165] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 5. | Hung MS, Fang YH, Lin YC, Lung JH, Hsieh MJ, Tsai YH. Survival-associated factors of first-line EGFR-tyrosine kinase inhibitor responders and non-responders in lung adenocarcinoma patients with common EGFR mutations. Mol Clin Oncol. 2018;8:421-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Jung HA, Park S, Sun JM, Lee SH, Ahn JS, Ahn MJ, Park K. Treatment and Outcomes of Metastatic Non-Small-Cell Lung Cancer Harboring Uncommon EGFR Mutations: Are They Different from Those with Common EGFR Mutations? Biology (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | He M, Capelletti M, Nafa K, Yun CH, Arcila ME, Miller VA, Ginsberg MS, Zhao B, Kris MG, Eck MJ, Jänne PA, Ladanyi M, Oxnard GR. EGFR exon 19 insertions: a new family of sensitizing EGFR mutations in lung adenocarcinoma. Clin Cancer Res. 2012;18:1790-1797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Lin YT, Liu YN, Wu SG, Yang JC, Shih JY. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor-sensitive Exon 19 Insertion and Exon 20 Insertion in Patients With Advanced Non-Small-cell Lung Cancer. Clin Lung Cancer. 2017;18:324-332.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 9. | Masood A, Kancha RK, Subramanian J. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in non-small cell lung cancer harboring uncommon EGFR mutations: Focus on Afatinib. Semin Oncol. 2019;46:271-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 10. | Yang S, Mao S, Li X, Zhao C, Liu Q, Yu X, Wang Y, Liu Y, Pan Y, Wang C, Gao G, Li W, Xiong A, Chen B, Sun H, He Y, Wu F, Chen X, Su C, Ren S, Zhou C. Uncommon EGFR mutations associate with lower incidence of T790M mutation after EGFR-TKI treatment in patients with advanced NSCLC. Lung Cancer. 2020;139:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Su J, Zhong W, Zhang X, Huang Y, Yan H, Yang J, Dong Z, Xie Z, Zhou Q, Huang X, Lu D, Yan W, Wu YL. Molecular characteristics and clinical outcomes of EGFR exon 19 indel subtypes to EGFR TKIs in NSCLC patients. Oncotarget. 2017;8:111246-111257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Xu J, Jiang Q, Xu H, Liu A, Huang L. Two Patients Having NSCLC With Novel Duplication Mutation in Their EGFR Gene (p.I740_K745dupIPVAIK) and Their Response to Osimertinib. J Thorac Oncol. 2020;15:e49-e51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg. 1975;70:606-612. [PubMed] |
| 14. | Kozower BD, Larner JM, Detterbeck FC, Jones DR. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e369S-e399S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 263] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 15. | Stella F, Luciano G, Dell'Amore A, Greco D, Ammari C, Giunta D, Bini A. Pulmonary Metastases from NSCLC and MPLC (Multiple Primary Lung Cancers): Management and Outcome in a Single Centre Experience. Heart Lung Circ. 2016;25:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |