Published online Feb 26, 2022. doi: 10.12998/wjcc.v10.i6.1869
Peer-review started: June 21, 2021
First decision: July 26, 2021
Revised: September 7, 2021
Accepted: January 19, 2022
Article in press: January 19, 2022
Published online: February 26, 2022
Processing time: 246 Days and 22 Hours
Tuberculous pericarditis (TP) remains a challenge for endemic countries. In developing countries, one to two percent of patients with pulmonary tuberculosis develops TP.
A 49-year-old woman presented with dyspnea, chest pain and dry cough. On physical examination, veiled heart sounds were found. The electrocardiogram showed low-voltage complexes and the transthoracic echocardiography revealed a large and free-looking pericardial effusion. The patient was taken for an open pericardiotomy. The pericardial fluid revealed high levels of adenosine deaminase and Ziehl-Neelsen stain showed acid-fast bacilli. Polymerase chain reaction study for Mycobacterium tuberculosis in pericardial fluid was positive. The patient received tetra conjugate management with adequate clinical response after the first week of treatment and resolution of fever and chest pain.
In cases of TP, obtaining pericardial fluid and/or pericardial biopsy is the most efficient strategy to confirm the diagnosis. Early diagnosis of this entity will allow physicians to initiate timely treatment, avoid complications and improve the patient's clinical outcome, so we consider the description of this case pertinent and its review in the literature.
Core Tip: Tuberculous pericarditis should be suspected in the evaluation of all cases of pericarditis that do not have a self-limited course. The present case identifies the usefulness of the study of Adenosine deaminase in the pericardial fluid and the performance of polymerase chain reaction for Mycobacterium tuberculosis in the biopsy, thanks to which the diagnosis could be confirmed. Management is based on the use of rifampin, isoniazid, ethambutol, and pyrazinamide.
- Citation: Lucero OD, Bustos MM, Ariza Rodríguez DJ, Perez JC. Tuberculous pericarditis-a silent and challenging disease: A case report. World J Clin Cases 2022; 10(6): 1869-1875
- URL: https://www.wjgnet.com/2307-8960/full/v10/i6/1869.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i6.1869
Tuberculosis (TB) is a transmissible disease and it is the main cause of morbimortality and one of the ten leading causes of death worldwide. It is also the leading cause of death from a single infectious agent [ahead of human immunodeficiency virus (HIV)/AIDS]. A quarter of the world’s population is infected with Mycobacterium tuberculosis and thus is at risk of developing the disease[1]. In developing countries, coinfection with the HIV is associated with a higher mortality[2]. Although the lung is the target organ for TB, any other organ can be affected. Extrapulmonary TB accounts for 10%-20% of the clinical presentation suffered by immunocompetent patients[3]. Approximately 1% to 2% of patients with pulmonary TB have tuberculous pericarditis (TP) and this carries a high mortality rate (17% to 40% over 6 mo)[4].
Pericarditis can be caused by infectious agents (e.g., viral and bacterial) or can be non-infectious in origin (e.g., systemic inflammatory diseases, cancer, and post-cardiac injury syndromes). TB is a major cause of pericarditis in developing countries, but accounts for less than 5% of cases in developed countries, where viral causes are responsible for 80% to 90% of the cases[5]. TP accounts for approximately 4% of acute pericarditis, 7% of cardiac tamponade and 6% of constrictive pericarditis cases[6]. Mycobacterium tuberculosis usually affects the pericardium by retrograde lymphatic spread from the peritracheal, peribronchial, or mediastinal lymph nodes or by hematogenous spread of a primary TB infection and is rarely affected by contiguous spread of a tuberculous lesion in the lung or hematogenous spread of a distant focus[7].
A 49-year-old woman came to the emergency room for unquantified fever peaks and intermittent chest pain lasting 2 mo.
Unquantified fever peaks and intermittent chest pain lasting 2 mo which increased in intensity.
During the 2 wk prior to admission, the patient experienced dyspnea, cough and hyaline expectoration.
The patient had a surgical history of liposuction and augmentation mammoplasty.
The patient was febrile with a temperature of 39.5 °C. Her heart rate 117 beats per minute, blood pressure 100/60 mmHg, respiratory rate of 24 breaths per minute and her oxygen saturation was 80%. The patient’s neck veins were distended and heart sounds were muffled.
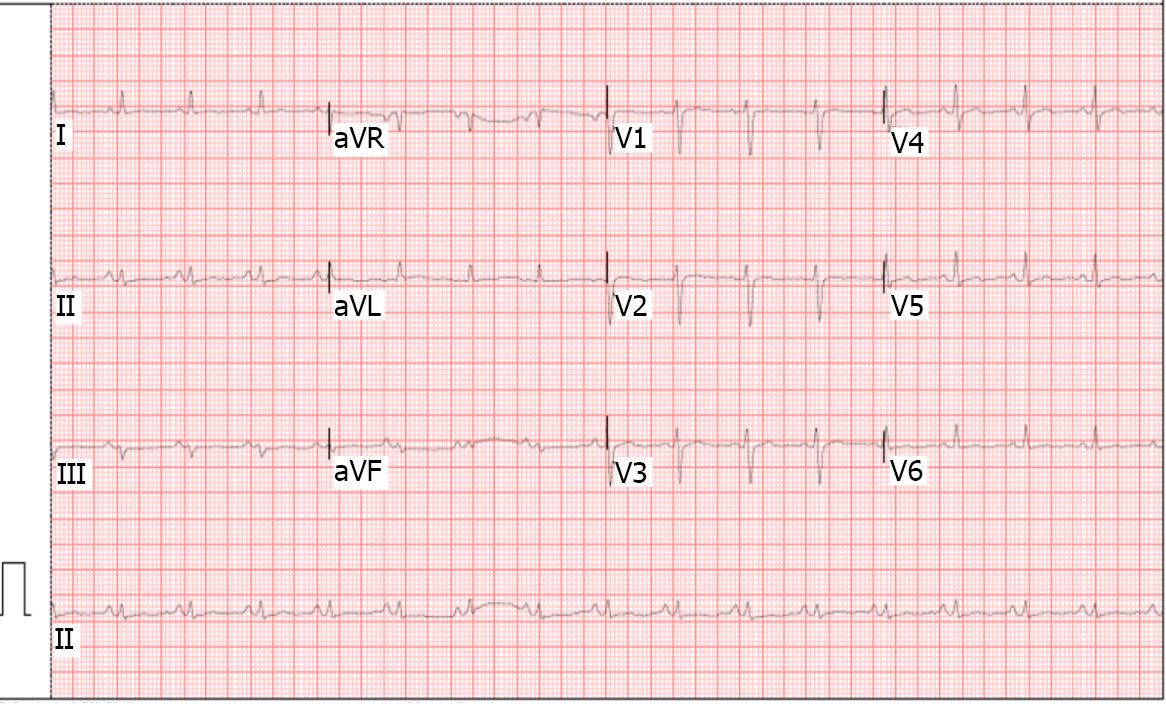
Electrocardiogram was done (Figure 1) and showed sinus tachycardia and low-voltage complexes in the limb and precordial leads. There were no alterations in PQ interval or ST-segment. Deaminase level in the pericardial fluid of 36.50 U/L (reference range, 0 to 9 U/L). The cytology of the pericardial fluid was of lymphocytic predominance and examination of the pericardial fluid revealed acid-fast bacilli. Polymerase chain reaction (PCR) study of pericardial fluid was positive for Mycobacterium tuberculosis.
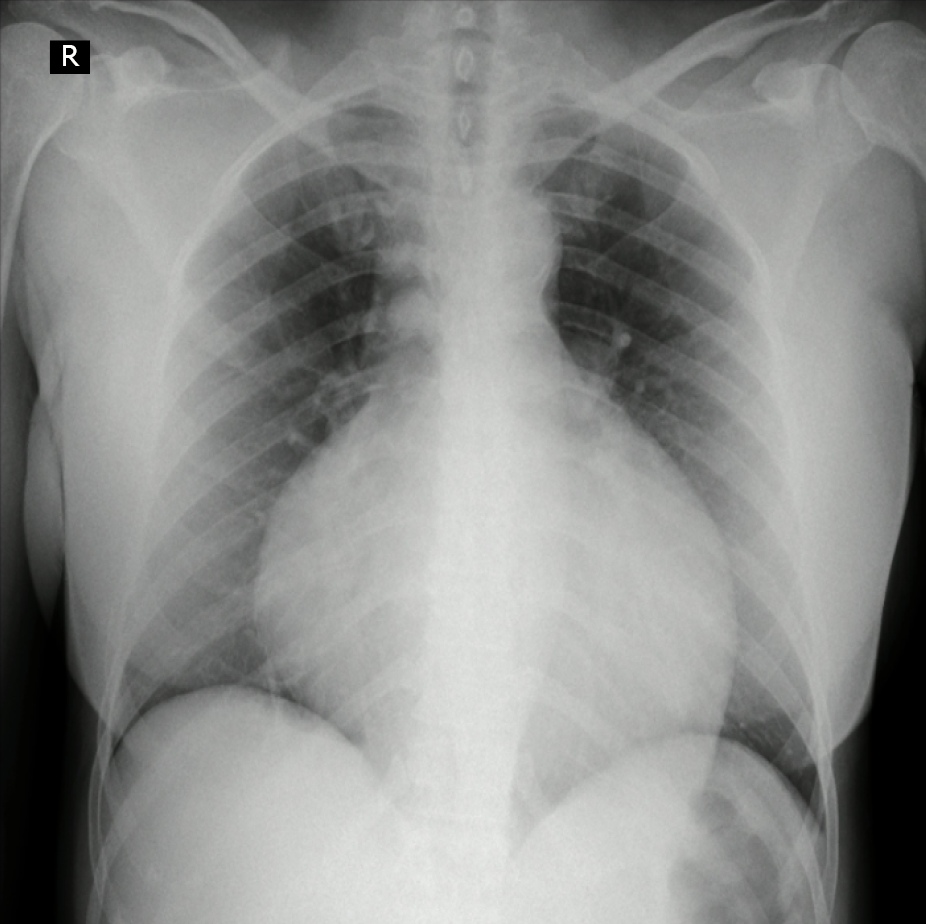
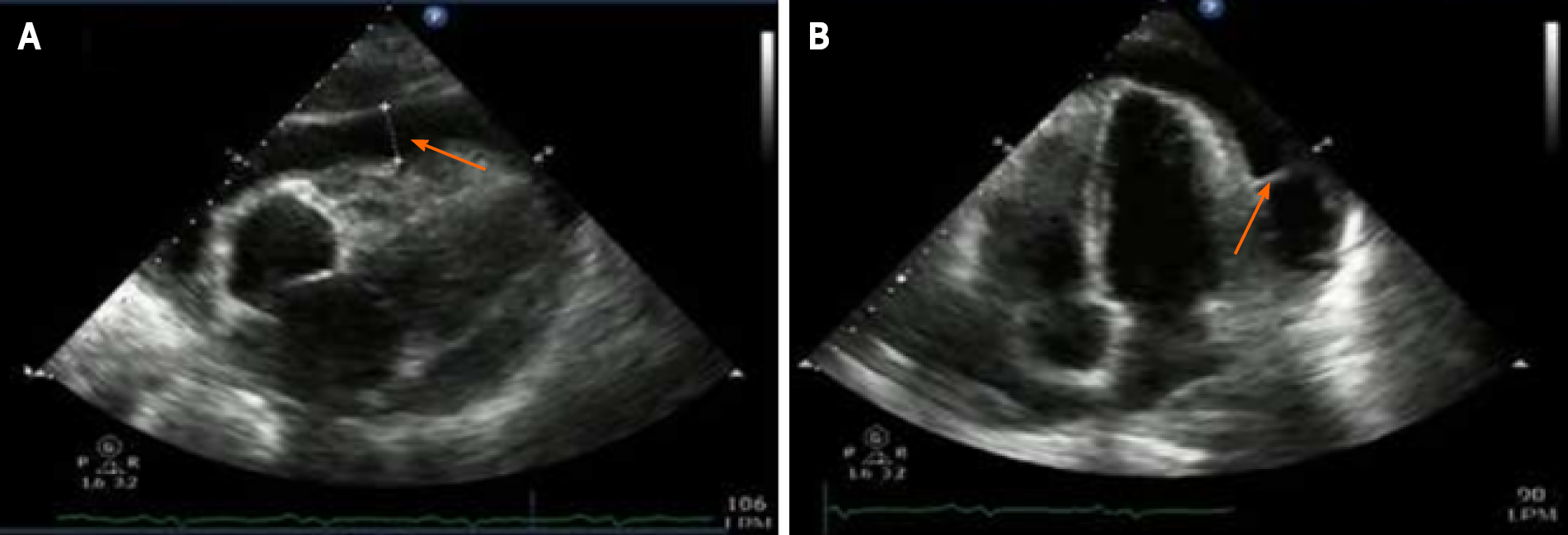
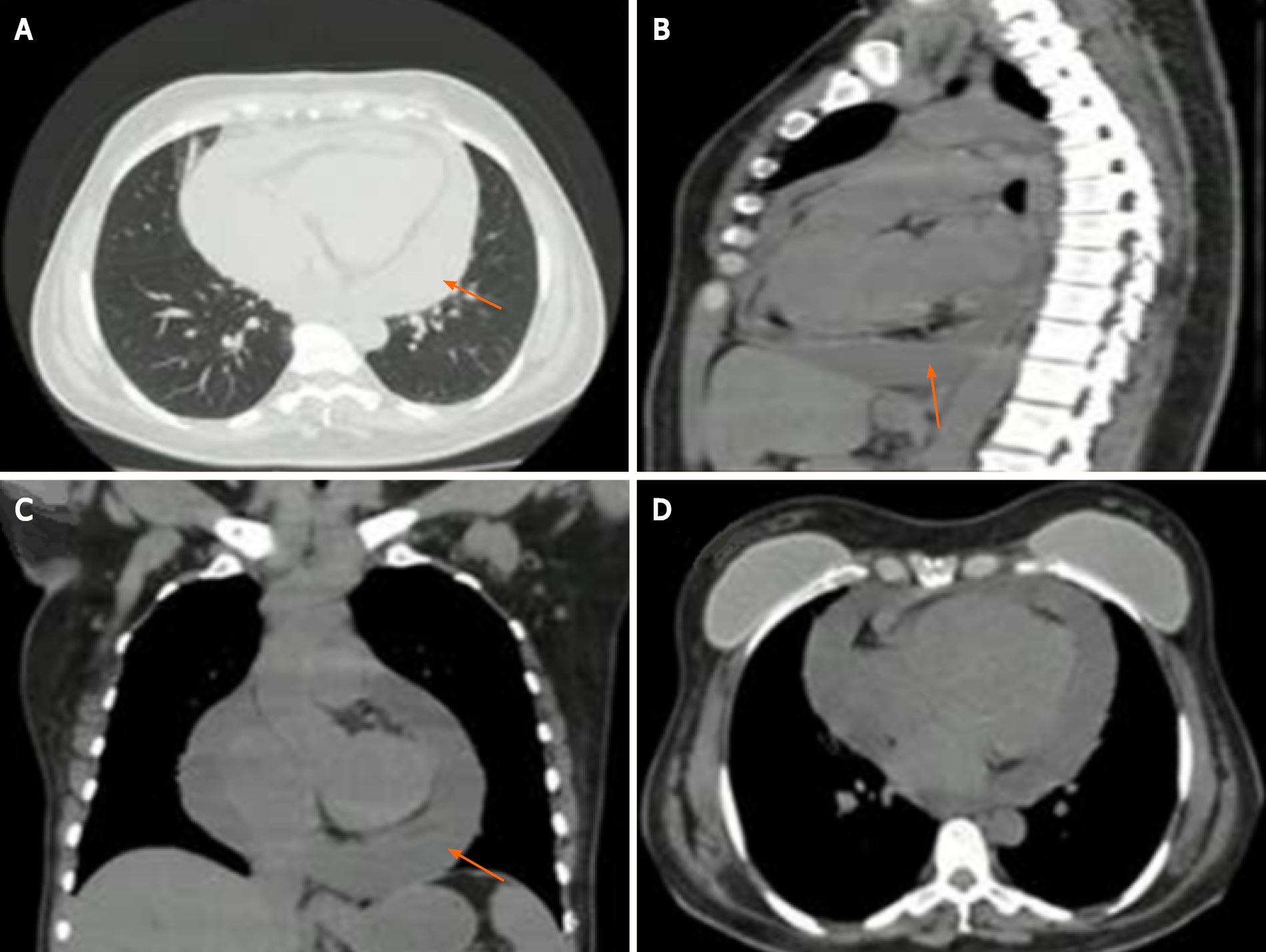
Chest x-ray was done (Figure 2) showing a generalized increase of the mediastinal cardiac silhouette, suggestive of pericardial effusion. There was an adequate pulmonary vascularization pattern. Pleuropulmonary lesions were not seen. Transthoracic echocardiography revealed a large and free-looking pericardial effusion, with an anterior interface of 17 mm and a posterior interface of 27 mm. The right cavities had a partial diastolic collapse, between 20%-30% (Figure 3). High-resolution chest tomography revealed a heart of normal size and there was evidence of pericardial effusion without alteration of the ventricular walls with a thickness of up to 25 mm (Figure 4).
TP was made. HIV was negative.
Tuberculous pericarditis.
The patient received treatment each day with isoniazid 300 mg, rifampin 600 mg, pyrazinamide 1600 mg, and ethambutol 1100 mg for 2 mo. This was followed by rifampin and isoniazid for an additional 4 mo along with prednisolone for 6 wk.
In the days following pericardiocentesis and initiation of tetraconjugate management for TB, the patient progressed satisfactorily with improved hemodynamic parameters and fever resolution.
TP has a diverse clinical picture and should be considered in the evaluation of non-self-limiting pericarditis cases. Despite the fact that the prevalence of TB has been reduced in general, the cases of extrapulmonary TB remains stable; approximately 1050000 new cases were reported in the world in 2018[8]. In developing countries, one to two percent of pulmonary TB patients develop TP. However, it can also appear as an isolated extrapulmonary form[9]. There are few studies that evaluate the prevalence of TP in Colombia, and generally, it is considered that there is an underdiagnosis of the disease. 13626 new cases of TB were reported during 2020, of which 83% corresponded to pulmonary TB and 17% to extrapulmonary TB, while 1.6% of these corresponded to TP[9]. Furthermore, in Colombia, a progressive increase in the extrapulmonary TB rate has been observed: 2.1 cases/100000 inhabitants in 1997; 3.7/100000 in 2006 and 4.4/100000 in 2018. Less than 1% of these cases correspond to TP, and generally, it occurs in patients coinfected with HIV who constitute about 85% of cases[10].
TP usually develops insidiously and with nonspecific systemic symptoms such as fever, night sweats, fatigue and weight loss. Although chest pain, cough, and dyspnea are common, the severe acute-onset pericardial pain characteristic of idiopathic pericarditis is rare[10]. Large pericardial effusion should be suspected when micro voltage is observed on the electrocardiogram. This is seen in complexes < 5 mm in the limb leads and < 10 mm in the precordial leads[11]. Chest radiography generally shows a widening of the cardiac silhouette in more than 90% of cases, with a globular image described as “Bottle of water” configuration. Generally, these findings are observed in conjunction with active lung TB and pleural effusion in 30% and 60% of cases, respectively[12]. On the echocardiogram, TP usually comes accompanied by pericardial effusion with thickening of the visceral pericardium and it is possible to identify fibrin bands or fibrosis that heals the pericardium[13].
TP diagnosis is confirmed if one of the following criteria is present: Positive culture for the bacillus of Koch in pericardial fluid, positive direct examination for Koch bacillus, or value greater than 50 IU/L on the Adenosine deaminase (ADA) test[14]. Furthermore, the diagnosis can be confirmed if the pericardial biopsy shows the following findings: Positive culture for the Koch bacillus and granulomas with caseous necrosis, or presence of Langhans-type multinucleated giant cells, or presence of tubercle bacilli in the sample[15].
The diagnosis of TP remains difficult due to the absence of a simple, rapid and accessible diagnostic test despite its associated morbidity and mortality. A protein-rich lymphocytic exudate that is often grossly hemorrhagic is a typical finding in the pericardial fluid. However, the TP fluid is paucibacillary and the estimated diagnostic accuracy based on the smear is only 5%[16]. The sensitivity of pericardial fluid culture ranges from 53% to 75%[17]. However, results are obtained in an average of 3 wk. PCR test for Mycobacterium tuberculosis DNA or RNA in pericardial fluid is more accessible and faster, and it has a lower cost than pericardial tissue PCR, but it also has a lower sensitivity (15% vs 80% respectively), and can yield up to 20% false positives[18]. PCR has been very useful since it is capable of identifying different nucleic acid sequences in samples with low bacilli concentrations and in addition, it identifies resistance to rifampicin encoded in the rpoB gene, which can be useful in settings where there is a high prevalence of multidrug-resistant TB. Finally, in recent years, the performance of the immunoassay has been studied which quantifies the release of interferon gamma (IFN-γ) (QuantiFERON®, ELISpot) for the diagnosis of pulmonary TB, extrapulmonary TB and latent TB. The sensitivity of pericardial biopsy varies from 10% to 64%[19].
Pericardial ADA levels ≥ 35 U/L are diagnostic of TP with a sensitivity and specificity of 90% and 74%, respectively[20]. On the other hand, it has been shown that IFN-γ, which is produced by CD-4 + and CD-8 + T lymphocytes in the context of TP, could be a precise diagnostic biomarker[21].
Treatment of TP is based on the use of rifampicin, isoniazid, pyrazinamide, and ethambutol for 2 mo, followed by isoniazid and rifampicin (a total of 6 mo of treatment)[22]. The effectiveness of treatment with corticosteroids in TP remains controversial. Steroids have not yet been found to have overwhel
TP occurs in 1%-2% of patients with pulmonary TB and carries a high mortality rate. The diagnosis of TP remains difficult due to the absence of a simple, rapid and accessible diagnostic test despite its associated morbidity and mortality. Treatment of TP consists of rifampicin, isoniazid, pyrazinamide, and ethambutol for 2 mo, followed by isoniazid and rifampicin (total of 6 mo of therapy). Early diagnosis of this entity will allow physicians to initiate timely treatment, avoid complications and improve the patient’s clinical outcome.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Colombia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Basu S, Keikha M, Samadder S S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | World Health Organization. Global tuberculosis report 2019. [cited 10 May 2021]. Available from: https://www.aidsdatahub.org/resource/global-tuberculosis-report-2019. |
| 2. | Furin J, Cox H, Pai M. Tuberculosis. Lancet. 2019;393:1642-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 547] [Article Influence: 91.2] [Reference Citation Analysis (0)] |
| 3. | Natarajan A, Beena PM, Devnikar AV, Mali S. A systemic review on tuberculosis. Indian J Tuberc. 2020;67:295-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 4. | Isiguzo G, Du Bruyn E, Howlett P, Ntsekhe M. Diagnosis and Management of Tuberculous Pericarditis: What Is New? Curr Cardiol Rep. 2020;22:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 5. | Munn-Mace G, Parmar D. Treatment of tuberculosis in complex emergencies in developing countries: a scoping review. Health Policy Plan. 2018;33:247-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Imazio M, Gaita F, LeWinter M. Evaluation and Treatment of Pericarditis: A Systematic Review. JAMA. 2015;314:1498-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 258] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 7. | López-López JP, Posada-Martínez EL, Saldarriaga C, Wyss F, Ponte-Negretti CI, Alexander B, Miranda-Arboleda AF, Martínez-Sellés M, Baranchuk A; Neglected Tropical Diseases, Other Infectious Diseases Affecting the Heart (the NET‐Heart Project). Tuberculosis and the Heart. J Am Heart Assoc. 2021;10:e019435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 8. | Daniels B, Kwan A, Pai M, Das J. Lessons on the quality of tuberculosis diagnosis from standardized patients in China, India, Kenya, and South Africa. J Clin Tuberc Other Mycobact Dis. 2019;16:100109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | López MP. Informe eventotuberculosis a periodo XI-2020.Bogotá: Instituto Nacional de Salud; 2020. [cited 10 May 2021]. Available from: https://www.ins.gov.co/buscador-eventos/Lineamientos/PRO_Tuberculosis.pdf. |
| 10. | Amado SB, Moreno S, Martínez S, Lasso JI, Lasserna AF. Tuberculosis extrapulmonar: un reto clínico vigente. Univ Med. 2020;61. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Jorquera-Román M, Araya-Cancino J, Enríquez-Montenegro J, Obando-Valdés J, Reyes-Cornejo F, Gutiérrez OB, Monasterio-Angulo V, Jofre-Tobar N, Verdugo JB, Rojas FR. [Tuberculous pericarditis an infrequent extrapulmonary manifestation of TB]. Rev Med Chil. 2021;149:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Ren Y, Qiu J, Li Z, Li C. P-wave terminal force in lead V1 is a predictive indicator for the diagnosis of tuberculous constrictive pericarditis. Heart Lung. 2019;48:155-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Howlett P, Du Bruyn E, Morrison H, Godsent IC, Wilkinson KA, Ntsekhe M, Wilkinson RJ. The immunopathogenesis of tuberculous pericarditis. Microbes Infect. 2020;22:172-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Huang YS, Zhang JX, Sun Y. Chronic massive pericardial effusion: a case report and literature review. J Int Med Res. 2020;48:300060520973091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Echeverri Dl, Matta L. Pericarditis tuberculosa. Biomédica. 2014;34. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Andrianto A, Mertaniasih NM, Gandi P, Al-Farabi MJ, Azmi Y, Jonatan M, Silahooij SI. Diagnostic test accuracy of Xpert MTB/RIF for tuberculous pericarditis: a systematic review and meta-analysis. F1000Res. 2020;9:761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | GBD 2019 Tuberculosis Collaborators. Global, regional, and national sex differences in the global burden of tuberculosis by HIV status, 1990-2019: results from the Global Burden of Disease Study 2019. Lancet Infect Dis. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 18. | Naicker K, Ntsekhe M. Tuberculous pericardial disease: a focused update on diagnosis, therapy and prevention of complications. Cardiovasc Diagn Ther. 2020;10:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Kyriakakis CG, Mayosi BM, de Vries E, Isaacs A, Doubell AF. An approach to the patient with suspected pericardial disease. S Afr Med J. 2016;106:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Hu X, Xing B, Wang W, Yang P, Sun Y, Zheng X, Shang Y, Chen F, Liu N, Yang L, Zhao Y, Tan J, Zhang X, Wang Y, Zhang Z, Liu Y. Diagnostic values of Xpert MTB/RIF, T-SPOT.TB and adenosine deaminase for HIV-negative tuberculous pericarditis in a high burden setting: a prospective observational study. Sci Rep. 2020;10:16325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 21. | Lima NA, Lino DODC, Coelho NM, Melgar T. Tuberculous constrictive pericarditis. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Uchi T, Hakuno D, Fukae T, Takahashi M, Takiguchi S, Li HC, Nishizawa K, Nozaki H, Sueyoshi K. Armored Heart Because of Tuberculous Constrictive Pericarditis. Circ Cardiovasc Imaging. 2019;12:e008726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Tzani A, Doulamis IP, Tzoumas A, Avgerinos DV, Koudoumas D, Siasos G, Vavuranakis M, Klein A, Kampaktsis PN. Meta-Analysis of Population Characteristics and Outcomes of Patients Undergoing Pericardiectomy for Constrictive Pericarditis. Am J Cardiol. 2021;146:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |