Published online Feb 26, 2022. doi: 10.12998/wjcc.v10.i6.1834
Peer-review started: July 27, 2021
First decision: October 3, 2021
Revised: October 16, 2021
Accepted: January 17, 2022
Article in press: January 17, 2022
Published online: February 26, 2022
Processing time: 211 Days and 7.3 Hours
D2 lymph node dissection for advanced gastric cancer is advocated, and station 8p lymph node should be considered in selected patients, which is, however, technically difficult.
To introduce a new and easy-to-perform procedure for dissection of the lymph nodes superior to the pancreas.
A series of patients who underwent laparoscopic gastrectomy for gastric cancer were retrospectively included with utilization of a new procedure for superior pancreatic lymphadenectomy (LND) with portal vein priority via the posterior common hepatic artery approach (SPLD-PPPH) based on a newly defined portal triangle. The surgical outcome of the patients, as well as the efficacy and safety of SPLD-PPPH are reported.
A total of 51 patients were included with most of them being male (n = 34, 66.7%). According to the 8th edition of AJCC TNM staging, there were four (7.8%) patients in stage I, 13 (25.5%) in stage II, 33 (64.7%) in stage III and one (2.0%) in stage IV. The average duration for LND was about 1 h (67.7 ± 6.9 min). After surgery, four patients developed morbidities, but all were treated successfully with no perioperative mortality. Among the 51 patients included, the percentage of patients who had lymph node metastasis at station 8p was 9.8%. Of note, with a total of 14 lymph nodes harvested at station 8p, the incidence of nodal metastasis was 14.3%.
About one in 10 patients with advanced gastric cancer had nodal metastasis at station 8p. The new approach of SPLD-PPPH is safe and effective for D2+ LND during laparoscopic radical gastrectomy.
Core Tip: D2 radical operation has been the standard treatment of gastric cancer. One in 10 advanced gastric cancer patients had lymph node metastasis at station 8p, but D2 lymphadenectomy (LND) remains technically difficult. The new superior pancreatic LND with portal vein priority via the posterior common hepatic artery approach achieved safe removal of 8p lymph nodes.
- Citation: Zhang YJ, Xiang RC, Li J, Liu Y, Xie SM, An L, Li HL, Mai G. Superior pancreatic lymphadenectomy with portal vein priority via posterior common hepatic artery approach in laparoscopic radical gastrectomy. World J Clin Cases 2022; 10(6): 1834-1842
- URL: https://www.wjgnet.com/2307-8960/full/v10/i6/1834.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i6.1834
Laparoscopic gastrectomy for gastric cancer has been demonstrated as safe, feasible and less invasive with comparable long-term outcomes vs open surgery[1-3]. At present, D2 lymphadenectomy (LND) has been the standard treatment for locally advanced gastric cancer[4-7]. However, the overall complication rate after D2 radical operation is about 20%[4-7]. The most serious complications are bleeding, pancreatic fistula, anastomotic leakage and abdominal infection. In fact, during the procedure of D2 radical LND, it is difficult to dissect the local lymph nodes due to the limitation of the range of movement of the instruments, and the pancreas is compressed during upper pancreatic dissection, resulting in a higher incidence of postoperative pancreatic fistula.
Although D2 LND has achieved global consensus for advanced gastric cancer, whether station 8p should be dissected routinely remains controversial[8-10]. Some studies have found that the incidence of station 8p nodal metastasis increases as the AJCC T category is upstaged, with rate of station 8p lymph node metastasis (LNM) of advanced gastric cancer ranging from 12.9% to 16.4%[3]. In addition, residual station 8p LNM was one of the major causes of local recurrence of gastric cancer after initial resection. An increased number of surgeons have advocated dissection of station 8p during surgical resection for advanced gastric cancer[11-13]. However, the lymph nodes of station 8p are located behind the common hepatic artery, making complete dissection difficult. How to safely remove the station 8p lymph node remains to be optimized. Based on the anatomy of the portal triangle, the current study investigated the safety and efficacy of superior pancreatic LND with portal vein priority via the posterior common hepatic artery approach (SPLD-PPPH) for laparoscopic radical gastrectomy.
Data from 51 consecutive patients with gastric cancer who underwent laparoscopic D2+ radical resection between June 2018 and June 2020 in our hospital were retrospectively included. Among all the patients included, superior pancreatic LND was performed with portal vein priority via the posterior common hepatic artery approach. The scope of regional LND included stations 5, 7, 8a, 8p, 9, 11p, 12a and 12p. A standard datasheet was utilized to collect data at each institution. Demographic factors, including age, gender and body mass index (BMI), operation time, intraoperative blood loss were documented, whereas the tumor-related characteristics, total number and station of lymph node examined, as well as number and station of the positive nodes were collected based on final pathological report. The study was approved by the Ethics Committee of Deyang City People’s Hospital. A waiver of informed consent was obtained, since the data were analyzed from the electronic medical record and reported without personal identifiers.
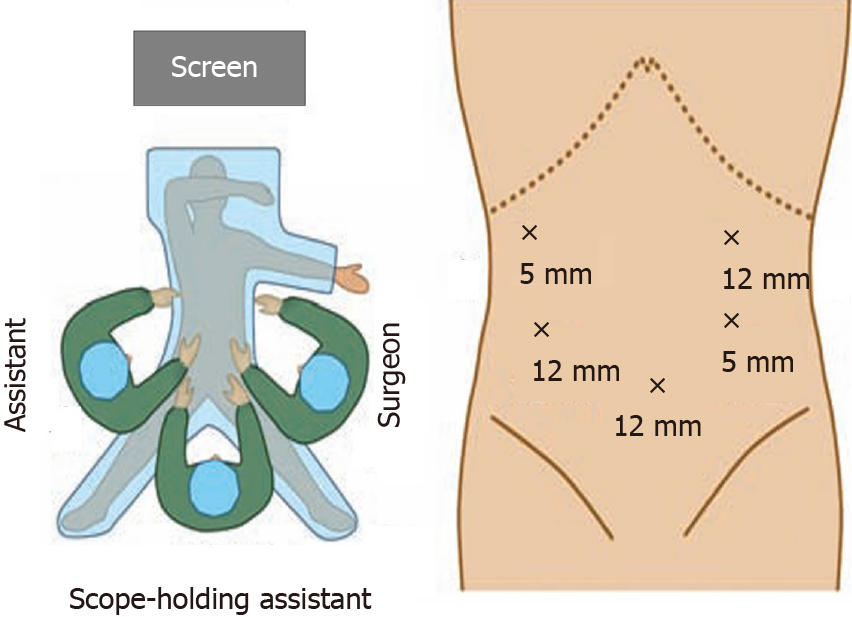
Patients were placed in the supine position with 10-15° height difference between the head and feet and lower limbs abduction. The surgeon stood on the left side of the patient, with the assistant on the right side, and the scope-holding assistant stood between the legs of the patients (Figure 1).
An arc shape 5-port method was utilized. The observation port was placed at the lower edge of the umbilicus with a 12-mm trocar, and a pneumoperitoneum was introduced to maintain the intra-abdominal CO2 pressure between 12 and 13 mmHg. Another 12-mm trocar was placed below the costal margin of the left anterior axillary line as the main operation port, whereas a 5-mm trocar on the left side of the umbilicus was used as an auxiliary operation port. In addition, 5-mm and 12-mm trocars were respectively placed at the right anterior axillary line and the umbilicus of the right midclavicular line as assistant operation ports (Figure 1).
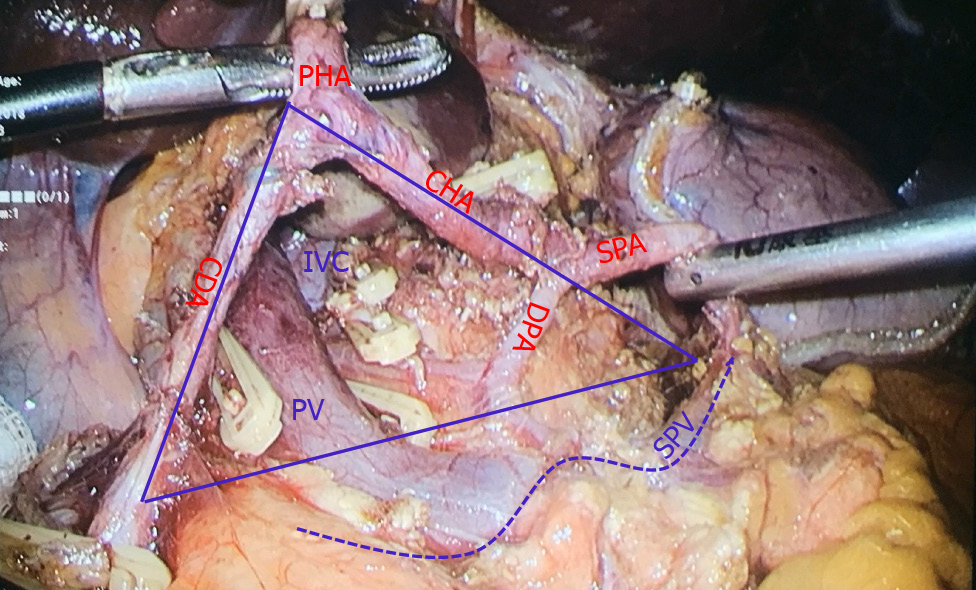
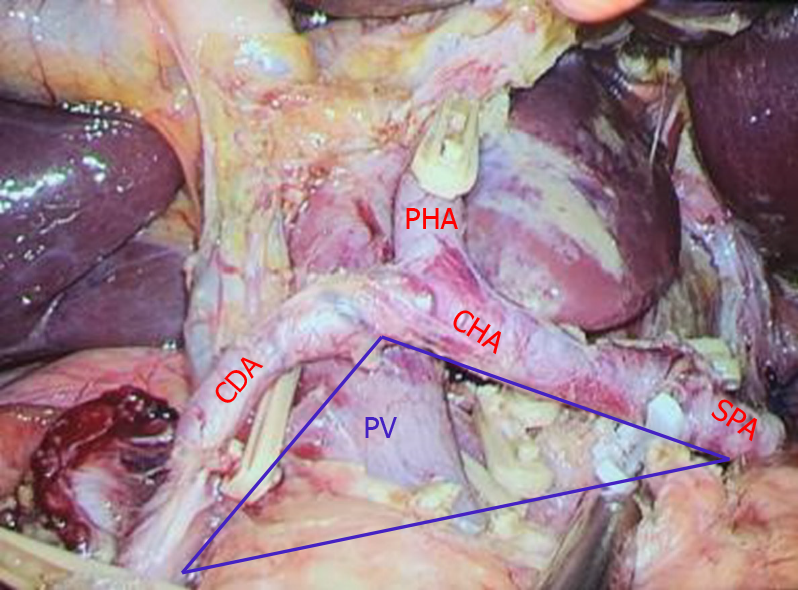
The concept of the portal triangle was defined as a triangular area formed by the upper edge of the pancreas, the left edge of the gastroduodenal artery, the right edge of the common hepatic artery and the beginning of the splenic artery (Figure 2). This triangle was the fixed projection of the beginning of the portal vein behind the common hepatic artery. It contained the dorsal pancreatic artery, initial segment of the portal vein, left gastric vein, station 8p lymph nodes, lymphatic vessels, and inferior vena cava (IVC).
The posterior part of the common hepatic artery was used as the approach to expose and protect the portal vein, and complete dissection of lymph nodes in stations 7, 8a, 8p, 9 and 11p of the superior pancreatic region. The specific surgical steps were as follows: (1) Judging the boundary of the portal triangular base of the portal vein (the upper edge of the pancreas): the assistant grabbed the bare area of the posterior wall of the stomach corresponding to the left gastric artery with their left hand and lifted it forward and upward to form the main tension of the posterior wall of the stomach. The right hand was always pulled upward with the corresponding point of the left hand to form an appropriate tension. The left hand of the main surgeon pulled or pressed the pancreas downward, and the right hand used the ultrasonic scalpel or electric hook to dissect along the upper edge of the pancreas; (2) Separation of the posterior pancreatic space: the ultrasonic scalpel or electric hook was used to separate the posterior pancreatic space parallel to the upper edge of the pancreas, from shallow to deep, from bottom to top, and from left to right. If the left gastric vein converged into the portal vein or splenic vein from the front of common hepatic artery, it should be separated, clipped and cut off; (3) Exposure of the dorsal pancreatic artery for protection or disconnection: in some patients, one or two branches of the dorsal pancreatic artery originated from the common hepatic artery, and the two ends of the artery could be dissected by the electric hook, and then clipped and disconnected, or preserved; (4) Exposure and protection of the portal vein: the tissue gap became loose after the dorsal pancreatic artery was cut off, and the light blue portal vein was faintly visible at this time. The anterior space of the portal vein was obtusely separated, and the portal vein was exposed by the dissection technique of the electric hook. The left hand continued to obtusely separate towards the hepatic hilar. In a small number of patients, the left gastric vein converged to the portal vein from the posterior direction of the common hepatic artery, and the left gastric vein was clipped close to the portal vein; (5) Along the space above the uncinate of the pancreas (this space continues with the Toldt space in front of the Gerota fascia), some patients could show metastatic and swollen lymph nodes and thick abdominal lymph vessels flowing into the intestinal trunk, which were clipped and cut off; (6) Continued separation back toward the IVC. The tissue in front of the site was loose, and there was no return vein branch or swollen lymph nodes; (7) The left adrenal gland was exposed by dissecting the starting segment of splenic artery and the left celiac artery along the anterior Toldt space of Gerota fascia above the splenic artery; and (8) The left gastric artery was dissected along the anterior space of the common hepatic artery and cut off. We continued to separate the proper hepatic artery for final confluence with the posterior space of the common hepatic artery. The lymph nodes in groups 7, 8a, 8p, 9 and 11p of the superior pancreatic region were removed completely (Figure 3).
A total of 51 patients were included; 34 were male (66.7%) and 28 (54.9%) were aged < 65 years. Most of the patients had a BMI < 24 (n = 34, 66.7%). In addition, according to the 8th edition of AJCC TNM staging, there were four (7.8%) patients in stage I, 13 (25.5%) in stage II, 33 (64.7%) in stage III and one (2.0%) in stage IV (Table 1). The average time duration for LND was about 1 h (67.7 ± 6.9 min), and the volume of intraoperative blood loss was < 100 mL (78.8 ± 17.8 mL). In addition, the average duration for postoperative exhaust was about 3 d. After surgery, four patients developed morbidities, including two with anastomotic leakage, one with the lung infection, and one with abdominal cavity infection; all of whom were treated successfully with percutaneous drainage and/or antibacterial treatments, with no need for reoperation, or in-hospital mortality (Table 1).
| Basic data | n = 51 |
| Sex (%) | |
| Male | 34 (66.7) |
| Female | 17 (33.3) |
| Age (%) | |
| < 65 yr | 28 (54.9) |
| ≥ 65 yr | 23 (45.1) |
| BMI (%) | |
| < 24 | 34 (66.7) |
| 24-27 | 11 (21.6) |
| > 27 | 4 (7.8) |
| TNM staging (%) | |
| I | 4 (7.8) |
| II | 13 (25.5) |
| III | 33 (64.7) |
| IV | 1 (2.0) |
| Duration of LND in the upper pancreas (min) | 67.7 ± 6.9 |
| Intraoperative blood loss (mL) | 78.8 ± 17.8 |
| Postoperative exhaust time (d) | 3.1 ± 0.9 |
| Postoperative complications (%) | 4 (7.8) |
Among the 51 patients, the percentage who had LNM at stations 5, 7, 8a, 8p, 9, 11p, 12a and 12p was 23.5%, 15.7%, 17.7%, 9.8%, 13.7%, 7.8%, 7.8% and 3.9%, respectively. Station 8p had 14 lymph nodes examined, and the incidence of LNM was 14.3% (Table 2). In contrast, the incidence of LNM at stations 5, 7, 8a, 9, 11p, 12a and 12p was 21.8%, 11.6%, 13.1%, 7.9%, 5.6%, 6.8% and 4.2%, respectively (Table 2).
| Nodal station | No. of patients with LNM | Rate of patients with LNM (%) | TNLE | No. of positive LNs | Rate of LNM (%) |
| 5 | 12 | 23.5 | 55 | 12 | 21.8 |
| 7 | 8 | 15.7 | 164 | 19 | 11.6 |
| 8a | 9 | 17.7 | 122 | 16 | 13.1 |
| 8p | 5 | 9.8 | 14 | 2 | 14.3 |
| 9 | 7 | 13.7 | 127 | 10 | 7.9 |
| 11p | 4 | 7.8 | 54 | 3 | 5.6 |
| 12a | 4 | 7.8 | 44 | 3 | 6.8 |
| 12p | 2 | 3.9 | 24 | 1 | 4.2 |
LNM is an independent risk factor for long-term outcome of gastric cancer after curative resection, and the station and number of lymph nodes removed definitely affect the tumor staging, guidance of postoperative adjuvant therapies, as well as long-term survival of patients[4,14,15]. Nowadays, the National Comprehensive Cancer Network (NCCN) Guidelines for Gastric Cancer in the United States and the Guidelines for the Treatment of Gastric Cancer in Japan and China have recommended D2 LND as the standard procedure during radical resection for advanced gastric cancer[16-18]. Typical D2 LND always exceeds 30 lymph nodes[19,20], In contrast, the national database identified that fewer than 15 lymph nodes were examined in a majority of American cases[21,22]. As such, it is likely that the majority of American patients did not undergo D2 LND. In fact, adequate D2 LND has been strongly recommended by the guidelines, as well as consideration of adjuvant therapies following standard gastrectomy (D2) for gastric cancer D2[23].
The 4th edition of the Japanese Guidelines for the Treatment of Gastric Cancer classified the station 8p lymph nodes outside the scope of D2 LND[8]. Whether it is necessary to expand the LND in station 8p is of debate[11,24]. Studies have reported that for advanced gastric cancer, removal of station 8p lymph nodes may improve the prognosis of patients with advanced gastric cancer[11]. In the current study, the incidence of station 8p LNM was 14.3%, which was similar to 12%-16% as reported previously, and the prognosis of patients with station 8p LNM was significantly worse[11,13]. As such, removal of station 8p lymph nodes might improve the prognosis of patients with gastrectomy, as well as inform adjuvant therapy strategies.
Given the difficult anatomic sites of station 8p lymph nodes (e.g., behind the common hepatic artery, adjacent to the portal vein and splenic vein), removal of them is technically challenging with high risk of portal vein injury, lymphatic fistula, and pancreatic injury. The current study is important as we introduced a new approach of SPLD-PPPH based on the concept of the portal triangle. The clinical significance of SPLD-PPPH approach included: (1) Complete clearance of the lymphatic tissues above the pancreas, including regional lymph nodes in station 8p, and avoidance of lymphatic residual or iatrogenic metastasis caused by lymph node transection via the front of the common hepatic artery; (2) The projection of the portal vein locates in this triangle, and its exposure can be actively performed for direct and effective protection of the vessel; (3) The left gastric vein can be dissected at the root of the portal vein to prevent retraction and bleeding; and (4) Lymphatic vessels can be clamped under direct vision to prevent postoperative lymphatic leakage.
In addition, intra- and postoperative complications related to the procedure should be cautioned against and can be avoided technically. First, intraoperative hemorrhage in the upper edge of pancreas: microvessels are common in the upper edge of the pancreas. Imprecise operation is the major reason for bleeding. Slow dissection with an ultrasonic knife and gauze compression can prevent bleeding. Portal vein injury: during the procedure of portal vein separation, fine dissection of loose tissue with an electric hook or blunt separation with forceps might be more appropriate to avoid portal vein injury. IVC injury: dissection of the lymphatic tissue to the front of the IVC is needed for complete clearance of the lymph nodes in the upper part of pancreas. The tissues in this area are loose with no venous branches, and blunt and sharp dissection with an ultrasonic knife is safe as long as the edge of the IVC is shown in the surgical scope. After surgery, the patients should be monitored for possible complications, including delayed massive hemorrhage of common hepatic artery, traumatic pancreatitis, lymph leakage, and intestinal dysfunction. Delayed hemorrhage of the common hepatic artery occurs mainly due to thermal injury and delayed necrosis of the vascular wall by ultrasonic knife. During surgery, the artery should be lifted up by the assistant, leaving the space behind the artery exposed, and the working face of the ultrasonic knife should avoid touching the vessels. Gentle dissection around the artery with the electric hook might be a better choice to avoid thermal injury of the vascular wall. Traumatic pancreatitis is mostly related to pancreatic membrane dissection during eradication of gastric cancer at the posterior wall of the stomach. Accurate judgement of the pancreatic tissue and the upper edge of the pancreas is critical to avoid pancreatic injury.
There were several limitations to the current study. First, all 51 patients underwent D2+ LND for advanced gastric cancer via SPLD-PPPH. As such, no control groups were available to define the advantage of this new approach. Nevertheless, we demonstrated that this new approach of SPLD-PPPH for D2 LND is easy and feasible in our patient cohort. However, validation of this new approach in other institutions is necessary. Second, the long-term survival of these patients was not reported due to the short period of follow-up, which needs to be further evaluated in the future.
About one in 10 patients with advanced gastric cancer had LNM at station 8p. Dissection of station 8p lymph nodes should be considered in patients with advanced gastric cancer. The current study introduced a new approach of SPLD-PPPH for D2+ LND during curative resection of advanced gastric cancer. With verification of this new approach in 51 patients, the SPLD-PPPH approach was safely and effectively performed with minimal morbidity of 7.8%. As such, this new approach can be adopted for station 8p LND in selected patients with gastric cancer.
D2 lymph node dissection of station 8p lymph nodes in gastric cancer is technically difficult.
How to safely and effectively dissect the lymph nodes superior to the pancreas during gastrectomy remains a clinical challenge for surgeons.
The current study introduced a new procedure for dissection of the lymph nodes superior to the pancreas.
Fifty-one patients who underwent laparoscopic gastrectomy for gastric cancer were retrospectively included with utilization of a new procedure for superior pancreatic lymphadenectomy (LND) with portal vein priority via the posterior common hepatic artery approach (SPLD-PPPH) based on a newly defined portal triangle.
All the procedures were safely performed. Among the 51 patients, 9.8% had lymph node metastasis at station 8p. Fourteen lymph nodes were harvested at station 8p, with an incidence of nodal metastasis of 14.3%.
The new procedure of SPLD-PPPH is safe and effective for D2+ LND during laparoscopic radical gastrectomy.
This new approach should be further evaluated with larger patient numbers.
The authors thank Dr. Zhang XF, from The First Affiliated Hospital of Xi’an Jiaotong University, China for the critical revision of the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ilhan E S-Editor: Gao CC L-Editor: Kerr C P-Editor: Gao CC
| 1. | Meng X, Wang L, Zhu B, Sun T, Guo S, Wang Y, Zhang J, Yang D, Zheng G, Zhang T, Zheng Z, Zhao Y. Totally Laparoscopic Gastrectomy Versus Laparoscopic-Assisted Gastrectomy for Gastric Cancer: A Systematic Review and Meta-Analysis. J Laparoendosc Adv Surg Tech A. 2021;31:676-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Trung LV, Loc NVV, Tien TPD, Vuong NL. Laparoscopic Proximal Gastrectomy with Jejunal Interposition for Early Proximal Gastric Cancer. J Gastrointest Cancer. 2021;52:536-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, Wang K, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Hu Y, Liu H, Zheng C, Li P, Xie J, Liu F, Li Z, Zhao G, Yang K, Liu C, Li H, Chen P, Ji J, Li G; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients With Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA. 2019;321:1983-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 527] [Article Influence: 87.8] [Reference Citation Analysis (1)] |
| 4. | Tokunaga M, Hiki N, Fukunaga T, Nohara K, Katayama H, Akashi Y, Ohyama S, Yamaguchi T. Laparoscopy-assisted distal gastrectomy with D2 Lymph node dissection following standardization--a preliminary study. J Gastrointest Surg. 2009;13:1058-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, Ryu SW, Lee HJ, Song KY. Morbidity and mortality of laparoscopic gastrectomy vs open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg. 2010;251:417-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 624] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 6. | Lee HJ, Yang HK. Laparoscopic gastrectomy for gastric cancer. Dig Surg. 2013;30:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Lee JH, Yom CK, Han HS. Comparison of long-term outcomes of laparoscopy-assisted and open distal gastrectomy for early gastric cancer. Surg Endosc. 2009;23:1759-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1915] [Article Influence: 239.4] [Reference Citation Analysis (1)] |
| 9. | Wang FH, Shen L, Li J, Zhou ZW, Liang H, Zhang XT, Tang L, Xin Y, Jin J, Zhang YJ, Yuan XL, Liu TS, Li GX, Wu Q, Xu HM, Ji JF, Li YF, Wang X, Yu S, Liu H, Guan WL, Xu RH. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun (Lond). 2019;39:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 313] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 10. | Guo DJ, Yang K, Zhang WH, Chen XL, Chen XZ, Zhang B, Zhou ZG, Hu JK. Prognostic Value of Metastatic No.8p LNs in Patients with Gastric Cancer. Gastroenterol Res Pract. 2015;2015:937682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Ye Z, Zeng Y, Wei S, Wang Y, Lin Z, Chen X, Chen L. [Feasibility of No.8p lymphadenectomy for the patients with advanced gastric cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2018;21:1129-1135. [PubMed] |
| 12. | Xu ZY, Hu C, Chen S, Du YA, Huang L, Yu PF, Wang LJ, Cheng XD. Evaluation of D2-plus radical resection for gastric cancer with pyloric invasion. BMC Surg. 2019;19:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Chen L, Wei S, Ye Z, Zeng Y, Zheng Q, Xiao J, Wang Y, Zhuo C, Lin Z, Li Y. [Analysis of risk factors and prognosis of No.8p lymph node metastasis in cases with advanced gastric cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2017;20:218-223. [PubMed] |
| 14. | Oh SE, Seo JE, An JY, Choi MG, Sohn TS, Bae JM, Kim S, Lee JH. Compliance with D2 Lymph node dissection in reduced-port totally laparoscopic distal gastrectomy in patients with gastric cancer. Sci Rep. 2021;11:3658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Aurello P, Catracchia V, Petrucciani N, D'Angelo F, Leonardo G, Picchetto A, Antolino L, Magistri P, Terrenato I, Lauro A, Ramacciato G. What is the role of nodal ratio as a prognostic factor for gastric cancer nowadays? Am Surg. 2013;79:483-491. [PubMed] |
| 16. | Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1140] [Cited by in RCA: 1308] [Article Influence: 87.2] [Reference Citation Analysis (1)] |
| 17. | Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, Sydes M, Fayers P. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522-1530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 994] [Cited by in RCA: 997] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 18. | Thiels CA, Hanson KT, Habermann EB, Boughey JC, Grotz TE. Integrated cancer networks improve compliance with national guidelines and outcomes for resectable gastric cancer. Cancer. 2020;126:1283-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Lee J, Lim DH, Kim S, Park SH, Park JO, Park YS, Lim HY, Choi MG, Sohn TS, Noh JH, Bae JM, Ahn YC, Sohn I, Jung SH, Park CK, Kim KM, Kang WK. Phase III trial comparing capecitabine plus cisplatin vs capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 Lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 580] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 20. | Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I, Meyer S, Plukker JT, Van Elk P, Obertop H, Gouma DJ, van Lanschot JJ, Taat CW, de Graaf PW, von Meyenfeldt MF, Tilanus H; Dutch Gastric Cancer Group. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1158] [Cited by in RCA: 1069] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 21. | Datta J, Lewis RS Jr, Mamtani R, Stripp D, Kelz RR, Drebin JA, Fraker DL, Karakousis GC, Roses RE. Implications of inadequate lymph node staging in resectable gastric cancer: a contemporary analysis using the National Cancer Data Base. Cancer. 2014;120:2855-2865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Deutsch GB, O'Connor V, Sim MS, Lee JH, Bilchik AJ. Incorporating surgical quality into the AJCC 7th edition improves staging accuracy in gastric cancer. Ann Surg Oncol. 2015;22:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Datta J, McMillan MT, Ecker BL, Karakousis GC, Mamtani R, Plastaras JP, Giantonio BJ, Drebin JA, Dempsey DT, Fraker DL, Roses RE. Implications of Lymph Node Staging on Selection of Adjuvant Therapy for Gastric Cancer in the United States: A Propensity Score-matched Analysis. Ann Surg. 2016;263:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 24. | Kumagai K, Sano T, Hiki N, Nunobe S, Tsujiura M, Ida S, Ohashi M, Yamaguchi T. Survival benefit of "D2-plus" gastrectomy in gastric cancer patients with duodenal invasion. Gastric Cancer. 2018;21:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |