Published online Feb 26, 2022. doi: 10.12998/wjcc.v10.i6.1815
Peer-review started: May 27, 2021
First decision: October 18, 2021
Revised: October 28, 2021
Accepted: January 19, 2022
Article in press: January 19, 2022
Published online: February 26, 2022
Processing time: 272 Days and 2.8 Hours
Noise-induced hearing loss (NIHL) is the second most common acquired hearing loss following presbycusis. Exposure to recreational noise and minimal use of hearing protection increase the prevalence of NIHL in young females. NIHL is irreversible. Identifying minor hearing pathologies before they progress to hearing problems that affect daily life is crucial.
To compare the advantages and disadvantages of extended high frequency (EHF) and otoacoustic emission and determine an indicator of hearing pathologies at the early sub-clinical stage.
This cross-sectional study was implemented in West China Hospital of Sichuan University from May to September 2019. A total of 86 participants, aged 18-22 years, were recruited to establish normative thresholds for EHF. Another 159 adults, aged 18-25 years with normal hearing (0.25-8 kHz ≤ 25 dBHL), were allocated to low noise and noise exposure groups. Distortion otoacoustic emission (DPOAE), transient evoked otoacoustic emissions (TEOAE), and EHF were assessed in the two groups to determine the superior technique for detecting early-stage noise-induced pathologies. The chi-square test was used to assess the noise and low noise exposure groups with respect to extended high-frequency audiometry (EHFA), DPOAE, and TEOAE. P ≤ 0.05 was considered statistically significant.
A total of 86 participants (66 females and 20 males) aged between 18 and 22 (average: 20.58 ± 1.13) years were recruited to establish normative thresholds for EHF. The normative thresholds for 9, 10, 11.2, 12.5, 14, 16, 18, and 20 kHz were 15, 10, 20, 15, 15, 20, 28, and 0 dBHL, respectively. A total of 201 participants were recruited and examined for eligibility. Among them, 159 adults aged between 18 and 25 years were eligible in this study. No statistical difference was detected between the noise exposure and the low noise exposure groups using EHFA, DPOAE, and TEOAE (P > 0.05) except in the right ear at 4 kHz using TEOAE (abnormal rate 20.4% vs 5.2%, respectively; P = 0.05).
These results showed TEOAE as the earliest indicator of minor pathology compared to DPOAE and EHFA. However, a multicenter controlled study or prospective study is essential to verify these results.
Core Tip: Noise-induced hearing loss is irreversible. Identifying minor pathologies of hearing before they progress to hearing problems that affect daily life is crucial. Our study recruited adults aged between 18 and 25 years with normal hearing (0.25-8 kHz ≤ 25 dBHL). The participants were allocated into a high noise exposure group or low noise exposure group based on their noise exposure history. The distortion otoacoustic emission (DPOAE), transient evoked otoacoustic emissions (TEOAE), and extended high frequency were assessed in the two groups to determine the superior technique for detecting early-stage noise-induced pathologies. The current study showed TEOAE as the earliest indicator of minor pathology compared to DPOAE and extended high-frequency audiometry.
- Citation: Meng ZL, Chen F, Zhao F, Gu HL, Zheng Y. Early detection of noise-induced hearing loss. World J Clin Cases 2022; 10(6): 1815-1825
- URL: https://www.wjgnet.com/2307-8960/full/v10/i6/1815.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i6.1815
Noise can be social or occupational[1]. Noise-induced hearing loss (NIHL) is caused by repeated exposure to loud sounds over an extended period, very loud impulse sound(s), or a combination of both[2]. NIHL can be divided into temporary threshold shift (TTS) and permanent threshold shift (PTS). TTS is defined as a threshold shift that recovers to baseline levels in hours, days, or weeks following exposure, while PTS is defined as a noise-induced threshold shift that persists after a period of recovery. It results from damage to and loss of cochlear hair cells[3].
NIHL is the second most common acquired hearing loss following presbycusis[4,5]. Occupational and social noise-induced PTS affects a large number of individuals with NIHL as the leading occupational disease[6]. Interestingly, a marked incidence was observed in the young population (12-35 years old) as a result of recreational noise exposure[4,7]. In addition, increased exposure to recreational noise and minimal use of hearing protection may be responsible for the increased prevalence of NIHL in young females[6]. Thus, social noise might play a major role in inducing NIHL in modern society.
Noise has several effects on human health, including concentration disturbance, memory loss, anxiety, depressive behavior, muscular contraction, tachycardia, and hypertension[5]. Although the noise exposure does not damage or result in loss of inner and outer hair cells, and the auditory detection thresholds are unaffected, the encoding of sound at suprathreshold levels is impaired[8,9]. In noise-exposed humans, this phenomenon manifests as difficulty in processing speech in a noisy background in the absence of clinically elevated thresholds[8,9].
NIHL is diagnosed based on the pure-tone audiogram. The typical patterns in hearing thresholds are a noise notch at 3, 4, and/or 6 kHz combined with a relatively normal threshold at 8 kHz[2]. The characteristic of NIHL is sensorineural hearing loss that is typically bilateral[10]. Although NIHL is irreversible and progressive while exposure to noise continues, it is also predictable and preventable[10]. Consequently, identifying minor pathologies of hearing before they progress to hearing problems that affect daily life is crucial for preventing the deterioration of hearing by changing the lifestyle, i.e., reducing noise exposure.
The commonly used methods to detect NIHL include conventional audiometry (CA), otoacoustic emission (OAE), and extended high-frequency audiometry (EHFA).
CA presents 0.25-8 kHz pure tones, which constitute the speech spectrum, and hence, the hearing loss in this frequency range can influence the daily communication that might be noticed by the affected individual. OAE comprises sounds of cochlear origin that can be recorded by a microphone fitted into the ear canal[11]. OAEs are sensitive to minor pathologies, thereby rendering them as an indicator of damage compared to CA[12]. EHFA presents a 9-20 kHz pure tone that has proved to be a promising tool for the early diagnosis of many hearing disorders[13]. The higher hearing thresholds of 10-16 kHz were observed in individuals < 31 years old following the use of personal listening devices for > 5 years[14]. Consequently, OAE and EHFA are promising tools for detecting NIHL at the early stages than by CA.
Some studies explored the types of measurements in the early detection of NIHL[5,15,16], albeit the results were inconsistent. Job et al[15] emphasized the use of distortion otoacoustic emission (DPOAE) measurements in public health and occupational noise prevention policies. Other studies stated that EHFA was sensitive in detecting NIHL[5,16]; these studies were designed differently and involved various participants.
This study aimed to compare the advantages and disadvantages of extended high frequency (EHF) and otoacoustic emission and determined an early indicator of minor pathologies of hearing in sub-clinical disease, so that further hearing loss can be prevented. Three measurements, EHFA, DPOAE, and transient-evoked otoacoustic emission (TEOAE), were compared in the patient group with normal hearing thresholds at CA. Several studies had compared the CA, EHFA, and DPOAE for the early diagnosis of NIHL[5,16,17]. These results were inconsistent and TEOAE was not discussed previously.
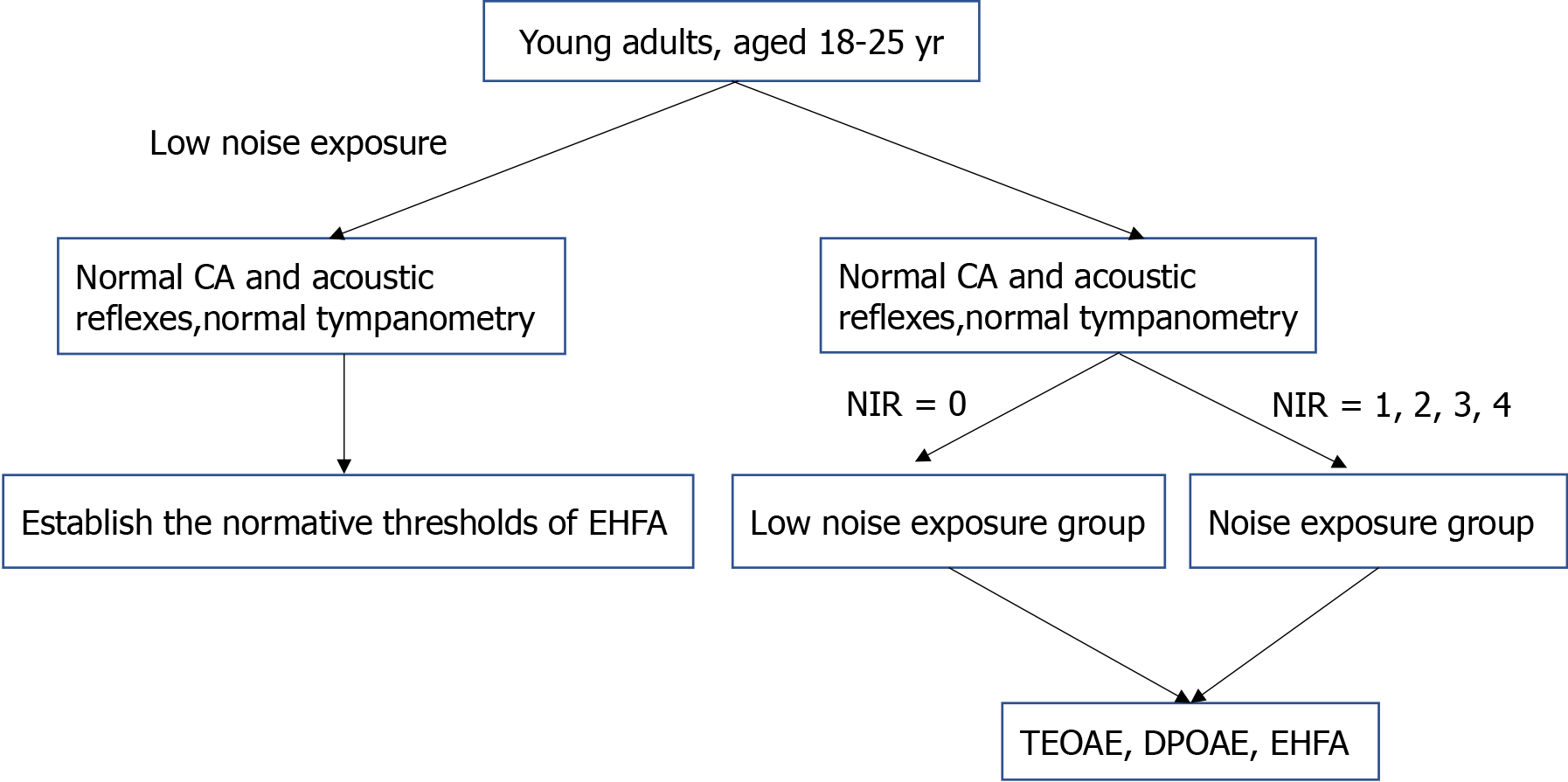
This cross-sectional study was implemented at the West China Hospital of Sichuan University from May 2019 to September 2019. Young adults, aged 18-25 years, were recruited randomly and sequentially according to the sequence to Hearing Center of West China Hospital. In the current study, only young adults aged 18-25 years were recruited. Because of the age factor, the early hearing pathology did not interfere with the test results. According to ISO 7029, little change occurred in this age group[12].
Participants with normal CA and acoustic reflexes were included in this study. Normal CA was defined as the threshold ≤ 25 dBHL at each frequency from 0.25-8 kHz. According to the World Health Organization (WHO) definition, normal hearing means the average thresholds of 0.5, 1, 2, and 4 kHz at ≤ 25 dBHL[18]. In this study, normal hearing was considered if all the frequencies from 0.25-8 kHz were ≤ 25 dBHL instead of the average thresholds at 0.5, 1, 2, and 4 kHz defined by the WHO. For early detection, only minor pathologies existed before the patient identified the problem. The normal hearing could be defined only if minor hearing loss occurred at one or two frequencies at 0.5, 1, 2, and 4 kHz according to the WHO’s normal hearing definition. We could not detect the minor hearing pathology early if the normal hearing was defined according to the WHO. Thus, the normal hearing was designated if all the frequencies from 0.25-8 kHz were ≤ 25 dBHL.
Participants with disorders of middle ear function, who underwent otoscopic examination, tympanometry, and audiometry, were excluded. The participants were considered to have middle ear disfunction in case of one of the following conditions: The tympanic membrane was perforated as observed by otoscopy, tympanogram was type B or type C, and the air-bone gap was > 10 dB at conventional audiometry[19].
Grouping of subjects
In the present society, all types of social media are popular, for example, listening to music through a cell phone, wearing headphones when playing games, prolonged use of the phone each day, playing a musical instrument, and attending a music concert. Consequently, it was difficult to differentiate young adults into noise and no noise exposure groups. Noise exposure cannot be avoided in the present society, and it is difficult to measure the amount of social and occupational noise exposure accurately. Thus, it is reasonable to differentiate young adults into noise exposure and low noise exposure groups instead of noise exposure and no noise exposure groups.
Participants were allocated to noise or low noise exposure groups according to their noise exposure history. The noise exposure was estimated using the Lutman structured noise questionnaires[12]. Occupational noise and social noise questionnaires were used during the interview with the participants[12]. The gunshot and explosive noise questionnaires were not used because none of the participants had this history. According to the information mentioned in the questionnaires, the noise exposure was estimated using the following equation: U = 10(L-A-90)/10 × Y × W × D × H/2080, where U is units of cumulative noise exposure, L is estimated noise level in dB(A), A is hearing protection attenuation in dB, Y is years of exposure, W is weeks/year of exposure, D is days/week of exposure, and H is hours/day of exposure[12]. Noise immission rating (NIR) was determined according to U = 10(L-A-90)/10 × Y × W × D × H/2080. Lutman et al categorized NIR into five degrees based on the units of cumulative noise exposure: 0 (U up to 5), 1 (U = 6-50), 2 (U = 51-500), 3 (U = 501-5000), and 4 (U = 5000+)[12]. The NIR values are equivalent to continuous exposure for 8 h/d, 5 d/wk, 48 wk/year, throughout a full 50-year working lifetime[12]. NIR = 0 is equivalent to continuous noise < 80 dB(A), NIR = 1 to 81-90 dB(A), NIR = 2 to 91-100 dB(A), NIR = 3 to 101-110 dB(A), and NIR = 4 to > 110 dB(A)[12]. Participants were grouped into the low noise group if NIR = 0 and the noise group if NIR = 1, 2, 3, or 4.
Study size
Assuming that there would be a 55% incidence of NIHL in the noise group and 32% in the low noise group[1], we calculated that 141 patients would need to be enrolled to provide 80% power to test for the difference between the groups in the incidence of NIHL, at a two-sided significance level of 5%. Assuming a 10% rate of loss to follow-up, we planned to enroll a total of 160 patients.
Blind method
CA, EHFA, DPOAE, and TEOAE were performed by a trained audiology technician who was unaware of the noise exposure history of each participant. The social noise exposure history of each participant was collected by another trained audiology technician who did not know the participant’s CA, EHFA, DPOAE, and TEOAE results.
Test tools
CA was performed in the range 0.25-8 kHz using Interacoustic AC-40 and TDH 39 headphones calibrated according to ANSI S3.6:2010 type 1 and ANSI S3.6-2010. EHFA was measured at 9, 10, 11.2, 11.5, 14, 16, 18, and 20 kHz using the same Interacoustic AC-40 andHDA200 high-frequency headphones calibrated according to ANSI S3.6-2010. Threshold values at each frequency were obtained using a modified Hughson-Westlake up-down procedure, which obtained thresholds at the lowest response in a minimum of 50% of the ascending trials, two per level, using a 10-dB descending and a 5-dB ascending measurement approach[20,21].
DPOAE and TEOAE were measured using an Interacoustic Titan that fulfilled the criterion of Medical Device Directive 93/42/EEC. For DPOAE, the ratio of the two frequencies was f2:f1 = 1.22; the intensity of the two frequencies was L1/L2 = 65/55 dBSPL. The frequency of f2 was 1, 1.5, 2, 3, 4, and 6 kHz, respectively. The result was considered a pass if the signal to noise ratio (SNR) ≥ 6 dB. The stimuli for TEOAE were 1, 1.5, 2, 3, and 4 kHz. The intensity of the stimulus was 83 dB peSPL. The result was considered pass if the SNR was ≥ 6dB, and the repetition was ≥ 70%.
Tympanometry was measured using Interacoustic AT235, according to the standard of Medical Device Directive 93/42/EEC. The ipsilateral (1 and 2 kHz) and contralateral (0.25, 0.5, 1, and 2 kHz) stimuli were calibrated according to ISO389-1 and 389-2, respectively.
Setting up normative thresholds of EHFA
The comparison of normative thresholds between conventional audiometry did not achieve a consensus that described the normal parameters for children or adults with respect to EHFA[13,22,23,24]. Thus, normative thresholds from 9-20 kHz need to be established, otherwise the EHFA results using DPOAE and TEOAE cannot be compared.
To develop a normative threshold of EHFA, participants aged 18-25 years, who had a low noise exposure history and complied with the inclusion criteria for a control group, were included in this study. Thresholds at 9, 10, 11.2, 12.5, 14, 16, 18, and 20 kHz were obtained using a modified Hughson-Westlake up-down procedure[20,21]. The percentile method was used to determine 95% normal range of 8-20 kHz.
Statistical analysis
Statistical analyses were performed by an expert statistician at the Chinese Evidence-Based Medicine/Cochrane Center using SPSS20.0 (SPSS Inc., Chicago, IL, USA). A Shapiro-Wilk normality test was used to assess the normal distribution for the group and set normative thresholds. As mentioned above, the percentile method was used to establish the normative thresholds from 9-20 kHz. The EHF results were converted into normal and abnormal according to the normative thresholds. The results of DPOAE and TEOAE were recorded as normal if they passed, or else were considered abnormal. The chi-square test was used to test for normal and abnormal in the noise and low noise exposure groups with respect to EHFA, DPOAE, and TEOAE. P ≤ 0.05 was considered statistically significant.
A total of 86 participants (66 females and 20 males), aged 18-22 (20.58 ± 1.13) years, were included to set up normative thresholds of EHFA. The Shapiro-Wilk test revealed that the sample did not conform to a normal distribution (P < 0.001) for 9, 10, 11.2, 12.5, 14, 16, 18, and 20 kHz, respectively. The upper limit of the one-sided clinical normal hearing threshold range was set up.
We used the percentage method to establish the normative thresholds of EHFA at the upper limit of 95% (Table 1). The normative thresholds for 9, 10, 11.2, 12.5, 14, 16, 18, and 20 kHz were 15, 10, 20, 15, 15, 20, 28, and 0 dBHL, respectively.
| EHF (kHz) | mean ± SD | Min | Max | 25th | 50th | 75th | 95th |
| 9 | 3.14 ± 5.26 | -5.00 | 15.00 | 0.00 | 5.00 | 5.00 | 15.00 |
| 10 | 0.93 ± 5.34 | -15.00 | 15.00 | -5.00 | 0.00 | 5.00 | 10.00 |
| 11.2 | 2.50 ± 7.19 | -15.00 | 25.00 | 0.00 | 0.00 | 5.00 | 20.00 |
| 12.5 | -0.52 ± 6.90 | -15.00 | 20.00 | -5.00 | 0.00 | 5.00 | 15.00 |
| 14 | 0.52 ± 7.97 | -10.00 | 20.00 | -5.00 | 0.00 | 5.00 | 15.00 |
| 16 | -3.08 ± 13.15 | -20.00 | 25.00 | -15.00 | -5.00 | 5.00 | 20.00 |
| 18 | -4.48 ± 16.09 | -20.00 | 30.00 | -20.00 | -10.00 | 10.00 | 28.25 |
| 20 | -13.78 ± 7.39 | -25.00 | 15.00 | -20.00 | -15.00 | -10.00 | 0.00 |
A total of 201 participants were recruited, of which 159 were eligible (Table 2) for this study. No data were missed. The procedure is illustrated in Figure 1. These individuals displayed an intact tympanic membrane, good gloss in the tympanic membrane, and a clear view of the manubrium of malleus and cone of light when examined through otoscopy by an ENT doctor. Also, the participants had type A tympanometry along with an ipsilateral acoustic reflex at 1 and 2 kHz and a contralateral acoustic reflex at 0.5, 1, 2, and 4 kHz. None of the participants had ≥ 15 dB air-bone gap in CA. The thresholds of conventional audiometry are shown in Table 3.
| Group | n | Female | Male | Mean ± SD | Min | Max | 25th | 50th | 75th |
| Low noise exposure | 61 | 45 | 16 | 21.59 ± 2.00 | 18 | 25 | 20.00 | 21.00 | 23.50 |
| Noise exposure | 98 | 64 | 34 | 21.17 ± 1.55 | 18 | 25 | 20.00 | 21.00 | 22.00 |
| Ear | Group | n | 0.25 kHz (mean ± SD) | 0.5 kHz (mean ± SD) | 1 kHz (mean ± SD) | 2 kHz (mean ± SD) | 4 kHz (mean ± SD) | 8 kHz (mean ± SD) |
| Right | Low noise exposure | 61 | 7.30 (± 5.21) | 7.82 (± 4.91) | 6.97 (± 4.50) | 6.97 (± 5.42) | 4.26 (± 5.69) | 7.54 (± 6.50) |
| Noise exposure | 98 | 6.99 (± 5.91) | 7.65 (± 5.14) | 8.06 (± 4.90) | 8.21 (± 5.62) | 5.87 (± 5.65) | 8.78 (± 6.70) | |
| Left | Low noise exposure | 61 | 6.80 (± 5.70) | 6.80 (± 4.48) | 5.66 (± 4.42) | 6.07 (± 5.99) | 5.57 (± 5.63) | 8.28 (± 6.18) |
| Noise exposure | 98 | 7.45 (± 5.38) | 8.32 (± 5.70) | 7.14 (± 5.13) | 6.99 (± 5.78) | 5.15 (± 5.54) | 9.03 (± 6.37) |
The chi-square test results of EHFA, DPOAE, and TEOAE are shown in Tables 4, 5, and 6, respectively. No statistical difference was detected between the noise and low noise exposure groups with respect to EHFA, DPOAE, and TEOAE (P > 0.05) except in the right ears at 4 kHz in TEOAE (P = 0.05).
| Ear | Frequency (kHz) | Group | Normal (N) | Abnormal (N) | Abnormalrate (%) | χ2 | P value |
| Right | 9 | 0 | 56 | 5 | 8.2 | 1.33 | 0.25 |
| 1 | 84 | 14 | 14.3 | ||||
| 10 | 0 | 51 | 10 | 16.4 | 2.62 | 0.11 | |
| 1 | 71 | 27 | 27.6 | ||||
| 11.2 | 0 | 58 | 3 | 4.9 | 2.36 | 0.12 | |
| 1 | 86 | 12 | 12.2 | ||||
| 12.5 | 0 | 54 | 7 | 11.5 | 2.13 | 0.15 | |
| 1 | 78 | 20 | 20.4 | ||||
| 14 | 0 | 51 | 10 | 16.4 | 1.47 | 0.23 | |
| 1 | 74 | 24 | 24.5 | ||||
| 16 | 0 | 52 | 9 | 14.8 | 1.09 | 0.30 | |
| 1 | 77 | 21 | 21.4 | ||||
| 18 | 0 | 60 | 1 | 1.6 | 1.00a | ||
| 1 | 95 | 3 | 3.1 | ||||
| 20 | 0 | 57 | 4 | 6.6 | 0.77a | ||
| 1 | 90 | 8 | 8.2 | ||||
| Left | 9 | 0 | 52 | 9 | 14.8 | 0.19 | 0.67 |
| 1 | 81 | 17 | 17.3 | ||||
| 10 | 0 | 45 | 16 | 26.2 | 0.74 | 0.39 | |
| 1 | 66 | 32 | 32.7 | ||||
| 11.2 | 0 | 53 | 8 | 13.1 | 1.02 | 0.31 | |
| 1 | 90 | 8 | 10.1 | ||||
| 12.5 | 0 | 51 | 10 | 16.4 | 0.03 | 0.86 | |
| 1 | 83 | 15 | 15.3 | ||||
| 14 | 0 | 50 | 11 | 18.0 | 0.05 | 0.83 | |
| 1 | 79 | 19 | 19.4 | ||||
| 16 | 0 | 50 | 11 | 18.0 | 0.05 | 0.83 | |
| 1 | 79 | 19 | 19.4 | ||||
| 18 | 0 | 57 | 4 | 6.6 | 0.77a | ||
| 1 | 90 | 8 | 8.2 | ||||
| 20 | 0 | 56 | 5 | 8.2 | 1.33 | 0.25 | |
| 1 | 84 | 14 | 14.3 |
| Ear | Frequency(kHz) | Group | Normal (n) | Abnormal (N) | Abnormal rate (%) | Fisher P value |
| Right | 1 | 0 | 60 | 1 | 1.6 | 0.41 |
| 1 | 93 | 5 | 5.1 | |||
| 1.5 | 0 | 61 | 0 | 0.0 | 0.52 | |
| 1 | 96 | 2 | 2.0 | |||
| 2 | 0 | 61 | 0 | 0.0 | 0.52 | |
| 1 | 96 | 2 | 2.0 | |||
| 3 | 0 | 61 | 0 | 0.0 | ||
| 1 | 98 | 0 | 0.0 | |||
| 4 | 0 | 61 | 0 | 0.0 | 1.00 | |
| 1 | 97 | 1 | 1.0 | |||
| 6 | 0 | 56 | 5 | 8.2 | 1.00 | |
| 1 | 90 | 8 | 8.2 | |||
| Left | 1 | 0 | 60 | 1 | 1.6 | 1.00 |
| 1 | 95 | 3 | 3.1 | |||
| 1.5 | 0 | 61 | 0 | 0.0 | 1.00 | |
| 1 | 97 | 1 | 1.0 | |||
| 2 | 0 | 61 | 0 | 0.0 | 0.52 | |
| 1 | 96 | 2 | 2.0 | |||
| 3 | 0 | 60 | 1 | 1.6 | 1.00 | |
| 1 | 96 | 2 | 2.0 | |||
| 4 | 0 | 59 | 2 | 3.3 | 0.15 | |
| 1 | 98 | 0 | 0.0 | |||
| 6 | 0 | 56 | 5 | 8.2 | 0.51 | |
| 1 | 93 | 5 | 5.1 |
| Ear | Frequency (kHz) | Group | Normal (n) | Abnormal (n) | Abnormal rate (%) | Fisher P value |
| Right | 1 | 0 | 61 | 0 | 0.0 | 1.00 |
| 1 | 97 | 1 | 1.0 | |||
| 1.5 | 0 | 61 | 0 | 0.0 | 1.00 | |
| 1 | 97 | 1 | 1.0 | |||
| 2 | 0 | 60 | 1 | 1.6 | 0.38 | |
| 1 | 98 | 0 | 0.0 | |||
| 3 | 0 | 59 | 2 | 3.3 | 1.00 | |
| 1 | 95 | 3 | 3.1 | |||
| 4 | 0 | 56 | 5 | 5.2 | 0.05a | |
| 1 | 78 | 20 | 20.4 | |||
| Left | 1 | 0 | 60 | 1 | 1.6 | 0.52 |
| 1 | 96 | 2 | 2.0 | |||
| 1.5 | 0 | 61 | 0 | 0.0 | ||
| 1 | 98 | 0 | 0.0 | |||
| 2 | 0 | 60 | 1 | 1.6 | 1.00 | |
| 1 | 96 | 2 | 2.0 | |||
| 3 | 0 | 60 | 1 | 1.6 | 1.00 | |
| 1 | 96 | 2 | 2.0 | |||
| 4 | 0 | 58 | 3 | 4.9 | 0.07 | |
| 1 | 84 | 14 | 14.3 |
The present study aimed to develop the tools to detect NIHL at the early stage. The definition of early detection is that the conventional frequency audiometry is normal. NIHL accrues progressively and often goes unnoticed until it has reached a degree of irreversible damage[25]. Thus, detecting NIHL before CA indicates a hearing loss. The evaluation of the sub-clinical condition is initiated by the patient to prevent the worsening of the noise-induced pathology such that it has an impact on the quality of life and clinical intervention.
Abnormal TEOAE was found at 4 kHz in one ear in this study. The result complied with the typical NIHL pattern with a noise notch at 3, 4, and 6 kHz[2]. Thus, we speculated that this phenomenon was an indication of the failure of the outer hair cells at 4 kHz and the first minor pathology at the site of sub-clinical conditions.
No statistical difference between noise exposure and low noise exposure groups was observed for DPOAE. This result complied with the trait of TEOAE that is sensitive to the changes in the cochlea manifested as subtle changes in the TEOAE waveform[11]. Compared to TEOAE, DPOAE offers a wider frequency range of observation (> 10 kHz) with a lower sensitivity to minor and sub-clinical conditions in adults[11].
Other studies comparing these measures stated that EHFA is more sensitive than OAE[5,16]. Mehrparvar et al compared CA, EHFA, and DPOAE for the early diagnosis of NIHL. The study concluded that EHFA is the most sensitive test for the detection of hearing loss in workers exposed to hazardous noise[5]. The participants worked in the tile and ceramic industry, and normal CA was not undertaken. The study also included participants > 50 years old[5]. Somma et al conducted a study in cement workers and demonstrated that EHFA was more sensitive than CA in detecting NIHL[16]. Other studies concluded that EHAF and DPOAE can reveal early changes in the auditory function compared to CA. For example, in the study by Knight et al, children and adolescents receiving platinum-based chemotherapy were evaluated[17]. The different conclusions may be due to the varying study designs and the various participants included. Only a few studies compared these measurements using TEOAE.
TEOAE is a rapid and convenient test tool. It can be used to detect early minor pathologies of hearing when the disease is sub-clinical. Thus, it can serve as a screening instrument among the population with a noise exposure history. It also can be used for those who complained of hearing problem and had noise exposure history but resented normal hearing in CA.
Since this was a cross-sectional study and the sample size was small, the results need to be interpreted cautiously. Further follow-up of the noise exposure group with TEOAE, DPOAE, and EHFA is still needed. A multicenter controlled study or prospective study is essential to substantiate the current findings.
In this study, we found that TEOAE is the optimal early indicator of minor pathology with normal CA compared to DPOAE and EHFA. However, multicenter, controlled, prospective studies are required to verify the results.
Research background
Noise-induced hearing loss (NIHL) is the second most common acquired hearing loss following presbycusis. A marked incidence was observed in the young population (12-35 years old) as a result of recreational noise exposure. Noise has several effects on human health, including concentration disturbance, memory loss, anxiety, depressive behavior, muscular contraction, tachycardia, and hypertension.
Research motivation
NIHL is irreversible and progressive while exposure to noise continues. Consequently, identifying minor pathologies of hearing before they progress to hearing problems that affect daily life is crucial for preventing the deterioration of hearing by changing the lifestyle, i.e., reducing noise exposure. The authors motivated to find an indicator that can predict the minor pathologies of hearing in sub-clinical disease, so that further hearing loss can be prevented.
Research objectives
To compare the advantages and disadvantages of extended high frequency (EHF) and otoacoustic emission and determine an indicator of hearing pathologies at the early sub-clinical stage.
Research methods
This cross-sectional study was implemented at West China Hospital of Sichuan University from May-September 2019. A total of 86 participants, aged 18-22 years, were recruited to establish normative thresholds for EHF. Another 159 adults, aged 18-25 years with normal hearing (0.25-8 kHz ≤ 25 dBHL), were allocated to low noise and noise exposure groups. Distortion otoacoustic emission (DPOAE), transient evoked otoacoustic emission (TEOAE), and EHF were assessed in the two groups to determine the superior technique for detecting early-stage noise-induced pathologies. The chi-square test was used to assess the noise and low noise exposure groups with respect to extended high-frequency audiometry (EHFA), DPOAE, and TEOAE. P ≤ 0.05 was considered statistically significant.
Research results
A total of 86 participants (66 females and 20 males) aged between 18 and 22 (average: 20.58 ± 1.13) years were recruited to establish normative thresholds for EHF. The normative thresholds for 9, 10, 11.2, 12.5, 14, 16, 18, and 20 kHz were 15, 10, 20, 15, 15, 20, 28, and 0 dBHL, respectively. A total of 201 participants were recruited and examined for eligibility. Among them, 159 adults aged between 18 and 25 years were eligible in this study. No statistical difference was detected between the noise exposure and the low noise exposure groups using EHFA, DPOAE, and TEOAE (P > 0.05) except in the right ear at 4 kHz using TEOAE (abnormal rate 20.4% vs 5.2%, respectively; P = 0.05).
Research conclusions
These results showed TEOAE as the earliest indicator of minor pathology compared to DPOAE and EHFA. However, multicenter, controlled, prospective studies are essential to verify these results.
Research perspectives
Since this was a cross-sectional study and the sample size was small, the results need to be interpreted cautiously. Further follow-up of the noise exposure group with TEOAE, DPOAE, and EHFA is still needed. Multicenter, controlled, prospective studies are essential to substantiate the current findings.
The authors acknowledge the support of Prof. Liu GJ affiliated to the Chinese Evidence-Based Medicine/Cochrane Center for the research design and statistical analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lee KS S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Prendergast G, Guest H, Munro KJ, Kluk K, Léger A, Hall DA, Heinz MG, Plack CJ. Effects of noise exposure on young adults with normal audiograms I: Electrophysiology. Hear Res. 2017;344:68-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 161] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 2. | Jansen EJ, Helleman HW, Dreschler WA, de Laat JA. Noise induced hearing loss and other hearing complaints among musicians of symphony orchestras. Int Arch Occup Environ Health. 2009;82:153-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Ryan AF, Kujawa SG, Hammill T, Le Prell C, Kil J. Temporary and Permanent Noise-induced Threshold Shifts: A Review of Basic and Clinical Observations. Otol Neurotol. 2016;37:e271-e275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 134] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 4. | Imam L, Hannan SA. Noise-induced hearing loss: a modern epidemic? Br J Hosp Med (Lond). 2017;78:286-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Mehrparvar AH, Mirmohammadi SJ, Davari MH, Mostaghaci M, Mollasadeghi A, Bahaloo M, Hashemi SH. Conventional Audiometry, Extended High-Frequency Audiometry, and DPOAE for Early Diagnosis of NIHL. Iran Red Crescent Med J. 2014;16:e9628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Henderson E, Testa MA, Hartnick C. Prevalence of noise-induced hearing-threshold shifts and hearing loss among US youths. Pediatrics. 2011;127:e39-e46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 149] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 7. | Pienkowski M. Loud Music and Leisure Noise Is a Common Cause of Chronic Hearing Loss, Tinnitus and Hyperacusis. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Paul BT, Bruce IC, Roberts LE. Evidence that hidden hearing loss underlies amplitude modulation encoding deficits in individuals with and without tinnitus. Hear Res. 2017;344:170-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Schaette R, McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci. 2011;31:13452-13457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 715] [Article Influence: 51.1] [Reference Citation Analysis (1)] |
| 10. | Metidieri MM, Rodrigues HF, Filho FJ, Ferraz DP, Neto AF, Torres S. Noise-Induced Hearing Loss (NIHL): literature review with a focus on occupational medicine. Int Arch Otorhinolaryngol. 2013;17:208-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Kemp DT. Otoacoustic emissions, their origin in cochlear function, and use. Br Med Bull. 2002;63:223-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 292] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 12. | Lutman ME, Davis AC, Ferguson MA. Epidemiological evidence for the effectiveness of the noise at work regulation. Health and Safety Executive. Research report. 2008;RR669. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Anastasio AR, Radael RD, Cavalcante JM, Hatzopoulos S. A report of extended high frequency audiometry thresholds in school-age children with no hearing complaints. Audiol Res. 2012;2:e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Škerková M, Kovalová M, Mrázková E. High-Frequency Audiometry for Early Detection of Hearing Loss: A Narrative Review. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Job A, Raynal M, Kossowski M, Studler M, Ghernaouti C, Baffioni-Venturi A, Roux A, Darolles C, Guelorget A. Otoacoustic detection of risk of early hearing loss in ears with normal audiograms: a 3-year follow-up study. Hear Res. 2009;251:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Somma G, Pietroiusti A, Magrini A, Coppeta L, Ancona C, Gardi S, Messina M, Bergamaschi A. Extended high-frequency audiometry and noise induced hearing loss in cement workers. Am J Ind Med. 2008;51:452-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Knight KR, Kraemer DF, Winter C, Neuwelt EA. Early changes in auditory function as a result of platinum chemotherapy: use of extended high-frequency audiometry and evoked distortion product otoacoustic emissions. J Clin Oncol. 2007;25:1190-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 18. | World Health Organization. Primary ear and hearing care training resource. WHO Library Cataloguing-in-Publication Data. 2006. [cited 20 February 2021]. Available from: https://www.who.int/pbd/deafness/activities/hearing_care/advanced.pdf?ua = 1. |
| 19. | Katez J, Chasin M, English K, Hood LJ, Tillery KL. Puretone Evaluation. In:Katz J. Clinical handbook of Audiology.7th edition. Philadelphia: Wolters Kluwer Health, 2015: 29-47. [DOI] [Full Text] |
| 20. | Carhart R, JergerJ. Preferred method for clinical determination of pure-tone thresholds. J Speech Hear Disord. 1959;24:330-345. [RCA] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 591] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 21. | Hughson W, Westlake H. Manual for program outline for rehabilitation of aural casualties both military and civilian. Trans Am Acad Ophthalmol Otolaryngol Suppl. 1944;48:1-15. [DOI] [Full Text] |
| 22. | Schechter MA, Fausti SA, Rappaport BZ, Frey RH. Age categorization of high-frequency auditory threshold data. J Acoust Soc Am. 1986;79:767-771. [PubMed] [DOI] [Full Text] |
| 23. | Stelmachowicz PG, Beauchaine KA, Kalberer A, Jesteadt W. Normative thresholds in the 8- to 20-kHz range as a function of age. J Acoust Soc Am. 1989;86:1384-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Sahyeb DR, Costa Filho AO, Alvarenga KF. High-frequency audiometry: study with normal audiological subjects. Braz J Otorhinolaryngol. 2003;69:93-99. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Bamiou D, Lutman ME. Interaction of NIHL and ageing: epidemiological aspects. In: Luxon L, Prasher D. Noise and its effects. 1th edition. Chichester: John Wiley, 2007: 64-84. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |