Published online Feb 26, 2022. doi: 10.12998/wjcc.v10.i6.1754
Peer-review started: August 24, 2021
First decision: November 17, 2021
Revised: November 25, 2021
Accepted: January 19, 2022
Article in press: January 19, 2022
Published online: February 26, 2022
Processing time: 183 Days and 0.2 Hours
Emerging evidence supports that the gut microbiome, reconsidered as a new organ in the human body, can not only affect the local gut, but also communicate with the brain via multiple pathways related to neuroendocrine, immune, and neural pathways, thereby proposing the new concept of the microbiome-gut-brain (MGB) axis. Recently, the role of short-chain fatty acids (SCFAs), which are the main anaerobic fermented metabolites of the gut microbiota in the MGB axis, has garnered significant attention. SCFAs are involved in a broad range of central neurological diseases, including neurodegenerative diseases, cerebral vascular diseases, epilepsy, neuroimmune inflammatory diseases, and mood disorders. However, the underlying mechanism of SCFA-related distant organ crosstalk is yet to be elucidated. Herein, we summarize current knowledge regarding interactions between SCFAs and the MGB axis, as well as their protective effects against central neurological diseases.
Core Tip: Recently, emerging evidence suggests that short-chain fatty acids (SCFAs) exert crucial functions on the brain. The levels of SCFAs can change in many neurological disorders such as Parkinson’s disease, Alzheimer’s disease, autism spectrum disorder, major depressive disorder, stroke, epilepsy, multiple sclerosis, and so on. Meanwhile, SCFAs might play a role in the pathogenesis of these diseases. In this review, we outline possible pathways of microbiota–gut–brain (MGB) axis, the interactions between SCFAs and MGB axis, as well as their relationships with different central neurological diseases, which helps to better understand the biological roles of SCFAs in neurological disorders via MGB axis and shed light on potential therapeutic approaches for these neurological disorders.
- Citation: Guo C, Huo YJ, Li Y, Han Y, Zhou D. Gut-brain axis: Focus on gut metabolites short-chain fatty acids. World J Clin Cases 2022; 10(6): 1754-1763
- URL: https://www.wjgnet.com/2307-8960/full/v10/i6/1754.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i6.1754
Microbes have existed on earth for hundreds of millions of years. However, it was not until 2000 that Lederberg first proposed the concept of “microbiota” and revealed its possible relationship with human diseases[1]. Henceforth, intestinal microbes, which have often been disregarded, but are currently regarded as a special organ of the human body, have become a research hotspot. It has been conservatively estimated that the gut contains more than 500 types of bacteria, over 10 trillion cells, i.e., 1.3 times more microbes than the human body, and constitute > 99% of the genes in our body[2-4]. It is fundamental to many physiological processes, including immunity, defense, digestion, and metabolism.
Over the past two decades, with the advancement of gene sequencing technology and the development of powerful bioinformatics analysis tools, researchers have gained a more comprehensive understanding of the role of intestinal flora in the development of human diseases. In addition, the scope of research has extended from digestive diseases to diseases of other systems such as the central nervous system (CNS). Recently, emerging evidence suggests a bidirectional interaction between intestinal microbiota and the brain. This crosstalk, known as the microbiota–gut–brain (MGB) axis, appears to be vital to many neurological diseases[5,6].
Short-chain fatty acids (SCFAs), primarily comprising acetate, propionate, and butyrate, are major microbial metabolites produced in the colony by the bacterial fermentation of specific dietary fibers, and they primarily serve as energy suppliers for colonocytes. Recently, many studies have supported the crucial function of SCFAs in the brain. Studies have shown that the levels of SCFAs change in many neurological diseases, including neurodegenerative diseases [Parkinson’s disease (PD), Alzheimer’s disease (AD), cerebral vascular diseases (stroke, transient ischemic attack, epilepsy, neuroimmune inflammatory diseases (multiple sclerosis, MS), neuromyelitis optical spectrum disorders (NMOSDs), and mood disorders [autism spectrum disorder (ASD), major depressive disorder (MDD)], which all imply that SCFAs might be vital to MGB axis communication[7]. Herein, we outline possible pathways of the MGB axis and illustrate the interactions between SCFAs and the MGB axis, as well as their relationships in different CNS diseases.
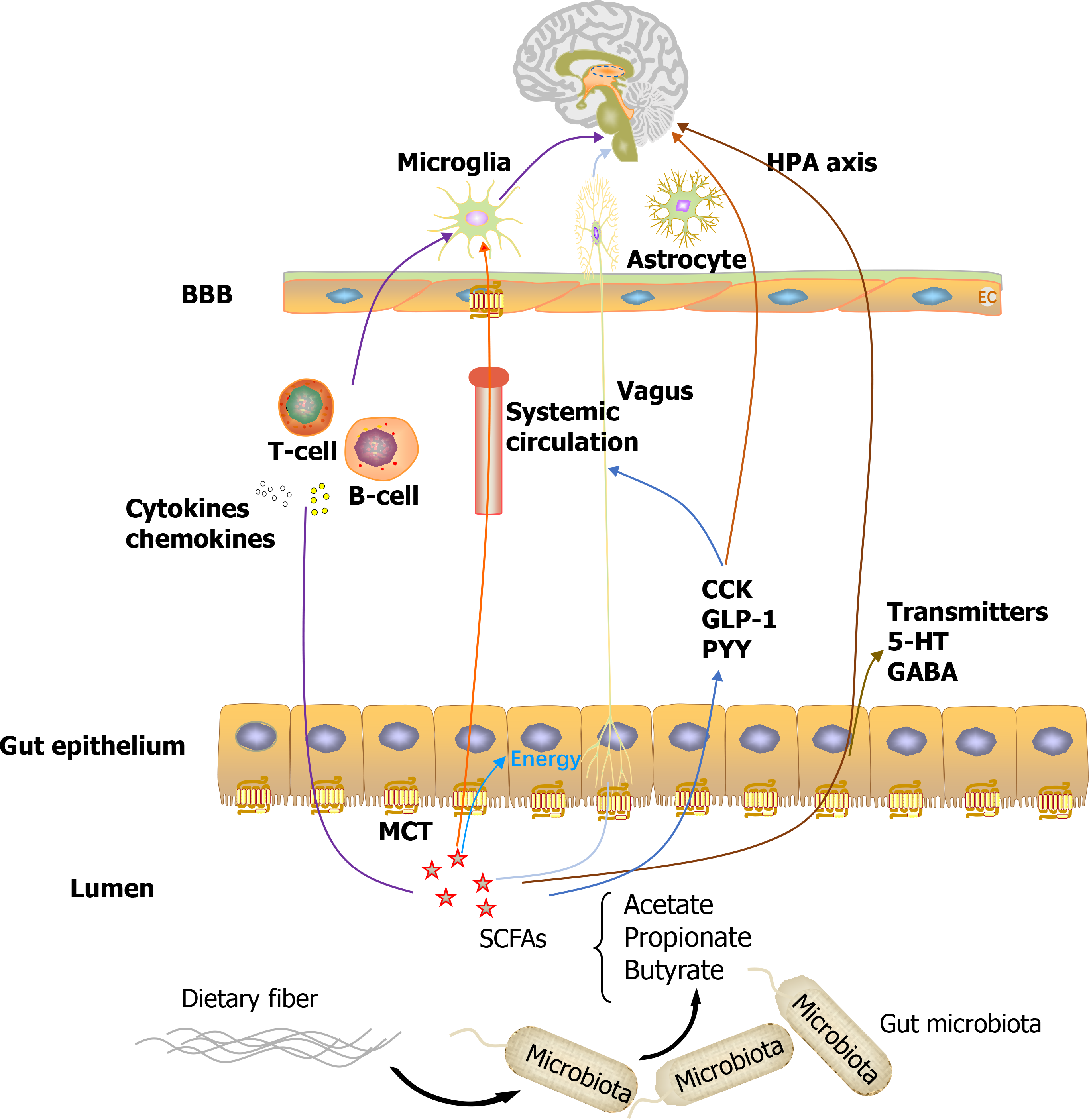
An increasing number of studies indicated that multiple direct and indirect pathways involving immune, neural, and humoral signaling exist, through which the gut microbiota can modulate the MGB axis and vice versa. Downward, the CNS can modulate the release of satiety peptides, affect the hypothalamic-pituitary-adrenal (HPA) axis, autonomic nervous system, and body immune system, thereby ultimately altering the state of intestinal epithelial cells and change the intestinal function. Conversely, the gut microbiota may affect the brain upward via the following mechanisms[4]: (1) The neural pathway: Some gut microbes can produce neuroactive metabolites (e.g., SCFAs) and neurotransmitters (e.g., GABA), and over 90% of 5-hydroxytryptamine (5-HT) is synthesized by enterochromaffin cells (EC). These microbial productions can be released into the blood circulation, pass through the blood-brain barrier (BBB), or activate other pathways, ultimately affecting neural function. The enteric nervous system (ENS) can directly communicate with the spinal cord and brain through the vagus nerve; (2) Endocrine pathway: The gut microbiota can regulate the HPA axis participating in stress responses. In addition, EC cells can synthesize hormones (e.g., peptide YY) that are involved in the modulation of eating, affecting either the hypothalamic centers of appetite control or indirectly affecting the vagal-brainstem-hypothalamic pathway; and (3) Immune pathway: The gastrointestinal tract has the densest concentration of immune cells and can release cytokines and chemokines that can infiltrate the blood and lymphatic systems, or affect neural messages carried by the vagal and spinal afferent neurons to the brain. Furthermore, gut microbiota can affect neuroinflammation via bacterial metabolite-mediated mechanisms, likely via SCFAs.
SCFAs are small organic monocarboxylic acids with a chain length of carbon atoms of less than six, of which more than 95% of them are acetate, propionate, and butyrate. The majority of SCFAs, reaching up to 50 to 200 mmol/L in the large intestine, are produced by the microbiota through the anaerobic fermentation of indigestible dietary fibers or resistant starch. Only a small proportion of SCFAs is acquired by the consumption of fermented foods[5,8]. The content of SCFAs in human feces is approximately 60 g/kg for acetic acid, 10-20 g/kg for propionic acid[9], and 3.5-32.6 g/kg for butyric acid[10], with a ratio of 60:20:20[11]. The exact levels of SCFAs vary among different individuals depending on the composition of the microbiota and the amount of complex carbohydrates in the diet. SCFAs, particularly butyrate, are absorbed by the colonic epithelium via monocarboxylate transporters (MCTs) and provide energy for the colon. SCFAs can cross the BBB, possibly owing to the abundant expression of MCTs in intracranial endothelial cells[12]. The remaining SCFAs are primarily utilized by hepatocytes, resulting in only a small fraction of SCFAs released in circulation, with concentrations of 1-15 μM for propionate and butyrate, and 100-200 μM for acetate in circulation[11].
In addition to their local protective effects on the gut, including enhancing gut motility and gut barrier integrity, SCFAs exhibit promising performance in the MGB axis. It has been reported that SCFAs function in the brain via two major cellular mechanisms. One is to bind to and activate G protein-coupled receptors, of which GPR43 and GPR41, which were later renamed as free fatty acid receptor 2 (FFAR2) and FFAR3, respectively, are the most investigated mechanisms. They are broadly expressed in the gastrointestinal mucosa and immune system[13]. FFAR3 has been shown to be highly expressed in brain tissue and the BBB[14], based on the finding that all three major SCFAs exist in the cerebrospinal fluid, although their concentrations were relatively low[15]. Another mechanism is to induce histone deacetylase (HDAC) inhibitory effects, with butyrate being the most potent inhibitor of class I and IIa HDACs[16]. Research has shown that the effects of SCFAs on HDACs are dose dependent. It is widely accepted that HDACs are involved in brain development and various neuropsychiatric diseases. Furthermore, SCFAs can interact with the brain through the three major MGB axis pathways mentioned above. SCFAs can interact with the neural pathway by reinforcing BBB integrity[17], affecting levels of neurotrophic factors[18], promoting serotonin biosynthesis[19], or directly activating vagal afferent[20]. Furthermore, SCFAs can promote the endocrine pathway by modulating the secretion of peptide YY and glucagonlike peptide 1[21]. Additionally, SCFAs can interfere with the immune pathway by directly affecting immune cells and immune modulators to maintain homeostasis. SCFAs can regulate the differentiation, recruitment, and activation of systemic inflammatory cells, including neutrophils, dendritic cells, macrophages, monocytes, and T cells[22,23], thereby affecting systemic inflammation as well as the microglial structure, maturation, and activation involved in neuroimmunity[24,25].
In summary, SCFAs might directly or indirectly communicate along the MGB axis by activating GPRs or inhibiting HDACs. They can enter the bloodstream, activate the vagus pathway, facilitate the secretion of other hormones or neurotransmitters, interfere with the immune response, and finally participate in neuropathologies.
AD is the most typical form of dementia among member of the older population. Recently, emerging evidence has shown that the gut microbiota participates in the pathophysiology of AD and exhibits a different composition in AD patients[26,27]. In 2019, an oral drug that affects reconditioned gut microbiota received its first approval in China for the treatment of mild to moderate AD to improve cognitive function. In this context, the roles of SCFAs in AD have garnered significant attention. A recent small sample, randomized, double-blind, pilot study in an AD group showed that the modified Mediterranean-ketogenic diet might alleviate AD symptoms by modulating SCFAs (reducing fecal lactate and acetate while increasing propionate and butyrate) as well as improved AD biomarkers in cerebral spinal fluid[28]. An animal study by Zhang et al[29] in APP/PS1 transgenic AD mice showed that the concentrations of butyric acid were lower in both feces and the brain, whereas the abundance of Butyricicoccus pullicaecorum, a butyrate producer, decreased, which may compromise cognitive decline in AD. Consistent with this, a study by Govindarajan et al[30] showed that treatment involving butyrate can improve memory impairment in AD mice even when administered at an advanced stage of pathology; this is attributable to its role in HDAC inhibition. Similarly, acetate was shown to be neuroprotective and exert an anti-neuroinflammatory effect in AD mice, likely via the upregulation of GPR41 and suppression of the ERK/JNK/NF-kappaB pathway[31]. Certain SCFAs, particularly valeric acid, butyric acid, and propionic acid, can interfere with initial protein-protein interactions in vitro, which are necessary for the formation of toxic soluble Aβ aggregates[32].
PD is the second most typical neurodegenerative disorder; it is clinically characterized by motor systems and non-motor symptoms, and pathogenetically characterized by the aggregation of Lewy bodies in the nervous system. The role of gut microbiota in PD has been investigated extensively and promising results have been obtained; researchers hypothesized that the pathological process in PD may spread from the gut to the brain[33]. It is widely accepted that the gut microbiota composition differs between patients with PD and healthy individuals, and target the gut microbiota could be a promising strategy for PD[34]. Emerging evidence indicates that SCFAs are crucial for correlating PD and the gut within the enteric nervous system. A recent study showed that fecal SCFA concentrations, as well as populations of SCFA-producing microbiota reduced significantly in PD patients compared with controls[35-39]; this will induce alterations in the ENS and contribute to gastrointestinal dysmotility in PD patients with digestive symptoms, such as constipation[40]. In addition, Shin[41] et al measured the plasma concentrations of SCFAs in PD patients and controls; they discovered that the acetic acid concentration was higher in the PD group and was positively correlated with age, whereas the propionic acid concentration was negatively correlated with the UPDRS part III score and use of entacapone. Meanwhile, the butyric acid concentration was correlated with the inhibitor and anticholinergic usages of monoamine oxidase. A-synuclein (aSyn) aggregation is regarded as critical in PD development. An animal study in an alpha-synuclein-overexpressing (ASO) mouse model of PD showed that SCFA-gavage ASO mice displayed significantly impaired performance in several motor tasks, and that aSyn aggregated more seriously in the brain compared with in untreated mice, possibly owing to the promotion of the microglial morphology to a more active status within affected brain regions[25]. Additionally, sodium butyrate might have caused α-synuclein degradation via an Atg5-dependent and PI3K/Akt/mTOR-related autophagy pathway. In an in vitro model of PD, propionic acid was suggested as a potential therapy for rotenone toxicity in PD.
Moreover, butyrate has been discovered in an animal model of Huntington’s disease to protect against neurotoxicity, resulting in improved motor performance by deacetylase inhibition.
These results imply the potential significance of SCFAs in the onset and development of neurodegenerative diseases and provide a new perspective for their future treatment.
Stroke is the second leading cause of death worldwide, and options for its treatment remain limited. Overwhelming evidence suggests that the gut microbiome is significantly associated with most of its modifiable risk factors, those that are associated with atherosclerosis, including hypertension, hyperlipidemia, diabetes, and obesity. Studies have revealed that stroke and transient ischemic attack (TIA) patients showed significant changes in gut microbial diversity (an increased abundance of Akkermansia muciniphila and an excessive abundance of clostridial species), the abundance of opportunistic pathogens, such as Enterobacter, Megasphaera, Oscillibacter, and Desulfovibrio were increased in stroke and TIA patients, and the commensal or beneficial genera including Bacteroides, Prevotella, and Faecalibacterium were decreased[42,43]. The levels of SCFAs changed after stroke, although the results were inconsistent[44-46]. Sun et al[47] discovered that the concentration of butyric acid decreased after stroke, whereas Li et al[46] and Dragana et al[42] presented the opposite conclusion. However, SCFAs were considered to be beneficial products in most studies. Sun et al[47] indicated that the oral gavage of Clostridium butyricum can attenuate cerebral ischemic-reperfusion injury and neuronal apoptosis by regulating the composition of intestinal microflora and restoring cerebral ischemic-reperfusion induced decreases of fecal microbiota diversity in diabetic mice. Additionally, the transplant of fecal microbiota rich in SCFAs, particularly butyric acid, exhibited protective effects in a rat model of middle cerebral artery occlusion, and alleviated post-stroke neurological deficits in aged stroke mice[44]. These functions might be related to their role in modulating the immune system. A recent study identified that SCFAs improved post-stroke recovery by altering contralesional cortex connectivity and changing synapse densities after stroke, which might be associated with their effect on the recruitment of T cells to the infarcted brain and the corresponding microglial activation[48]. However, studies regarding the association between cerebral vascular diseases and SCFAs are limited, most of which are focused on ischemic stroke and completed in animal models. High-quality clinical studies pertaining to its roles in chronic vascular diseases, such as small vessel diseases or vascular dementia, should be further investigated.
Epilepsy is a chronic brain condition characterized by persistent unprovoked seizures caused by the abnormal function of the CNS due to the excessive and synchronous discharge of neurons. More than 50 million individuals worldwide are affected by epilepsy, and this can result in cognitive decline and depression in the patients. Recent clinical studies have confirmed that the intestinal flora of patients with epilepsy differs from that of normal individuals. Fusobacteria phylum, Proteobacteria phylum and the genera of Campylobacter, Delftia, Haemophilus, Lautropia, Neisseria among Proteobacteria phylum were found to be higher in patients compared with the healthy persons[49]. Similarly, in an animal model of epilepsy, it has been confirmed that a ketogenic diet exerts an anti-epileptic effect by changing the composition of gut microbiota, consequently resulting in increased expression of GABA in the hippocampus of mice. The transplantation of feces from epileptic mice with ketogenic diet intervention in the normal diet group can reduce the frequency of seizures[50]. However, studies regarding the role of gut microbiota in epilepsy remain limited, and most of them are related to treatment through a ketogenic diet[51]. Their roles in epilepsy are likely to be optimistic. A previous study based on a mouse vascular dementia model showed that injection with Clostridium butyricum significantly reduced cognitive impairment and histopathological changes in the hippocampus of mice by regulating the gut-brain axis, Clostridium butyricum increased the levels of BDNF and Bcl-2 but decreased level of Bax and induced Akt phosphorylation, ultimately reduced neuronal apoptosis[52]. Furthermore, SCFAs reversed functional abnormalities in the mitochondrial respiratory chain complex in the prefrontal cortex, hippocampus, striatum, and amygdala regions of rats in manic animal models, as well as reversed depressive and manic behaviors which was associated with histone deacetylase inhibition[53]. The studies above indirectly indicated that SCFAs can affect the anatomical structure associated closely with epilepsy. Hence, further studies regarding the relationship between SCFAs and epilepsy are warranted.
MS is a chronic T cell-mediated autoimmune disease of the CNS that is characterized by demyelination and axonal damage in the brain and spinal cord. Recently, gut microbiota has received increasing attention in regard to their roles in the development of MS, as well as SCFAs[54]. It was discovered that fecal levels of SCFAs (acetate, propionate, and butyrate) reduced in a Chinese cohort study of MS compared to health controls, corresponding to their alterations in blood circulation[55,56]. SCFAs have been shown to exert anti-inflammatory effects in MS, possibly by interfering with T cell differentiation[57]. Specifically, oral treatment with SCFAs ameliorated the symptoms of experimental autoimmune encephalomyelitis (EAE), i.e., the most typically used animal model of MS, and reduced axonal damage by suppressing the differentiation of pro-inflammatory Th17 cells, while promoting differentiation of anti-inflammatory Tregs[58,59]. Similarly, another experiment that treated ordinary EAE mice with fecal samples from those rich in SCFAs resulted in better EAE clinical scores[60]. Moreover, butyrate might alleviate CNS demyelination and promote remyelination by facilitating oligodendrocyte maturation and differentiation[61]. Acetate supplementation is assumed to increase histone acetylation by inducing more acetyl-CoA metabolism, resulting in preserved spinal cord lipid content and reduced clinical symptoms of EAE[62]. A similar finding has been discovered in NMOSDs, which are characterized by severe immune-mediated demyelination and axonal damage predominantly affecting the optic and spinal cord nerves[63].
A recent study demonstrated that levels of SCFAs reduced in patients with anti-leucine-rich glioma-inactivated 1 encephalitis[64], which is a rare autoimmune encephalitis, related to a group of immune-mediated inflammatory neurological diseases with antibodies against CNS components, characterized by subacute disturbances of memory, behavior, mood, and seizures. Compared to health controls, the anti-leucine-rich glioma-inactivated 1 encephalitis patients exhibited a decreased microbial diversity and an altered composition of gut microbiome, the Faecalibacterium, Roseburia, Lachnospira, Ruminococcus, and Blautia, which had the ability to produce SCFAs, were obviously reduced in the patient group. However, more studies are warranted to explore the relationships or internal mechanisms between gut microbiota and anti-leucine-rich glioma-inactivated 1 encephalitis.
As both microbiota and its metabolites SCFAs have been widely proven to be associated closely to the immune system, we can expect their application in future immune-modulating therapy.
ASD is collectively referred to as autism, Asperger’s syndrome, and pervasive developmental disorder. It is characterized by impairment in communication skills, as well as repetitive or restrictive patterns in behavior, interests, and activities. The results of the relationship between SCFAs and ASD are controversial. In a clinical study, the concentrations of fecal acetic, butyric, iso-butyric, valeric and isovaleric acids except for caproic acid were all significantly higher in children with ASD compared with controls, which indicated that the fermentation products were associated with the occurrence and progress of ASD[65]. However, a recent study reported that children with ASD had lower fecal acetate and butyrate levels, but higher fecal valeric acid level than the controls, which was related with the altered composition of the gut microbiota in ASD individuals, the abundances of butyrate-producing taxa (Ruminococcaceae, Eubacterium, Lachnospiraceae and Erysipelotrichaceae) were decreased and the abundance of valeric acid associated bacteria (Acidobacteria) was increased in autistic individuals[66]. Rats treated with propionic acid showed restricted interest and impaired social behavior, as observed in ASD[67]. Therefore, it can be speculated that the pathogenesis of ASD might be caused by the overproduction of propionate by gut microbiota[67]. The modulate of gut microbiota might be a promising method for the treatment of ASD.
MDD is the most typical mental disorder among the disabilities worldwide. A clinical study had shown that fecal SCFA levels decreased in patients with depression although the size of groups in this clinical study is small and more participants are needed[68]. Similarly, in an animal study, three major fecal SCFAs (acetic acid, propionic acid and pentanoic acid) in the hypothalamus were discovered to be lower in depressed mice than in control mice[69]. Recently, it was reported that high-dietary fiber significantly attenuated depressive symptoms in maternal mice after weaning offspring by elevating the formation of SCFAs[70]. These studies indicate that SCFAs might be vital to the pathogenesis of MDD and may be a possible treatment strategy for MDD in the future.
Explosive basic and clinical studies have implicated gut microbiota dysbiosis and SCFAs to play critical roles in various neurological diseases (Figure 1). Despite the low levels of SCFA in peripheral circulation, they may actively interact with the MGB axis involving various biological processes by binding to GRPs or by inhibiting HDAC. In addition, they may interfere with immune response, exerting anti-inflammatory functions, or activate the vagus nerve and finally communicate with the brain. It is proposed that the fecal SCFA levels are decreased in most neurological disorders; this may be related to the intracranial pathology. These promising results also suggest their potential protective roles in CNS diseases, and SCFAs supplementation may be anticipated as an effective therapy in the future. An earlier systematic review summarized the human randomized clinical trials regarding the effects of gut microbiota shaping on cognitive functions, including probiotics, prebiotics, synbiotics, and fecal microbiota transplant (FMT). The results showed that probiotic supplementation and FMT could improve cognitive functions in subjects, irrespective of their health; however, supplementation with prebiotics in unhealthy subjects did not provide any cognitive improvement[71]. Most studies are descriptive research. Most current results are revealed in animal studies, and the remaining small number of clinical studies generally include small sample sizes, resulting in a low-level evidence. Relatively few studies are published in the field of epilepsy and certain other fields, such as chronic vascular diseases, that are also speculated to be possibly related to microbiota dysbiosis. Moreover, most studies are elucidated around the trends of SCFAs in specified neurological diseases, whereas deeper linking or mechanisms between SCFAs and the brain are still ambiguous. Further, larger samples of clinical studies and basic mechanism research are urgently warranted.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neurosciences
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kikuchi K, Quaglio AEV S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Lederberg J. Infectious history. Science. 2000;288:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 358] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 2. | Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474:1823-1836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1710] [Cited by in RCA: 2056] [Article Influence: 257.0] [Reference Citation Analysis (7)] |
| 3. | Sender R, Fuchs S, Milo R. Are We Really Vastly Outnumbered? Cell. 2016;164:337-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1022] [Cited by in RCA: 1292] [Article Influence: 143.6] [Reference Citation Analysis (0)] |
| 4. | Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, Guzzetta KE, Jaggar M, Long-Smith CM, Lyte JM, Martin JA, Molinero-Perez A, Moloney G, Morelli E, Morillas E, O'Connor R, Cruz-Pereira JS, Peterson VL, Rea K, Ritz NL, Sherwin E, Spichak S, Teichman EM, van de Wouw M, Ventura-Silva AP, Wallace-Fitzsimons SE, Hyland N, Clarke G, Dinan TG. The Microbiota-Gut-Brain Axis. Physiol Rev. 2019;99:1877-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 2781] [Article Influence: 463.5] [Reference Citation Analysis (2)] |
| 5. | Cox LM, Weiner HL. Microbiota Signaling Pathways that Influence Neurologic Disease. Neurotherapeutics. 2018;15:135-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 6. | Cryan JF, O'Riordan KJ, Sandhu K, Peterson V, Dinan TG. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19:179-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 779] [Article Influence: 155.8] [Reference Citation Analysis (0)] |
| 7. | Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16:461-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 1846] [Article Influence: 307.7] [Reference Citation Analysis (1)] |
| 8. | Silva YP, Bernardi A, Frozza RL. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne). 2020;11:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 780] [Cited by in RCA: 1605] [Article Influence: 321.0] [Reference Citation Analysis (0)] |
| 9. | Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 1305] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 10. | McOrist AL, Miller RB, Bird AR, Keogh JB, Noakes M, Topping DL, Conlon MA. Fecal butyrate levels vary widely among individuals but are usually increased by a diet high in resistant starch. J Nutr. 2011;141:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 11. | Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1896] [Cited by in RCA: 2190] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 12. | Vijay N, Morris ME. Role of monocarboxylate transporters in drug delivery to the brain. Curr Pharm Des. 2014;20:1487-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 296] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 13. | Mohajeri MH, Brummer RJM, Rastall RA, Weersma RK, Harmsen HJM, Faas M, Eggersdorfer M. The role of the microbiome for human health: from basic science to clinical applications. Eur J Nutr. 2018;57:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 276] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 14. | Hoyles L, Snelling T, Umlai UK, Nicholson JK, Carding SR, Glen RC, McArthur S. Microbiome-host systems interactions: protective effects of propionate upon the blood-brain barrier. Microbiome. 2018;6:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 377] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 15. | Wishart DS, Feunang YD, Marcu A, Guo AC, Liang K, Vázquez-Fresno R, Sajed T, Johnson D, Li C, Karu N, Sayeeda Z, Lo E, Assempour N, Berjanskii M, Singhal S, Arndt D, Liang Y, Badran H, Grant J, Serra-Cayuela A, Liu Y, Mandal R, Neveu V, Pon A, Knox C, Wilson M, Manach C, Scalbert A. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 2018;46:D608-D617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2659] [Cited by in RCA: 2544] [Article Influence: 363.4] [Reference Citation Analysis (0)] |
| 16. | Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem Int. 2016;99:110-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 601] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 17. | Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, Korecka A, Bakocevic N, Ng LG, Kundu P, Gulyás B, Halldin C, Hultenby K, Nilsson H, Hebert H, Volpe BT, Diamond B, Pettersson S. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1699] [Article Influence: 169.9] [Reference Citation Analysis (0)] |
| 18. | Varela RB, Valvassori SS, Lopes-Borges J, Mariot E, Dal-Pont GC, Amboni RT, Bianchini G, Quevedo J. Sodium butyrate and mood stabilizers block ouabain-induced hyperlocomotion and increase BDNF, NGF and GDNF levels in brain of Wistar rats. J Psychiatr Res. 2015;61:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 19. | Reigstad CS, Salmonson CE, Rainey JF 3rd, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29:1395-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 900] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 20. | Goswami C, Iwasaki Y, Yada T. Short-chain fatty acids suppress food intake by activating vagal afferent neurons. J Nutr Biochem. 2018;57:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 21. | Byrne CS, Chambers ES, Alhabeeb H, Chhina N, Morrison DJ, Preston T, Tedford C, Fitzpatrick J, Irani C, Busza A, Garcia-Perez I, Fountana S, Holmes E, Goldstone AP, Frost GS. Increased colonic propionate reduces anticipatory reward responses in the human striatum to high-energy foods. Am J Clin Nutr. 2016;104:5-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 22. | Corrêa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MA. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunology. 2016;5:e73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 596] [Cited by in RCA: 885] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 23. | Luu M, Visekruna A. Short-chain fatty acids: Bacterial messengers modulating the immunometabolism of T cells. Eur J Immunol. 2019;49:842-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 24. | Erny D, Hrabě de Angelis AL, Prinz M. Communicating systems in the body: how microbiota and microglia cooperate. Immunology. 2017;150:7-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 25. | Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet MF, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson's Disease. Cell. 2016;167:1469-1480.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2094] [Cited by in RCA: 2408] [Article Influence: 267.6] [Reference Citation Analysis (0)] |
| 26. | Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 969] [Cited by in RCA: 1125] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 27. | Doifode T, Giridharan VV, Generoso JS, Bhatti G, Collodel A, Schulz PE, Forlenza OV, Barichello T. The impact of the microbiota-gut-brain axis on Alzheimer's disease pathophysiology. Pharmacol Res. 2021;164:105314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 208] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 28. | Nagpal R, Neth BJ, Wang S, Craft S, Yadav H. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer's disease markers in subjects with mild cognitive impairment. EBioMedicine. 2019;47:529-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 423] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 29. | Zhang L, Wang Y, Xiayu X, Shi C, Chen W, Song N, Fu X, Zhou R, Xu YF, Huang L, Zhu H, Han Y, Qin C. Altered Gut Microbiota in a Mouse Model of Alzheimer's Disease. J Alzheimers Dis. 2017;60:1241-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 337] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 30. | Govindarajan N, Agis-Balboa RC, Walter J, Sananbenesi F, Fischer A. Sodium butyrate improves memory function in an Alzheimer's disease mouse model when administered at an advanced stage of disease progression. J Alzheimers Dis. 2011;26:187-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 293] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 31. | Liu J, Li H, Gong T, Chen W, Mao S, Kong Y, Yu J, Sun J. Anti-neuroinflammatory Effect of Short-Chain Fatty Acid Acetate against Alzheimer's Disease via Upregulating GPR41 and Inhibiting ERK/JNK/NF-κB. J Agric Food Chem. 2020;68:7152-7161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 32. | Ho L, Ono K, Tsuji M, Mazzola P, Singh R, Pasinetti GM. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer's disease-type beta-amyloid neuropathological mechanisms. Expert Rev Neurother. 2018;18:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 260] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 33. | Braak H, Rüb U, Gai WP, Del Tredici K. Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm (Vienna). 2003;110:517-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1153] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 34. | Mulak A, Bonaz B. Brain-gut-microbiota axis in Parkinson's disease. World J Gastroenterol. 2015;21:10609-10620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 373] [Cited by in RCA: 390] [Article Influence: 39.0] [Reference Citation Analysis (5)] |
| 35. | Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, Mutlu E, Shannon KM. Colonic bacterial composition in Parkinson's disease. Mov Disord. 2015;30:1351-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 680] [Cited by in RCA: 897] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 36. | Pietrucci D, Cerroni R, Unida V, Farcomeni A, Pierantozzi M, Mercuri NB, Biocca S, Stefani A, Desideri A. Dysbiosis of gut microbiota in a selected population of Parkinson's patients. Parkinsonism Relat Disord. 2019;65:124-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 37. | Nishiwaki H, Ito M, Ishida T, Hamaguchi T, Maeda T, Kashihara K, Tsuboi Y, Ueyama J, Shimamura T, Mori H, Kurokawa K, Katsuno M, Hirayama M, Ohno K. Meta-Analysis of Gut Dysbiosis in Parkinson's Disease. Mov Disord. 2020;35:1626-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 232] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 38. | Vascellari S, Palmas V, Melis M, Pisanu S, Cusano R, Uva P, Perra D, Madau V, Sarchioto M, Oppo V, Simola N, Morelli M, Santoru ML, Atzori L, Cossu G, Manzin A. Gut Microbiota and Metabolome Alterations Associated with Parkinson's Disease. mSystems. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 192] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 39. | Wallen ZD, Appah M, Dean MN, Sesler CL, Factor SA, Molho E, Zabetian CP, Standaert DG, Payami H. Characterizing dysbiosis of gut microbiome in PD: evidence for overabundance of opportunistic pathogens. NPJ Parkinsons Dis. 2020;6:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 150] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 40. | Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Bürmann J, Faßbender K, Schwiertz A, Schäfer KH. Short chain fatty acids and gut microbiota differ between patients with Parkinson's disease and age-matched controls. Parkinsonism Relat Disord. 2016;32:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 813] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 41. | Qiao CM, Sun MF, Jia XB, Shi Y, Zhang BP, Zhou ZL, Zhao LP, Cui C, Shen YQ. Sodium butyrate causes α-synuclein degradation by an Atg5-dependent and PI3K/Akt/mTOR-related autophagy pathway. Exp Cell Res. 2020;387:111772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 42. | Stanley D, Moore RJ, Wong CHY. An insight into intestinal mucosal microbiota disruption after stroke. Sci Rep. 2018;8:568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 43. | Yin J, Liao SX, He Y, Wang S, Xia GH, Liu FT, Zhu JJ, You C, Chen Q, Zhou L, Pan SY, Zhou HW. Dysbiosis of Gut Microbiota With Reduced Trimethylamine-N-Oxide Level in Patients With Large-Artery Atherosclerotic Stroke or Transient Ischemic Attack. J Am Heart Assoc. 2015;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 405] [Cited by in RCA: 488] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 44. | Chen R, Xu Y, Wu P, Zhou H, Lasanajak Y, Fang Y, Tang L, Ye L, Li X, Cai Z, Zhao J. Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol Res. 2019;148:104403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 265] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 45. | Yamashiro K, Tanaka R, Urabe T, Ueno Y, Yamashiro Y, Nomoto K, Takahashi T, Tsuji H, Asahara T, Hattori N. Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke. PLoS One. 2017;12:e0171521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 189] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 46. | Li N, Wang X, Sun C, Wu X, Lu M, Si Y, Ye X, Wang T, Yu X, Zhao X, Wei N. Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol. 2019;19:191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 201] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 47. | Sun J, Wang F, Ling Z, Yu X, Chen W, Li H, Jin J, Pang M, Zhang H, Yu J, Liu J. Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Res. 2016;1642:180-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 48. | Sadler R, Cramer JV, Heindl S, Kostidis S, Betz D, Zuurbier KR, Northoff BH, Heijink M, Goldberg MP, Plautz EJ, Roth S, Malik R, Dichgans M, Holdt LM, Benakis C, Giera M, Stowe AM, Liesz A. Short-Chain Fatty Acids Improve Poststroke Recovery via Immunological Mechanisms. J Neurosci. 2020;40:1162-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 232] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 49. | Şafak B, Altunan B, Topçu B, Eren Topkaya A. The gut microbiome in epilepsy. Microb Pathog. 2020;139:103853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 50. | Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell. 2018;173:1728-1741.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 638] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 51. | De Caro C, Iannone LF, Citraro R, Striano P, De Sarro G, Constanti A, Cryan JF, Russo E. Can we 'seize' the gut microbiota to treat epilepsy? Neurosci Biobehav Rev. 2019;107:750-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 52. | Liu J, Sun J, Wang F, Yu X, Ling Z, Li H, Zhang H, Jin J, Chen W, Pang M, Yu J, He Y, Xu J. Neuroprotective Effects of Clostridium butyricum against Vascular Dementia in Mice via Metabolic Butyrate. Biomed Res Int. 2015;2015:412946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 53. | Moretti M, Valvassori SS, Varela RB, Ferreira CL, Rochi N, Benedet J, Scaini G, Kapczinski F, Streck EL, Zugno AI, Quevedo J. Behavioral and neurochemical effects of sodium butyrate in an animal model of mania. Behav Pharmacol. 2011;22:766-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 54. | Melbye P, Olsson A, Hansen TH, Søndergaard HB, Bang Oturai A. Short-chain fatty acids and gut microbiota in multiple sclerosis. Acta Neurol Scand. 2019;139:208-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 55. | Zeng Q, Junli Gong, Liu X, Chen C, Sun X, Li H, Zhou Y, Cui C, Wang Y, Yang Y, Wu A, Shu Y, Hu X, Lu Z, Zheng SG, Qiu W, Lu Y. Gut dysbiosis and lack of short chain fatty acids in a Chinese cohort of patients with multiple sclerosis. Neurochem Int. 2019;129:104468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 56. | Saresella M, Marventano I, Barone M, La Rosa F, Piancone F, Mendozzi L, d'Arma A, Rossi V, Pugnetti L, Roda G, Casagni E, Cas MD, Paroni R, Brigidi P, Turroni S, Clerici M. Alterations in Circulating Fatty Acid Are Associated With Gut Microbiota Dysbiosis and Inflammation in Multiple Sclerosis. Front Immunol. 2020;11:1390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 127] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 57. | Colpitts SL, Kasper LH. Influence of the Gut Microbiome on Autoimmunity in the Central Nervous System. J Immunol. 2017;198:596-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 58. | Haghikia A, Jörg S, Duscha A, Berg J, Manzel A, Waschbisch A, Hammer A, Lee DH, May C, Wilck N, Balogh A, Ostermann AI, Schebb NH, Akkad DA, Grohme DA, Kleinewietfeld M, Kempa S, Thöne J, Demir S, Müller DN, Gold R, Linker RA. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity. 2015;43:817-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 641] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 59. | Mizuno M, Noto D, Kaga N, Chiba A, Miyake S. The dual role of short fatty acid chains in the pathogenesis of autoimmune disease models. PLoS One. 2017;12:e0173032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 169] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 60. | Chitrala KN, Guan H, Singh NP, Busbee B, Gandy A, Mehrpouya-Bahrami P, Ganewatta MS, Tang C, Chatterjee S, Nagarkatti P, Nagarkatti M. CD44 deletion leading to attenuation of experimental autoimmune encephalomyelitis results from alterations in gut microbiome in mice. Eur J Immunol. 2017;47:1188-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 61. | Chen T, Noto D, Hoshino Y, Mizuno M, Miyake S. Butyrate suppresses demyelination and enhances remyelination. J Neuroinflammation. 2019;16:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 151] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 62. | Chevalier AC, Rosenberger TA. Increasing acetyl-CoA metabolism attenuates injury and alters spinal cord lipid content in mice subjected to experimental autoimmune encephalomyelitis. J Neurochem. 2017;141:721-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 63. | Gong J, Qiu W, Zeng Q, Liu X, Sun X, Li H, Yang Y, Wu A, Bao J, Wang Y, Shu Y, Hu X, Bellanti JA, Zheng SG, Lu Y, Lu Z. Lack of short-chain fatty acids and overgrowth of opportunistic pathogens define dysbiosis of neuromyelitis optica spectrum disorders: A Chinese pilot study. Mult Scler. 2019;25:1316-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 64. | Ma X, Ma L, Wang Z, Liu Y, Long L, Ma X, Chen H, Chen Z, Lin X, Si L, Chen X. Clinical Features and Gut Microbial Alterations in Anti-leucine-rich Glioma-Inactivated 1 Encephalitis-A Pilot Study. Front Neurol. 2020;11:585977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 65. | Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig Dis Sci. 2012;57:2096-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 284] [Article Influence: 21.8] [Reference Citation Analysis (1)] |
| 66. | Liu S, Li E, Sun Z, Fu D, Duan G, Jiang M, Yu Y, Mei L, Yang P, Tang Y, Zheng P. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci Rep. 2019;9:287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 295] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 67. | Shultz SR, Aziz NA, Yang L, Sun M, MacFabe DF, O'Brien TJ. Intracerebroventricular injection of propionic acid, an enteric metabolite implicated in autism, induces social abnormalities that do not differ between seizure-prone (FAST) and seizure-resistant (SLOW) rats. Behav Brain Res. 2015;278:542-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 68. | Skonieczna-Żydecka K, Grochans E, Maciejewska D, Szkup M, Schneider-Matyka D, Jurczak A, Łoniewski I, Kaczmarczyk M, Marlicz W, Czerwińska-Rogowska M, Pełka-Wysiecka J, Dec K, Stachowska E. Faecal Short Chain Fatty Acids Profile is Changed in Polish Depressive Women. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 176] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 69. | Wu M, Tian T, Mao Q, Zou T, Zhou CJ, Xie J, Chen JJ. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl Psychiatry. 2020;10:350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 70. | Liu Z, Li L, Ma S, Ye J, Zhang H, Li Y, Sair AT, Pan J, Liu X, Li X, Yan S. High-Dietary Fiber Intake Alleviates Antenatal Obesity-Induced Postpartum Depression: Roles of Gut Microbiota and Microbial Metabolite Short-chain Fatty Acid Involved. J Agric Food Chem. 2020;68:13697-13710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 71. | Baldi S, Mundula T, Nannini G, Amedei A. Microbiota shaping - the effects of probiotics, prebiotics, and fecal microbiota transplant on cognitive functions: A systematic review. World J Gastroenterol. 2021;27:6715-6732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (1)] |