Published online Feb 6, 2022. doi: 10.12998/wjcc.v10.i4.1432
Peer-review started: September 5, 2021
First decision: October 11, 2021
Revised: October 21, 2021
Accepted: December 23, 2021
Article in press: December 23, 2021
Published online: February 6, 2022
Processing time: 140 Days and 16.5 Hours
Inflammatory myofibroblastic tumors (IMTs) are defined as tumors composed of differentiated myofibroblastic spindle cells, usually accompanied by numerous plasma cells and lymphocytes, and classified as intermediate (occasionally metastatic) by the World Health Organization. Its pathogenesis and biological behavior have not yet been elucidated. Breast IMT is extremely rare, and prosthesis implantation combined with IMT has not been reported. This study reports a case of IMT following resection of a malignant phyllodes tumor of the left breast and implantation of a prosthesis.
A 41-year-old female presented to our hospital with a mass in the left breast for 3 mo. The patient had undergone resection of a large mass in her left breast pathologically diagnosed as a malignant phyllodes tumor and implantation of a prosthesis five years prior. Ultrasonic examination revealed an oval mass in the left breast, and the patient underwent left breast mass resection and prosthesis removal. Light microscopy revealed the spindle cells to be diffusely proliferated, with a large number of neutrophils, lymphocytes, and plasma cell infiltration. Immunohistochemical staining revealed that the spindle cells were partially positive for smooth muscle actin, which is positive for BCL-2 and cluster of differentiation (CD) 99 but were negative for anaplastic lymphoma kinase, cytokeratin, S-100 protein, desmin, and CD34. The final diagnosis was IMT. No recurrence or metastasis was observed during the 5-year postoperative follow-up.
Prosthesis implantation may be one of the causes of IMT, but further investigation is necessary to prove it.
Core Tip: We believe that our study makes a significant contribution to the literature because inflammatory myofibroblastic tumors (IMTs) of the breast are rare and unique; however, whether they are reactive or neoplastic in nature remains unelucidated. This case presented the opportunity to review studies regarding cases of inflammatory myofibroblastic breast tumors and determine whether they are reactive lesions due to an exaggerated response to tissue injury or indicate a true neoplastic process. This report prompts that prosthesis implantation may cause IMT.
- Citation: Zhou P, Chen YH, Lu JH, Jin CC, Xu XH, Gong XH. Inflammatory myofibroblastic tumor after breast prosthesis: A case report and literature review. World J Clin Cases 2022; 10(4): 1432-1440
- URL: https://www.wjgnet.com/2307-8960/full/v10/i4/1432.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i4.1432
Inflammatory myofibroblastic tumors (IMTs) are rare lesions of mesenchymal origin, with a global incidence of approximately 0.04%–0.7%[1]. They primarily occur in the lungs, abdomen, pelvis, and retroperitoneum of adolescents. Unlike IMTs in other organs, most breast IMTs occur in middle-aged women > 40 years old[2]. Although reported in various organs, the occurrence of IMT in the breast is rare, and to the best of our knowledge, only 35 cases have been reported. Herein, we report a case of IMT following resection of a malignant phyllodes tumor of the left breast and implantation of a prosthesis. In addition, we review current studies on breast IMT.
A 41-year-old female had a mass in the left breast for 3 mo.
During the 3 mo, the breast mass had slowly enlarged, but the patient did not have clinical symptoms, such as fever and pain.
The patient had undergone implantation of a prosthesis five years prior and resection of a large mass in her left breast, pathologically diagnosed as a malignant phyllodes tumor.
The patient had no relevant family history.
Physical examination revealed an abnormal shape of the left breast and prosthesis, which was palpable. Additionally, an approximately 4 cm × 3 cm non-tender mass, with a clear boundary and poor activity, was identified at the 9 o’clock position.
No abnormalities were found in the patient’s laboratory examinations.
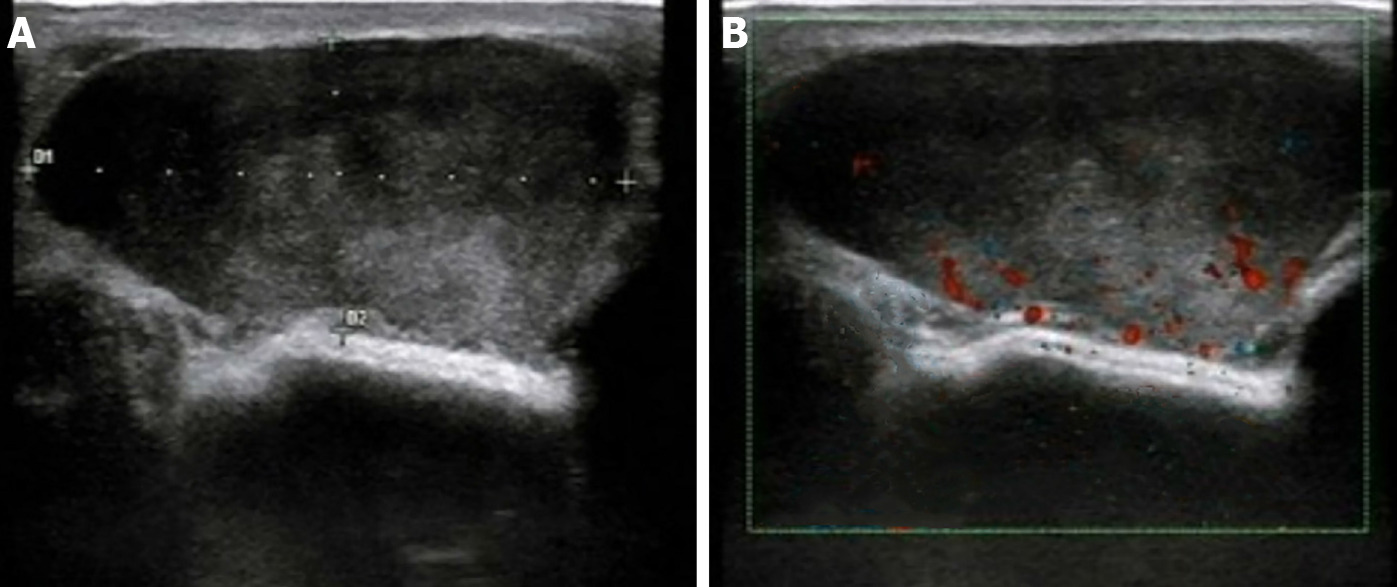
Ultrasonic examination (Esaote M7, Genova, Italy) revealed an oval, hypoechoic mass (approximately 4.2 cm × 1.8 cm in size) with clear borders and smooth edges at the 9 o'clock position in the left breast that is 3.5 cm from the nipple. Internal echo was heterogeneous, with scattered small fleck echo and slightly enhanced rear echo. A disc-shaped anechoic area was observed behind the left breast, with good internal sound transmission. Color Doppler flow imaging (CDFI) indicated a limited blood flow signal within the hypoechoic mass (Figure 1).
The diagnosis was IMT.
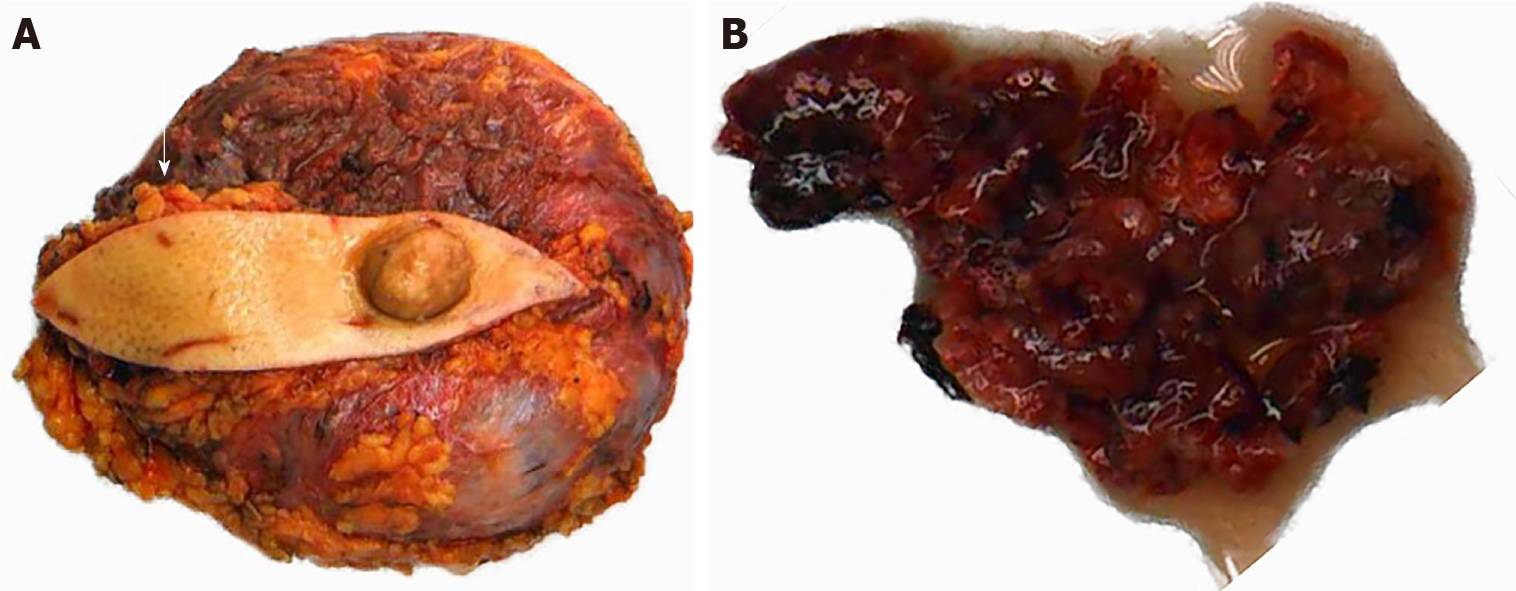
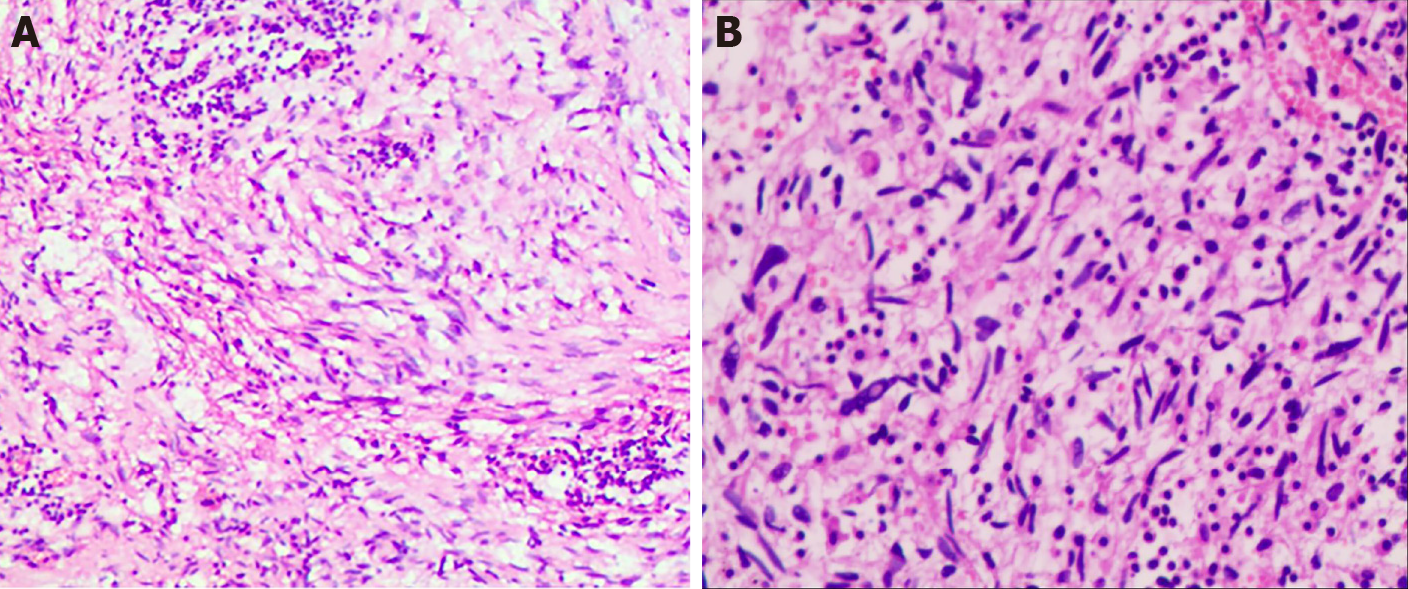
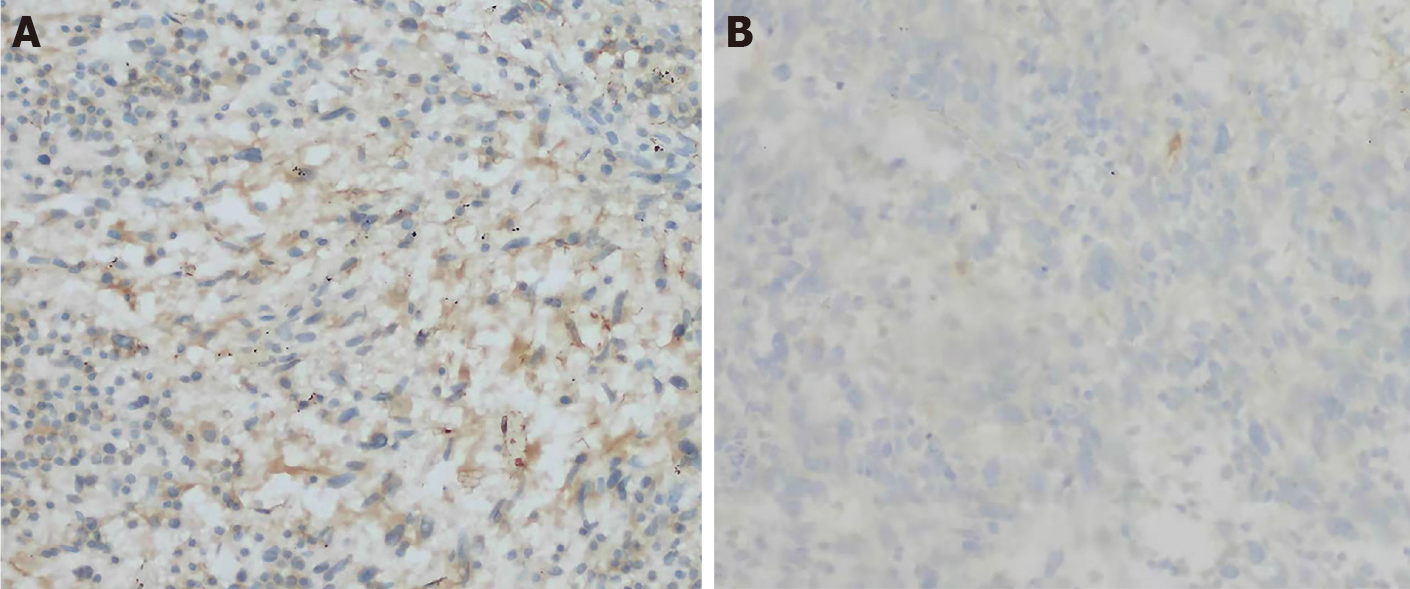
The patient underwent left breast mass resection and prosthesis removal in our hospital due to the abnormal shape of the left breast and the large mass. Specimens mainly included the breast glandular tissue, tumor tissue, prosthesis, spindle-shaped flap, and nipple (Figure 2). A complete prosthesis was identified behind the breast tissue, with a knot observed under the skin 3.5 cm from the nipple section (2.5 cm × 2.5 cm × 1.5 cm), which was gray-red and soft, with missing central tissue. Light microscopy revealed that the spindle cells to be diffusely proliferated, irregularly arranged, and scattered in the nucleus. Additionally, mildly atypical cells, mitosis, interstitial vascular proliferation, and dilation and congestion with hemorrhage were evident, as well as a large number of neutrophils and lymphocytes and plasma cell infiltration (Figure 3). Immunohistochemical staining revealed that the spindle cells were partially positive for smooth muscle actin (SMA), positive for BCL-2 and cluster of differentiation (CD) 99 but were negative for anaplastic lymphoma kinase (ALK), cytokeratin (CK), S-100 protein, desmin, and CD34. The Ki-67 score was approximately 5%, which is atypical for an IMT (Figure 4).
The patient was followed up every year. Each follow-up examination included a physical examination, a chest X-ray, a breast ultrasound (US), an abdominal US, and a routine blood examination. During the 5-year postoperative follow-up, the patient had no symptoms or imaging evidence of recurrence or metastasis.
IMTs of the breast are rare and unique; however, whether they are reactive or neoplastic in nature remains unelucidated. IMTs were widely considered inflammatory lesions and have been referred to as inflammatory pseudotumors, plasma cell granulomas, fibrous xanthomas, and inflammatory myofibrohistiocytic proliferation. Conversely, cases of local recurrence and metastasis have challenged the theory of reactive post-inflammatory lesions. As proven through cytogenetic analysis, approximately 50% of IMTs are positive for rearrangements involving the ALK gene, while cytogenetic abnormalities support the neoplastic nature of IMTs[3]. Nevertheless, the pathogenesis of ALK-negative IMT remains controversial. These lesions might not undergo gene rearrangements and might be caused by trauma, surgery, infection, or other factors that cause excessive inflammation in human tissues, which activate the abnormal proliferation of myofibroblasts[4].
Among the 35 cases of breast IMT reported in the literature, all but one occurred in females[5]. The patients’ ages ranged from 13 to 86 years, with a mean age of 47.1 years (Table 1). In our case, the patient was a 41-year-old middle-aged woman who sought medical attention due to a palpable mass. Retrospective analysis of the ultrasonograms revealed that the uneven, low echo in the mass was primarily related to the diffuse proliferation and irregular array of spindle cells in the tumor. Conversely, the scattered and small high echo could have been caused by considerable mixed acute and chronic inflammatory cell infiltration, while the spotty blood flow signal detected in the mass may be related to interstitial vascular hyperplasia with hemorrhage determined via light microscopy. The above-mentioned ultrasound manifestations lacked specificity; thus, it was difficult to distinguish them from those of phyllodes tumors or giant fibroadenomas. IMTs may also manifest with the imaging features of malignant tumors and show the diversity and lack of specificity in ultrasound imaging (Table 2). Furthermore, some scholars believe that the definitive diagnosis of IMT is difficult based on cytology alone. A reliable diagnosis may require histological samples because IMT cytology may mimic other benign or malignant breast lesions without specific features[6]. Therefore, the final diagnosis still requires postoperative histopathological examination.
| No. | Ref. | Year | Age | Sex | Site | ALK | Follow up |
| 1 | Pettinato et al[18] | 1988 | 29 | F | Right | NED, 30 mo | |
| 2 | Coffin et al[19] | 1995 | 13 | F | Right | NED, 12 mo | |
| 3 | Chetty et al[20] | 1997 | 16 | F | Right | NED, 12 mo | |
| 4 | Chetty et al[20] | 1997 | 46 | F | Right | NED, 12 mo | |
| 5 | Chetty et al[20] | 1997 | 18 | F | Right | NED, 6 mo | |
| 6 | Yip et al[12] | 1997 | 66 | F | bilateral | Bilateral recurrence at 5th month; after second excision NED 9 mo | |
| 7 | Gobbi et al[21] | 1999 | 86 | F | Left | NA | |
| 8 | Sastre et al[22] | 2002 | 64 | F | Right | Neg | NED, 33 mo |
| 9 | Haj et al[23] | 2003 | 31 | F | Right | NA | |
| 10 | Zardawi et al[11] | 2003 | 79 | F | Right | Neg | Bilateral recurrences in 9 yr |
| 11 | Ilvan et al[24] | 2005 | 60 | F | Right | NED, 85 mo | |
| 12 | Khanafsa et al[13] | 2005 | 33 | F | Left | Neg | Recurrence at 3 mo;after second excision, 12 mo |
| 13 | Khanafsa et al[13] | 2005 | 75 | F | Left | Neg | NED, 14 mo |
| 14 | Khanafsa et al[13] | 2005 | 47 | F | Right | Neg | NED, 12 mo |
| 15 | Zen et al[25] | 2005 | 46 | F | Left | NED, 12 mo | |
| 16 | Akbulut et al[6] | 2007 | 38 | F | Left | Neg | NED, 12 mo |
| 17 | Kim et al[26] | 2009 | 60 | F | Left | Neg | NED, 24 mo |
| 18 | Park et al[27] | 2010 | 47 | F | Right | NED, 36 mo | |
| 19 | Hill et al[28] | 2010 | 53 | F | Right | Neg | NA |
| 20 | Vecchio et al[5] | 2011 | 22 | M | Left | Neg | NA |
| 21 | Zhou et al[17] | 2013 | 46 | F | Right | Pos | NED |
| 22 | Li et al[29] | 2013 | 39 | F | Left | Pos | NED, 24 mo |
| 23 | Zhao et al[14] | 2013 | 56 | F | Right | Pos | Local recurrence and metastasis to left groin area |
| 24 | Bosse et al[3] | 2014 | 23 | F | Left | Pos | NED, 12 mo |
| 25 | Kovács et al[4] | 2015 | 31 | F | Left | Pos | NED, 5 years |
| 26 | Markopoulos et al [30] | 2015 | 67 | F | Left | Neg | NA |
| 27 | Choi et al[31] | 2015 | 27 | F | Right | Neg | NED, 12 mo |
| 28 | Greenleaf et al[32] | 2016 | 69 | F | Right | NA | |
| 29 | Goto et al[33] | 2016 | 52 | F | Left | NED, 9 mo | |
| 30 | Talu et al[34] | 2016 | 38 | F | Left | Neg | NED, 16 mo |
| 31 | Mao et al[2] | 2018 | 43 | F | Left | Neg | NED, 12 mo |
| 32 | Inoue et al[35] | 2018 | 16 | F | Right | Pos | NED, 9 mo |
| 33 | Dani et al[36] | 2018 | 73 | F | Bilateral | Neg | NED, 7 years |
| 34 | Fernández et al[9] | 2018 | 52 | F | Right | Neg | NED, 8 mo |
| 35 | Lv et al[1] | 2020 | 44 | F | Right | Neg | NED, 4 mo |
| 36 | Present case | 2021 | 41 | F | Left | Neg | NED, 5 years |
| No. | Ref. | Year | Ultrasonographic findings |
| 1 | Haj et al[23] | 2003 | Well-defined, homogeneous hypoechoic mass with an irregular border. No change in the rear echo. |
| 2 | Kim et al[26] | 2009 | Irregularly shaped, ill-defined, homogeneous hypoechoic mass, with anechogenic halo. CDFI: Moderate vascularity in the peripheral halo. |
| 3 | Park et al[27] | 2010 | Irregular, mostly hypoechoic, complex mass, with ill-defined margins and acoustic enhancement. CDFI: Increased vascular flow within the mass. |
| 4 | Bosse et al[3] | 2014 | A heterogeneous hypoechoic mass with irregular margins and indifferent acoustic shadowing. CDFI: Negative. |
| 5 | Markopoulos et al[30] | 2015 | Heterogeneous and oval with an echogenic rim. |
| 6 | Choi et al[31] | 2015 | Irregularly shaped, microlobulated, and hypoechoic, with combined posterior features. CDFI: Increased vascular flow to the peripheral portion. |
| 7 | Inoue et al[35] | 2018 | Well-circumscribed, oval, and hypoechoic, with a central hyperechoic area. |
| 8 | Mao et al[2] | 2018 | Irregularly shaped with unclear boundaries; the internal echo was heterogeneous with a strong fleck echo evident. CDFI: Limited blood flow signal. |
| 9 | Present case | 2021 | Clear borders and smooth edges; the internal echo was heterogeneous, with scattered small fleck echo, and slightly enhanced rear echo. CDFI: Limited blood flow signal. |
This case is a patient we diagnosed and treated five years ago. Due to insufficient experience and inefficient equipment at the time, we only performed gray-scale ultrasound and CDFI on the patient, which was insufficient. In current practice, we will recommend contrast-enhanced ultrasound (CEUS) to patients with similar cases before surgery. CEUS as a pure blood pool phenomenon technology, especially the rapid development of high-frame-rate CEUS in recent years, can show the richness of the blood supply and the blood supply pattern of the tumor, which help differentiate benign and malignant tumors. In addition, CEUS can further clarify the boundary of the tumor and show whether the surrounding normal tissues have been invaded. If there is an invasion, it can show the range of invasion, which helps determine the scope of surgical resection and ensure that the resection margin is negative.
The patient had undergone prosthesis implantation in our hospital 5 years prior and resection of a large mass (approximately 10 cm × 10 cm × 5 cm in size) pathologically diagnosed as a malignant phyllodes tumor following a surgery in the left breast. Due to this history of phyllodes tumor, we first considered the possibility of lobular tumor recurrence. Histological analysis of phyllodes tumors indicates that they are typically arranged in a slit-like epithelial bilayer component rich in cells surrounding the mesenchymal overgrowth and interstitial inflammatory cell-free components. In the present embodiment, the optical microscope did not meet the performance; thus, phyllodes tumor recurrence may be excluded. Since metaplastic breast carcinoma appears similar to IMT under the light microscope, there is a marked difference in management and prognosis. Therefore, immunohistochemistry should be performed to rule out this possibility. Following an immunohistochemical assay, we found that the spindle cells were reactive for SMA, while the tumor cells were negative for CK, which ruled out the possibility of metaplastic carcinoma. Based on a comprehensive assessment of the patient history and histopathological and immunohistochemical results, IMT was considered the final diagnosis.
This case presented the opportunity to critically review the literature regarding the cases of breast IMTs (Table 1) to determine whether they are reactive lesions due to an exaggerated response to tissue injury or indicate a true neoplastic process. Although approximately half of all IMTs across anatomical sites undergo clonal rearrangements of the ALK gene on chromosome 2p23 activating ALK protein expression (Table 1), ALK overexpression in breast IMTs is rare. In this article, we discuss ALK-negative breast IMT especially its possible etiology of trauma and surgery, which could challenge the theory that the tumorigenic nature of chromosomal abnormalities supports IMTs. The patient had undergone left breast mass resection and prosthesis implantation due to a large malignant lobular tumor of the left breast. Moreover, newly developed IMT presented 5 years following surgery. Notably, most cases of breast IMT are spontaneous, and only a few cases that had a history of trauma and tumor resection before IMTs have been reported[5]. Vecchio et al[5] reported a male patient with breast IMT that had developed 4 mo following mechanical trauma; its location was consistent with the site of the trauma. Mao et al[2] reported a 43-year-old female who developed IMT 18 mo following resection of a left breast fibroadenoma. Both studies of Vecchio et al[5] and Mao et al[2] speculated that trauma and surgery could be important factors that promote the development of IMT. Moreover, Vecchio et al[5] reported that IMT was essentially reactive due to an absence of ALK expression and benign clinical behavior without any evidence of metastasis. IMT and inflammatory pseudotumors are thus different variants of the same disease[5]. Based on the view that chronic inflammation is considered the cause, we humbly propose a new viewpoint that prosthesis implantation also causes IMT.
Considering the origin cells of IMTs, myofibroblasts are mainly involved in the growth, repair, and scarring of normal tissue. An abnormal inflammatory response induces the over proliferation of myofibroblasts, thereby forming IMTs. In this case, a large number of acute and chronic inflammatory cells, such as neutrophils, lymphocytes, and plasma cells, were observed under the light microscope. We hypothesized that this abnormal inflammatory response could have been attributed to the surgical trauma caused by the resection of the large malignant tumor and the prolonged stimulation of the prosthesis as a foreign body. Previous studies reported that prosthesis implantation was closely related to the incidence of breast fibromatosis[7]. Notably, breast fibromatosis and IMT originate from myofibroblasts. In addition, this case and those reported by Vecchio et al[5] and Mao et al[2] were negative for ALK, indirectly indicating that ALK gene fusion may not have occurred. This supports the speculation that these mammary lesions are reactive in nature; however, through genetic testing, some studies have observed that ALK gene fusion can occur in very few ALK-negative IMTs from the lung[8]. Whether a similar phenomenon can occur in ALK-negative breast IMT has not been reported. Unfortunately, genetic testing was not performed in this case as ALK was not expressed, with no evidence to prove that ALK gene changes had occurred. While it can be inferred from Table 1 that ALK-negative IMT has almost no recurrence, whether ALK-negative expression is a good prognostic factor for IMT remains unelucidated[9].
The malignant potential of IMT is incompletely characterized. Radical resection is the preferred method of treatment for breast IMTs. Kovach et al[10] confirmed that the recurrence rate of the primary surgical approach was 8%. Moreover, if there are no contraindications related to patient anatomy or morbidity, surgical resection of all lesions is recommended. In our review of 35 cases of breast IMTs, all tumors were initially treated with surgery, and the outcome of most breast IMTs is favorable. Recurrences occurred in four cases[11-14], including two cases of bilateral metastasis[11,12]. In one case, local recurrence occurred, and metastasis to the groin area was confirmed[14]. In addition to surgery, some scholars believe that ALK-targeted inhibitors, such as crizotinib, are used to treat patients with metastatic or unresectable ALK-positive IMT and provide surgical opportunities[15]. Sporadic cases show that treatment with corticosteroids improves the outcome[16]; however, these results are still under discussion, whereas adjunctive therapy after surgery needs further clinical investigation. Although most patients achieved satisfactory results, follow-up remains essential. Notably, no clear molecular cytogenetics or clinical characteristics following resection could predict the risk of recurrence or metastasis[17]. Therefore, ultrasonography has great value in the timely detection of breast IMTs, preoperative lesion range determination, postoperative monitoring, and follow-up due to its convenience and radiation-free nature.
Breast IMT is extremely rare; prosthesis implantation may cause IMT, although further investigation is necessary to prove it. Its clinical manifestations lack specificity, and imaging manifestations are diverse. Therefore, Sonographers should perform a comprehensive analysis of the medical history for the diagnosis, especially in patients with pathogenic factors, such as trauma or prosthesis implantation surgery, the possibility of IMT should be considered. Radical resection and postoperative close follow-up are recommended, although the pathogenesis and biological behavior of IMT remain unelucidated.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Abdominal Ultrasound Professional Committee of Chinese Society of Ultrasound Medical Engineering.
Specialty type: Radiology, Nuclear Medicine and Medical Imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liang YJ, Mohey NM S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Lv X, Ye J, Jiang G, Wang Y, Lv J. Simultaneous multiple primary cancers with concomitant inflammatory myofibroblastic tumor: a case report. Int J Clin Exp Pathol. 2020;13:1212-1215. [PubMed] |
| 2. | Mao X, Liu H, Du J, Yu N, Chen L, Zhang L. Imaging findings of inflammatory myofibroblastic tumor in breast: A case report. Medicine (Baltimore). 2018;97:e11804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Bosse K, Ott C, Biegner T, Fend F, Siegmann-Luz K, Wallwiener D, Hahn M. 23-Year-Old Female with an Inflammatory Myofibroblastic Tumour of the Breast: A Case Report and a Review of the Literature. Geburtshilfe Frauenheilkd. 2014;74:167-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Kovács A, Máthé G, Mattsson J, Stenman G, Kindblom LG. ALK-Positive Inflammatory Myofibroblastic Tumor of the Nipple During Pregnancy-An Unusual Presentation of a Rare Disease. Breast J. 2015;21:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Vecchio GM, Amico P, Grasso G, Vasquez E, La Greca G, Magro G. Post-traumatic inflammatory pseudotumor of the breast with atypical morphological features: A potential diagnostic pitfall. Report of a case and a critical review of the literature. Pathol Res Pract. 2011;207:322-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Akbulut M, Gunhan-Bilgen I, Zekioglu O, Duygulu G, Oktay A, Ozdemir N. Fine needle aspiration cytology of inflammatory myofibroblastic tumour (inflammatory pseudotumour) of the breast: a case report and review of the literature. Cytopathology. 2007;18:384-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Neuman HB, Brogi E, Ebrahim A, Brennan MF, Van Zee KJ. Desmoid tumors (fibromatoses) of the breast: a 25-year experience. Ann Surg Oncol. 2008;15:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Takeuchi K, Soda M, Togashi Y, Sugawara E, Hatano S, Asaka R, Okumura S, Nakagawa K, Mano H, Ishikawa Y. Pulmonary inflammatory myofibroblastic tumor expressing a novel fusion, PPFIBP1-ALK: reappraisal of anti-ALK immunohistochemistry as a tool for novel ALK fusion identification. Clin Cancer Res. 2011;17:3341-3348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Fernández-Aceñero MJ, Rejas M, Vázquez Á, Varela S, Jiménez-Ayala B. [Inflammatory myofibroblastic tumor of the breast: A rare entity]. Rev Esp Patol. 2018;51:193-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Kovach SJ, Fischer AC, Katzman PJ, Salloum RM, Ettinghausen SE, Madeb R, Koniaris LG. Inflammatory myofibroblastic tumors. J Surg Oncol. 2006;94:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 203] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 11. | Zardawi IM, Clark D, Williamsz G. Inflammatory myofibroblastic tumor of the breast. A case report. Acta Cytol. 2003;47:1077-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Yip CH, Wong KT, Samuel D. Bilateral plasma cell granuloma (inflammatory pseudotumour) of the breast. Aust N Z J Surg. 1997;67:300-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Khanafshar E, Phillipson J, Schammel DP, Minobe L, Cymerman J, Weidner N. Inflammatory myofibroblastic tumor of the breast. Ann Diagn Pathol. 2005;9:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Zhao HD, Wu T, Wang JQ, Zhang WD, He XL, Bao GQ, Li Y, Gong L, Wang Q. Primary inflammatory myofibroblastic tumor of the breast with rapid recurrence and metastasis: A case report. Oncol Lett. 2013;5:97-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Wu S, Xu R, Zhao H, Zhu X, Zhang L, Zhao X. Inflammatory myofibroblastic tumor of renal pelvis presenting with iterative hematuria and abdominal pain: A case report. Oncol Lett. 2015;10:3847-3849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Lee MH, Lee HB, Lee YC, Rhee YK, Lee EJ, Chung MJ, Jin GY, Kweon EY, Park SJ. Bilateral multiple inflammatory myofibroblastic tumors of the lung successfully treated with corticosteroids. Lung. 2011;189:433-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Zhou Y, Zhu J, Zhang Y, Jiang J, Jia M. An inflammatory myofibroblastic tumour of the breast with ALK overexpression. BMJ Case Rep. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Pettinato G, Manivel JC, Insabato L, De Chiara A, Petrella G. Plasma cell granuloma (inflammatory pseudotumor) of the breast. Am J Clin Pathol. 1988;90:627-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1030] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 20. | Chetty R, Govender D. Inflammatory pseudotumor of the breast. Pathology. 1997;29:270-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Gobbi H, Atkinson JB, Kardos TF, Simpson JF, Page DL. Inflammatory myofibroblastic tumour of the breast: report of a case with giant vacuolated cells. Breast. 1999;8:135-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Sastre-Garau X, Couturier J, Derré J, Aurias A, Klijanienko J, Lagacé R. Inflammatory myofibroblastic tumour (inflammatory pseudotumour) of the breast. Clinicopathological and genetic analysis of a case with evidence for clonality. J Pathol. 2002;196:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Haj M, Weiss M, Loberant N, Cohen I. Inflammatory pseudotumor of the breast: case report and literature review. Breast J. 2003;9:423-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Ilvan S, Celik V, Paksoy M, Cetinaslan I, Calay Z. Inflammatory myofibroblastic tumor (inflammatory pseudotumor) of the breast. APMIS. 2005;113:66-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Zen Y, Kasahara Y, Horita K, Miyayama S, Miura S, Kitagawa S, Nakanuma Y. Inflammatory pseudotumor of the breast in a patient with a high serum IgG4 Level: histologic similarity to sclerosing pancreatitis. Am J Surg Pathol. 2005;29:275-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | Kim SJ, Moon WK, Kim JH, Cho N, Chang CM. Inflammatory pseudotumor of the breast: a case report with imaging findings. Korean J Radiol. 2009;10:515-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Park SB, Kim HH, Shin HJ, Gong G. Inflammatory pseudotumor (myoblastic tumor) of the breast: a case report and review of the literature. J Clin Ultrasound. 2010;38:52-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Hill PA. Inflammatory pseudotumor of the breast: a mimic of breast carcinoma. Breast J. 2010;16:549-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Li J, Yun W, Qin J, Zhao J, Liu X, Wu J, Ji M, Tang J. Inflammatory myofibroblastic tumor of the breast coexisting with breast cancer: a case report. Breast Care (Basel). 2013;8:290-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Markopoulos C, Charalampoudis P, Karagiannis E, Antonopoulou Z, Mantas D. Inflammatory myofibroblastic tumor of the breast. Case Rep Surg. 2015;2015:705127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Choi EJ, Jin GY, Chung MJ, Moon WS, Youn HJ. Primary Inflammatory Myofibroblastic Tumors of the Breast with Metastasis: Radiographic and Histopathologic Predictive Factors. J Breast Cancer. 2015;18:200-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Greenleaf EK, Williams NC, Leung AM. Inflammatory Pseudotumor of the Breast. Am Surg. 2016;82:e106-e107. [PubMed] |
| 33. | Goto W, Kashiwagi S, Takada K, Asano Y, Morisaki T, Takashima T, Noda S, Onoda N, Ohsawa M, Hirakawa K, Ohira M. [A Case of Inflammatory Pseudotumor of the Mammary Gland]. Gan To Kagaku Ryoho. 2016;43:2029-2031. [PubMed] |
| 34. | Talu CK, Çakır Y, Hacıhasanoğlu E, Leblebici C, Aksoy Ş, Nazlı MA. Inflammatory Myofibroblastic Tumor of the Breast Coexisting with Pseudoangiomatous Stromal Hyperplasia. J Breast Health. 2016;12:171-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Inoue M, Ohta T, Shioya H, Sato S, Takahashi H, Nakata N, Taniguchi C, Hirano M, Nishioka M, Yamakawa H. Inflammatory myofibroblastic tumors of the breast with simultaneous intracranial, lung, and pancreas involvement: ultrasonographic findings and a review of the literature. J Med Ultrason (2001). 2018;45:331-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Dani M, Pinder S, Fentiman I. Bilateral Inflammatory Pseudotumour of the Breast: A Case Report and Review of the Literature. Eur J Breast Health. 2018;14:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |