Published online Feb 6, 2022. doi: 10.12998/wjcc.v10.i4.1417
Peer-review started: September 3, 2021
First decision: November 11, 2021
Revised: November 22, 2021
Accepted: December 25, 2021
Article in press: December 25, 2021
Published online: February 6, 2022
Processing time: 142 Days and 19.6 Hours
Ornithine transcarbamylase deficiency (OTCD) is a common ornithine cycle disorder, and OTC gene variation is the main pathogenic factor of this disease. This study explored and validated a variant in the OTC gene.
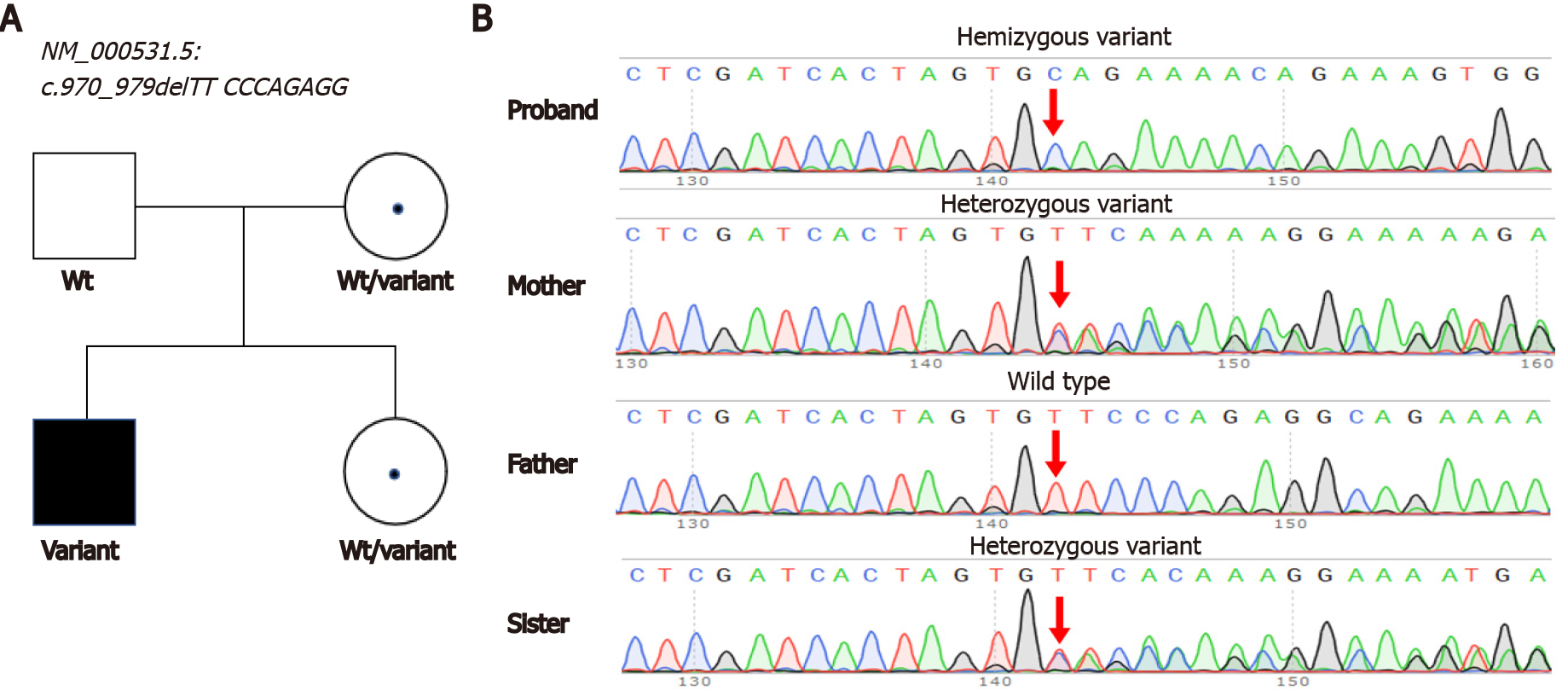
The neonate exhibited high blood ammonia, lactic acid, and homocysteine levels on the fifth day after birth. A novel deletion variant in the OTC gene [NM_000531.5, c.970_979delTTCCCAGAGG, p.Phe324GlnfsTer16] was uncovered by exome sequencing. The variant caused a protein-coding frameshift and resulted in early translation termination at the 16th amino acid after the variant site.
Our results provide a novel pathogenic variant in OTC and related clinical features for further OTCD screening and clinical consultation.
Core Tip: In this study, we introduce one boy with ornithine transcarbamylase deficiency caused by an unreported hemizygous variant of OTC gene. Our study delivered the importance of OTC gene testing in metabolism disease. We believe that our study will inspire more doctors to apply genetics testing when facing complex clinical features for neonatal cases.
- Citation: Wang LP, Luo HZ, Song M, Yang ZZ, Yang F, Cao YT, Chen J. Hemizygous deletion in the OTC gene results in ornithine transcarbamylase deficiency: A case report. World J Clin Cases 2022; 10(4): 1417-1422
- URL: https://www.wjgnet.com/2307-8960/full/v10/i4/1417.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i4.1417
Ornithine transcarbamylase deficiency (OTCD, OMIM: 311250), also known as hyperammonemia type II, is an X-linked genetic disorder of the ornithine cycle (urea cycle)[1]. The incidence of OTCD is approximately 1/80000–1/56500. OTCD is the most common type of ornithine circulation disorder and accounts for 50%-66% of total ornithine circulation disorders. Both neonates and adults can be affected by complex clinical symptoms of this disorder, with varying degrees of severity. Due to this lack of specificity, OTCD is often misdiagnosed[1-3]. OTCD has a high mortality rate in neonates, and survivors often have varying degrees of neurological sequelae. Early diagnosis, individualized diet, medication, and liver transplantation are the main strategies for reducing the mortality and disability rates of patients with OTCD.
The OTC gene (OMIM: 300461) is located on chromosome Xp11.4, contains 10 exons and 9 introns, and encodes a 354 amino acid protein. The OTC gene is highly expressed in the liver[4]. Pathogenic variants in the OTC gene lead to a reduction or absence of OTC enzyme activity and the shutdown of citrulline synthesis and the ornithine cycle, resulting in an ammonia metabolism disorder and an increase in levels of ammonia in the blood[5]. Excessive accumulation of ammonia is highly toxic to the central nervous system, interferes with the energy metabolism of brain cells, and causes cytotoxic cerebral edema and acute or chronic traumatic encephalopathy as well as neuropsychiatric damage[5].
Based on the time of onset, patients with OTCD are divided into neonatal onset and late onset (age of onset > 28 d) groups[6]. Enzyme activity between the two groups is notably different. In the neonatal onset group, enzyme activity is completely reduced, and in the late onset group, enzyme activity is partially reduced. Most patients with neonatal-onset OTCD are males with hemizygous variants[7]. They demonstrate no symptoms at birth but gradually refuse to feed and begin to exhibit symptoms of vomiting, irritability, hyperventilation, and lethargy within a few hours to days after birth. Onset is sudden with rapid and complex clinical features, such as convulsions, coma, hypothermia, and respiratory failure[8]. Due to the lack of specificity in clinical features, patients are often misdiagnosed with neonatal sepsis, neonatal hypoxic-ischemic encephalopathy, birth injury, food poisoning, acute gastroenteritis, encephalitis, epilepsy, encephalopathy combined with visceral steatosis (Wright's syndrome), neurodegenerative disease, or schizophrenia. Elevated ammonia in the blood is a main abnormal indicator in patients with OTCD, and OTC gene variants are another crucial factor in the diagnosis of OTCD[9]. To date, over 530 variants in the OTC gene have been reported, but no hotspot mutations have been found. Therefore, the collection of as many pathogenic variants as possible is important for clinical diagnosis and screening.
This study involved a male neonate with a pathogenic variant in OTC. We comprehensively investigated the clinical features and enzymatic activity through genetic testing.
A five-day-old boy who did not feed and showed no movement or responsiveness was referred to our department for further treatment.
The proband was delivered via cesarean section due to "fetal distress" at 40 wk. There was no meconium-stained amniotic fluid, no abnormalities in the umbilical cord, and no premature rupture of membranes. His birth weight was 2350 g, and his Apgar score was normal. He was diagnosed as a "low birth weight infant with gastrointestinal bleeding” and showed improvement after unknown treatment. The amount of ordinary formula milk fed was increased gradually until he consumed 30 mL of milk at each feeding. Five days later, he stopped feeding, showed no movement, and exhibited poor responsiveness, which was accompanied by an abnormal increase in muscle tone, shortness of breath, moaning, foaming at the mouth, screaming, pumping, vomiting, abdominal distention, and blood in the stool.
Physical examination revealed a low body temperature (35 °C), low blood pressure (35/15 mmHg), bradycardia (97/min), and lack of spontaneous breathing.
The final blood glucose level of the patient was 2.6 mmol/L. He had high blood ammonia [461.0 μmol/L (ref: 18–72)], high lactic acid [10.80 mmo1/L (ref: 1.06–2.09)], and high homocysteine [29.21 μmol/L (ref: < 15)] levels (Table 1).
| Item | Result | Ref. |
| Body temperature | 35 ℃ | 36–37 ℃ |
| Blood pressure | 35/15 mmHg | 66–75/45 mmHg |
| Heart rate | 97/min | 120–140/min |
| Blood ammonia | 461.0 μmol/L | 18–72 μmol/L |
| Lactic acid | 10.80 mmo1/L | 1.06–2.09 mmo1/L |
| Homocysteine | 29.21 μmol/L | < 15 μmol/L |
A hemizygous variant in the OTC [NM_000531.5, c.970_979delTTCCCAGAGG, p.Phe324GlnfsTer16] gene was identified by exome sequencing. The variant caused a 10-bp deletion and early translation termination in the OTC gene. Sanger sequencing confirmed that this variant was inherited from his mother (Figure 1). The variant was absent in public databases (gnomAD, Exome Aggregation Consortium, or 1000 Genomes). The variant was classified as likely pathogenic according to the ACMG guidelines (Table 2). Pathogenic variants in other genes associated with hyperammonemia have not yet been identified. We reported this variant in the ClinVar database (accession number: VCV001256051).
| Gene | Variant | Inheritance | MAF | SIFT | Polyphen2 | Mutation taster | Evidence | ACMG category | ||
| ExAc | gnomAD | 1000 genomes | ||||||||
| OTC | c.970_979del | Hemi | NE | NE | NE | - | - | - | PS2 + PM2_supporting + PP3 | Likely pathogenic |
| TTCCCAGAGG | ||||||||||
The male infant patient was diagnosed with OTCD caused by an OTC mutation.
After admission, we ensured that the patient's airway was unblocked, warmed the body, monitored vital signs, assisted breathing with the use of a ventilator, and corrected the blood pH with the administration of sodium bicarbonate. Meropenem and penicillin were utilized to combat infection, phenobarbital for spasms, dopamine for circulation, and 10% glucose to maintain the stability of the internal environment.
Arginine was used to reduce blood ammonia, and levocarnitine was used to promote metabolism. Lidocaine was used for nonparoxysmal ventricular arrhythmia, which was indicated by an electrocardiogram.
The patient remained in a coma since admission with weak heart sounds. After active rescue and treatment, the patient’s condition remained critical, with no remission or spontaneous breathing. His blood pressure, oxygen saturation, and heart rate were unstable; there was no response to stimulation. The patient was still in a coma when discharged and died soon after.
OTCD diagnosis is mainly based on clinical symptoms, blood ammonia levels, and other general biochemical tests, such as blood amino acids, urine organic acids, and genetic tests. For suspected cases, such as those in which patients present intermittent or progressive encephalopathy and high blood ammonia levels with unknown causes, blood amino acid analysis and urine organic acid analysis should be performed as early as possible. If blood citrate is reduced or normal and urine whey acid or uracil is increased[9], OTCD diagnosis can be confirmed by combining these results with genetic testing. For neonates whose blood amino acid screening by tandem mass spectrometry indicates reduced citrulline levels, dynamic observation should be carried out, and urine organic acid and genetic tests should be performed.
The clinical symptoms and related examinations of OTCD patients lack specificity and should be differentiated from those of hyperammonemia caused by other factors, including different ornithine circulatory disorders; miscellaneous genetic metabolic diseases, including organic acid hematic disease, fatty acid oxidation disorder, beta oxygen defects, high insulin, and hepatic encephalopathy; severe liver damage; exogenous toxicity (e.g., carbamidine); and drugs (e.g., valproic acid). All these factors can elevate blood ammonia levels and should be identified according to the patient’s medical history and clinical symptoms[9]. Genetic testing of the OTC gene is another crucial factor in the diagnosis of this disease.
Liver transplantation is regarded as an effective treatment for OTCD. For patients with neonatal onset, liver transplantation should be performed at the earliest identification of disease. Surgery is recommended between three months (and/or body weight > 5 kg) and one year[9]. Once OTCD is suspected, clinical examinations for blood ammonia, blood amino acids, and urine organic acids should be performed rapidly in a specialized metabolic laboratory. Our case shows high blood ammonia [461.0 μmol/L (ref: 18-72)], high lactic acid [10.80 mmo1/L (ref: 1.06-2.09)], and high homocysteine [29.21 μmol/L (ref: < 15)] levels. After interpreting the sequencing results, we confirmed the diagnosis of OTCD in this patient.
Genetic testing is a crucial method for the diagnosis of OTCD. As a routine practice of next-generation sequencing (NGS), high-throughput sequencing will rapidly uncover many pathogenic variants in neonates who are suspected to have OTCD with abnormal hyperammonemia. Array CGH or multiplex ligation-dependent probe amplification fails to detect OTC gene deficiency in SNVs or microinsertions and deletions in patients[10,11], whereas NGS has the advantage of detecting these variations. Our study uncovered an unreported variant in the OTC gene [NM_ 000531.5, c.970_979delTTCCCAGAGG, p.Phe324GlnfsTer16], which caused the early termination of OTC. Our results provide a reference for the accurate diagnosis of patients with the same variant. A previous study reported that approximately 15% of female carriers become symptomatic[12]. Our findings also suggest heterozygote detection of at-risk female relatives as a promising direction for further investigation.
In our case study of one individual, a rare variant in the OTC gene was identified and confirmed by Sanger sequencing. This finding broadens the OTC variant spectrum and provides evidence for further OTCD screening and clinical consultation.
We thank the patient's family members for their participation in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bernstein HG, Naz S S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Lichter-Konecki U, Caldovic L, Morizono H, Simpson K. Ornithine Transcarbamylase Deficiency. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mirzaa G, Amemiya A, editors. GeneReviews((R)). Seattle (WA), 1993. |
| 2. | Wilcken B. Problems in the management of urea cycle disorders. Mol Genet Metab. 2004;81 Suppl 1:S86-S91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Summar ML, Koelker S, Freedenberg D, Le Mons C, Haberle J, Lee HS, Kirmse B; European Registry and Network for Intoxication Type Metabolic Diseases (E-IMD). Electronic address: http://www.e-imd.org/en/index.phtml; Members of the Urea Cycle Disorders Consortium (UCDC). Electronic address: http://rarediseasesnetwork.epi.usf.edu/ucdc/. The incidence of urea cycle disorders. Mol Genet Metab. 2013;110:179-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 225] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 4. | Caldovic L, Abdikarim I, Narain S, Tuchman M, Morizono H. Genotype-Phenotype Correlations in Ornithine Transcarbamylase Deficiency: A Mutation Update. J Genet Genomics. 2015;42:181-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 5. | Braissant O. Current concepts in the pathogenesis of urea cycle disorders. Mol Genet Metab. 2010;100 Suppl 1:S3-S12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Posset R, Garbade SF, Boy N, Burlina AB, Dionisi-Vici C, Dobbelaere D, Garcia-Cazorla A, de Lonlay P, Teles EL, Vara R, Mew NA, Batshaw ML, Baumgartner MR, McCandless SE, Seminara J, Summar M, Hoffmann GF, Kölker S, Burgard P; Additional individual contributors of the UCDC and the E-IMD consortium. Transatlantic combined and comparative data analysis of 1095 patients with urea cycle disorders-A successful strategy for clinical research of rare diseases. J Inherit Metab Dis. 2019;42:93-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Burgard P, Kölker S, Haege G, Lindner M, Hoffmann GF. Neonatal mortality and outcome at the end of the first year of life in early onset urea cycle disorders--review and meta-analysis of observational studies published over more than 35 years. J Inherit Metab Dis. 2016;39:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Gordon N. Ornithine transcarbamylase deficiency: a urea cycle defect. Eur J Paediatr Neurol. 2003;7:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Häberle J, Burlina A, Chakrapani A, Dixon M, Karall D, Lindner M, Mandel H, Martinelli D, Pintos-Morell G, Santer R, Skouma A, Servais A, Tal G, Rubio V, Huemer M, Dionisi-Vici C. Suggested guidelines for the diagnosis and management of urea cycle disorders: First revision. J Inherit Metab Dis. 2019;42:1192-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 305] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 10. | Yokoi K, Nakajima Y, Inagaki H, Tsutsumi M, Ito T, Kurahashi H. Exonic duplication of the OTC gene by a complex rearrangement that likely occurred via a replication-based mechanism: a case report. BMC Med Genet. 2018;19:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Shchelochkov OA, Li FY, Geraghty MT, Gallagher RC, Van Hove JL, Lichter-Konecki U, Fernhoff PM, Copeland S, Reimschisel T, Cederbaum S, Lee B, Chinault AC, Wong LJ. High-frequency detection of deletions and variable rearrangements at the ornithine transcarbamylase (OTC) locus by oligonucleotide array CGH. Mol Genet Metab. 2009;96:97-105. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | McCullough BA, Yudkoff M, Batshaw ML, Wilson JM, Raper SE, Tuchman M. Genotype spectrum of ornithine transcarbamylase deficiency: correlation with the clinical and biochemical phenotype. Am J Med Genet. 2000;93:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |