Published online Feb 6, 2022. doi: 10.12998/wjcc.v10.i4.1341
Peer-review started: August 5, 2021
First decision: November 6, 2021
Revised: November 11, 2021
Accepted: December 25, 2021
Article in press: December 25, 2021
Published online: February 6, 2022
Processing time: 171 Days and 17.7 Hours
Traditional chemotherapy has benefited many patients with non-Hodgkin's lymphoma, but results in a very poor response in patients with rare lymphomas or refractory lymphomas. Previous studies have shown that chidamide has potential anti-lymphoma activity and reverses lymphoma cell chemoresistance to increase the chemosensitivity of lymphoma cells to traditional chemotherapy.
A 14-year-old boy was admitted to our hospital with a 5-d history of generalized erythema, papules, and blisters. Initially, the disease was refractory to potent anti-allergic and anti-infective treatment, and his condition progressively worsened. Skin biopsy revealed primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma. Considering that the disease is extremely rare in clinical practice, existing case reports have shown poor efficacy with traditional chemotherapy alone. We recommend chidamide combined with traditional chemotherapy for treatment. The regimen was as follows: Chidamide 30 mg/biw, cyclophosphamide 1100 mg/d1, pirarubicin 70 mg/d1, vincristine 2 mg/d1, dexamethasone 20 mg/d1-5, etoposide 100 mg/d1-5, in a 21 d cycle. The trea
This case suggests that the combination of chidamide and traditional chemo
Core Tip: The long-term efficacy of traditional chemotherapy in the treatment of primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma is poor, and the main mechanism is the emergence of chemoresistance in lymphoma cells. Chidamide induces apoptosis and growth arrest of lymphoid and hematologic tumor cells and enhances the sensitivity of lymphoma cells to traditional chemo
- Citation: He ZD, Yang HY, Zhou SS, Wang M, Mo QL, Huang FX, Peng ZG. Chidamide combined with traditional chemotherapy for primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma: A case report. World J Clin Cases 2022; 10(4): 1341-1348
- URL: https://www.wjgnet.com/2307-8960/full/v10/i4/1341.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i4.1341
Primary cutaneous aggressive epidermotropic CD8+ cytotoxic cell T-cell lymphoma is a rare subtype of cutaneous T-cell lymphoma, accounting for less than 1% of all cutaneous T-cell lymphomas. Only dozens of cases have been reported worldwide, and there is no optimal treatment. According to existing reports, the majority of patients with primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma currently receive doxorubicin-based traditional chemotherapy. There are case reports of treatment with CHOP, CHOPE, and Hy-CVAD regimens, but the efficacy is poor. All result in short-term benefits, with an overall survival of 12-32 mo[1]. The main reason for the poor long-term efficacy of primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma to traditional chemotherapy is that lymphoma cells are prone to chemoresistance[2]. Chidamide has potential anti-hematological tumor activity and enhances the chemosensitivity of lymphoma cells. It has been recognized for the treatment of relapsed or refractory peripheral T-cell lymphoma. Based on the above theory, we boldly tried the combination of chidamide with traditional chemotherapy (CHOPE) in a patient with primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma with promising results.
A 14-year-old boy was admitted to the Department of Dermatology with a 5-d history of generalized erythema, papules, and blisters and was transferred to the Department of Medical Oncology.
The patient had generalized erythema, papules, and blisters with itching since August 18, 2017. Three days after the onset of symptoms, some blisters formed blood blisters with tan crusts on the surface, accompanied by pain, and fever, fear of cold, and chills, with a maximum body temperature of 41 °C.
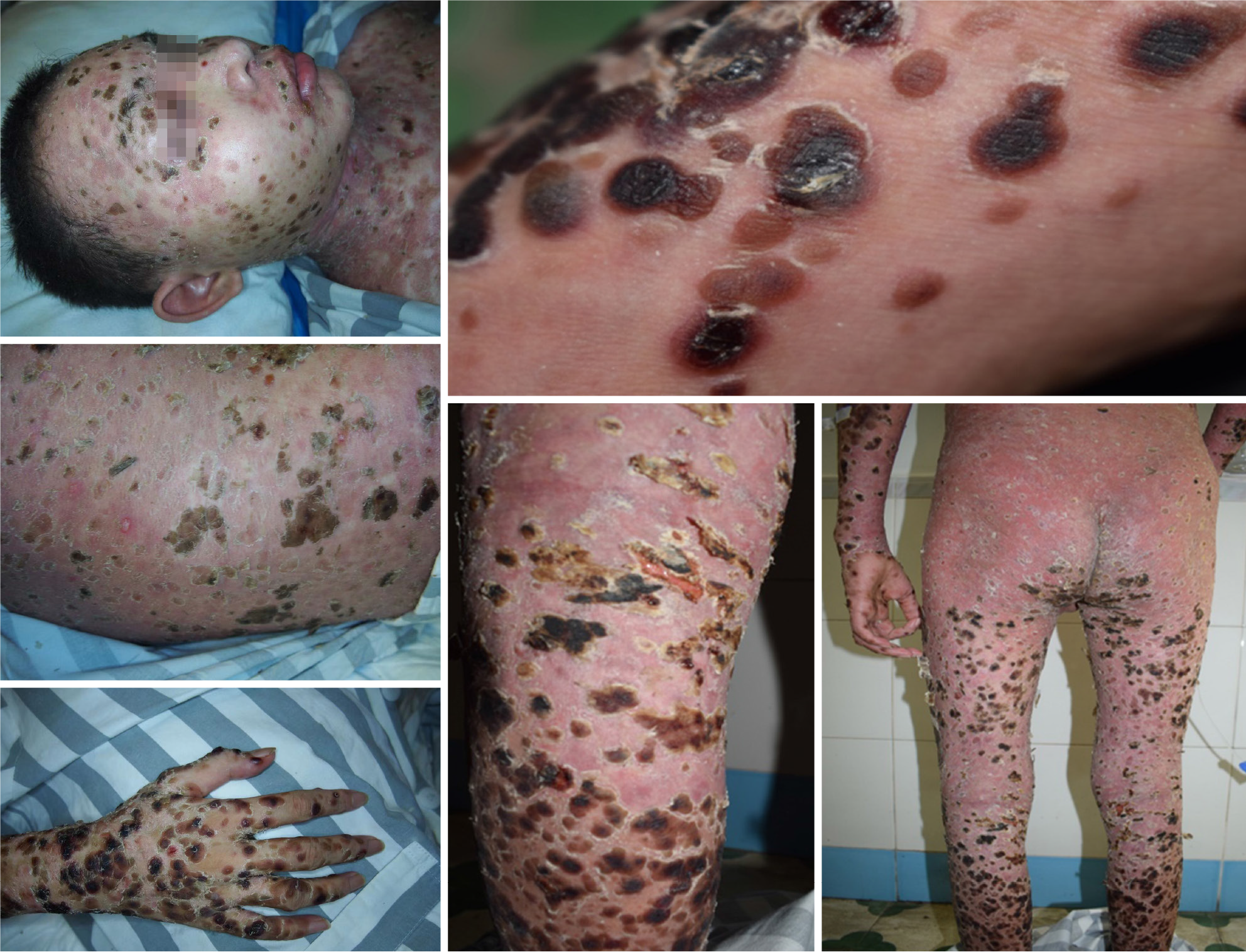
The patient's body temperature was 37.8 °C, heart rate was 110 bpm, respiratory rate was 20 breaths/min, and blood pressure was 99/67 mmHg. Scattered blood blisters were observed throughout the body, with the most severe blood blisters on the trunk, consistent with the distribution of skin transverse striae (Figure 1). Erosive surfaces of varying sizes were seen in the oral cavity, trunk, extremities, and perineum. Physical examination of the heart, lungs, and abdomen was unremarkable.
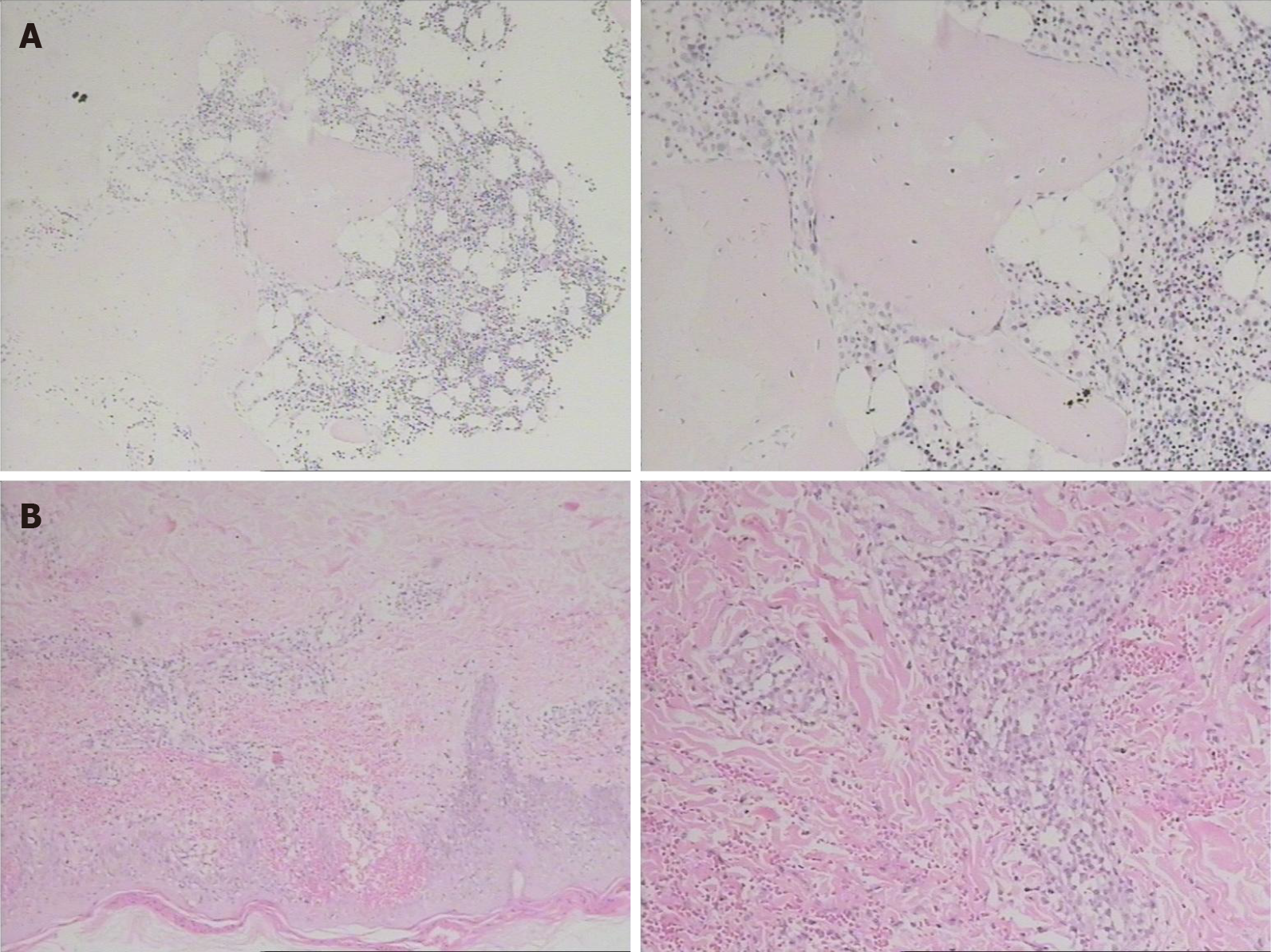
After admission, a blood test was performed and the patient’s white blood cell count was 5.86 × 109/L, hemoglobin concentration was 146.70 g/L, and platelets were 129.80 × 109/L. His biochemical results were as follows: Total protein, 61.2 g/L; albumin, 29.5 g/L; creatinine, 54 μmol/L; lactate dehydrogenase, 351 U/L; β-2 microglobulin, 3.6 mg/L; interleukin-6, 13.34 pg/mL. The results of bone marrow biopsy showed slight microscopic bone marrow hyperplasia, cell volume accounted for 40%, tertiary hematopoietic cells were present, granulocyte/erythrocyte ratio was slightly increased, and cell morphology was normal (Figure 2A). The skin of the left thigh was biopsied, and the pathology results indicated primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma (Figure 2B). The results of immunohistochemistry revealed the following: CD3 (+), CD2 (-/+), CD4 (-/+), CD5 (-/+), CD7 (+), CD8 (+ +), CD56 (-), TiA-1 (+), GB (+), AlK (-), CD30 (-), CD20 (-), and CD10 (-); EBERs (-); TCR-r gene rearrangement (-).
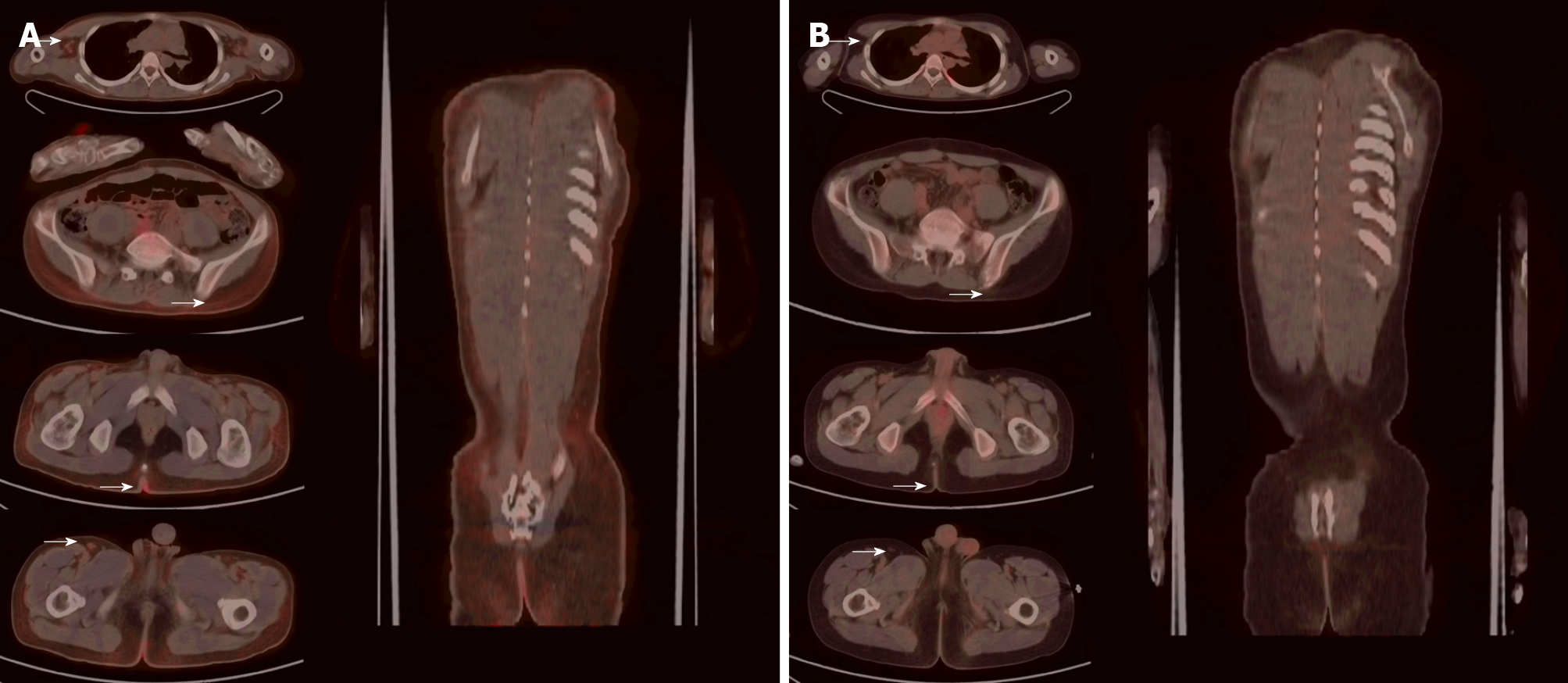
Positron emission tomography/computed tomography (PET/CT) showed mildly increased systemic cutaneous glucose metabolism, which was considered to be cutaneous lymphoma. Mild systemic skin swelling and a diffuse mild increase in glucose metabolism, especially in the local skin of both armpits, right upper quadrant, and posterior coccyx were observed (Figure 3). Multiple small lymph nodes of different sizes with increased glucose metabolism were observed in both armpits and groins.
Based on pathological biopsy, laboratory examinations, imaging examination and clinical manifestations, the patient was finally diagnosed with primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma.
The patient was diagnosed more than 20 d after the onset of symptoms, and chidamide combined with CHOPE regimen was administered as the patient's first cycle of treatment as follows: Chidamide 30 mg/biw, cyclophosphamide 1100 mg/d1, doxorubicin 70 mg/d1, vincristine 2 mg/d1, dexamethasone 20 mg/d1-5, etoposide 100 mg/d1-5, in a 21 d cycle. The patient completed a total of 6 cycles of treatment and subsequently entered maintenance therapy.
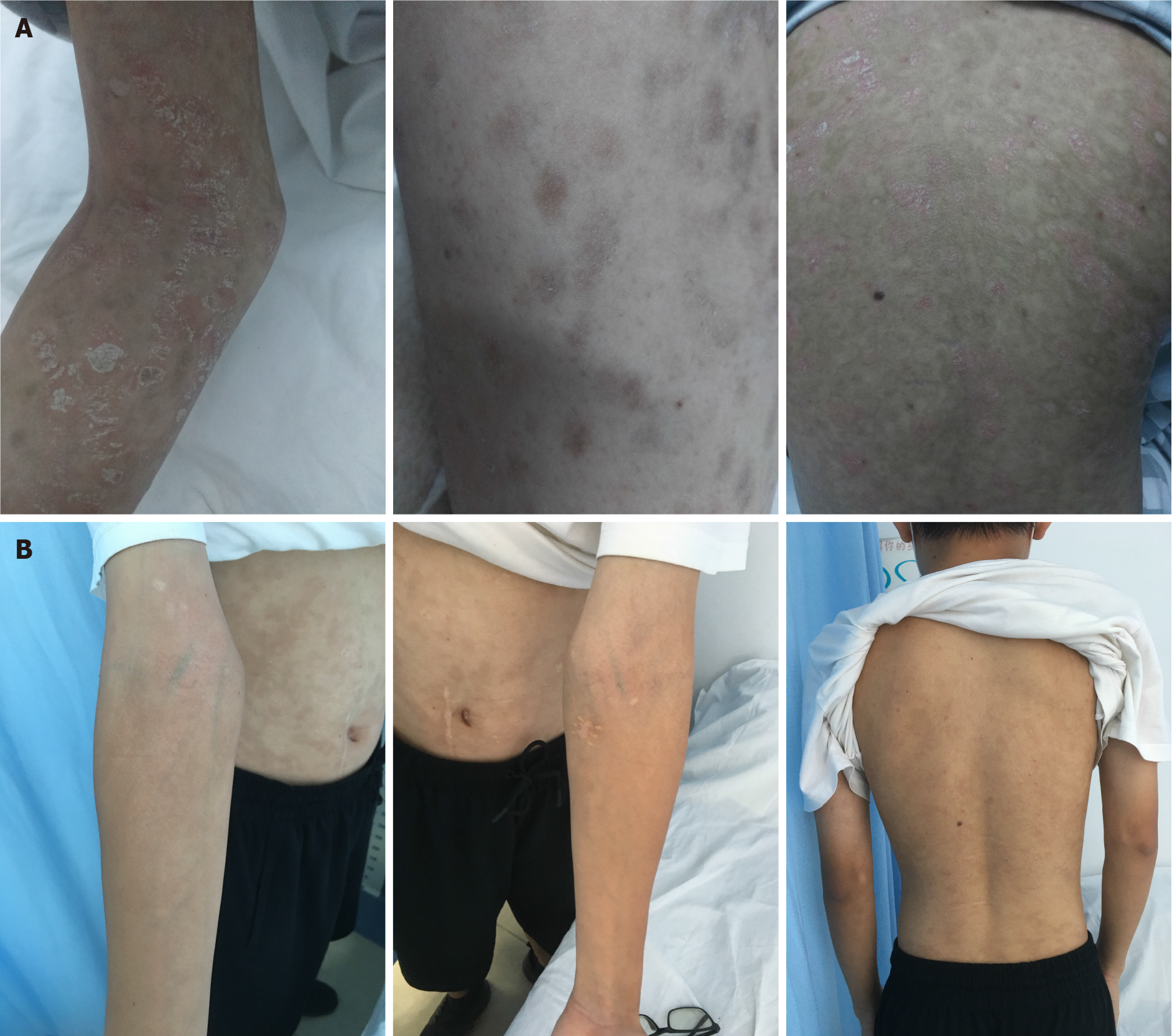
After 4 cycles of treatment, the patient returned to the hospital for follow-up, and his skin rash on the face, neck, trunk, and extremities had disappeared (Figure 4A). The blood tests showed that the white blood cell count was 6.14 × 109/L, hemoglobin concentration was 123 g/L, and platelets were 225 × 109/L. His biochemical results were as follows: Total protein, 68.2 g/L; albumin, 36 g/L; creatinine, 60 μmol/L; lactate dehydrogenase, 225 U/L; β-2 microglobulin 1.96 mg/L; interleukin-6, 5.3 pg/mL. PET/CT showed that after chemotherapy for cutaneous lymphoma, the original lesions were inactivated and no new lesions were observed (Deauville score: 1 point). No clear structural or glucose metabolism abnormalities were noted (Figure 3B). Complete remission was observed. The patient had grade III bone marrow suppression during the 5th cycle of chemotherapy, and continued to complete chemotherapy as planned after repeated blood tests were normal after leukocyte-elevating therapy. After completing 6 cycles of treatment, he regularly took chidamide 20 mg/biw as maintenance therapy for 1 year. No significant hematological toxicity or gastrointestinal adverse events occurred during maintenance therapy with chidamide. The last follow-up was performed 2 wk ago and the patient's condition was stable without recurrence (Figure 4B).
Primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma was first reported as a rare histopathological subtype of cutaneous T-cell lymphoma in 1999 by Berti et al[3]. It was first classified as a non-specific provisional entity by the World Health Organization/European Organization for Research and Treatment of Cancer in 2005[4]. This type of lymphoma is characterized by the presence of localized or diffuse papules, nodules, or tumors, which present as central ulceration or necrosis, or plaques with surface hyperkeratosis[5]. Primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma is highly aggressive and can rapidly spread to various organs, such as the lungs, testes, central nervous system, or oral mucosa, with a poor response to traditional chemotherapy[6]. Some patients can benefit from doxorubicin-based multiagent chemotherapy for a short time, but most patients relapse a short time after treatment and even develop resistance to traditional chemotherapy and then it transforms into relapsed or refractory lymphoma. Treatment is still a great clinical challenge.
Primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma is clinically heterogeneous, and its course progresses rapidly. Treatment options for advanced disease are limited, and significantly effective treatment options deserve active exploration. Numerous studies have shown that histone deacetylase inhibitors (HDAC inhibitors) are used to treat malignant tumors, such as cutaneous T-cell lymphoma and peripheral T-cell lymphoma. Moreover, HDAC inhibitors are now increasingly being used in combination with other types of anticancer drugs for the treatment of various malignancies. Chidamide is an innovative drug independently developed in China, which can exert anti-tumor activity by reversing tumor cell resistance, increasing tumor cell chemosensitivity, potently regulating immunity and potential direct anti-tumor effects[7,8]. In December 2014, chidamide was approved by China Food and Drug Administration for the treatment of relapsed or refractory peripheral T-cell lymphoma. Previous studies have shown that chidamide can induce apoptosis and growth arrest of lymphoid or hematologic tumor cells, and can also reverse tumor cytochemical resistance to increase tumor cell chemosensitivity. Wei Guan et al[9] retrospectively analyzed 17 cases of refractory or relapsed T-lymphocytic lymphoma/leukemia (T-LBL/ALL) and found that chidamide has pleiotropic regulatory immune function and can enhance the sensitivity of tumor cells to chemotherapeutic drugs. Jiang et al[10] found that chidamide inhibited the proliferation and induced apoptosis of tumor cells and exerted potential anti-leukemia activity. Chidamide can also increase the chemosensitivity of tumor cells by disrupting the Smo/gli-1 pathway and the downstream signaling target p-AKT. Yan et al[8] found that the combination of chidamide and syndilimab enhanced the efficacy of syndilimab in NK/T cell lymphoma. Based on the above theory, we speculate that a treatment regimen combining chidamide may be a promising therapeutic strategy for refractory T-cell lymphoma. Common adverse events of chidamide include thrombocytopenia, leukopenia, neutropenia, QTc prolongation, fatigue, fever, and gastro
The present patient with primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma achieved complete remission following treatment with chidamide combined with CHOPE regimen. On the one hand, this may have been due to the potential antitumor activity of chidamide, and on the other hand, it may have been because chidamide reverses tumor cell chemoresistance to increases tumor cell chemosensitivity. In this case, the patient experienced short-term hematological toxicity during chemotherapy with chidamide combined with CHOPE regimen, but recovered quickly after leukocyte-elevating therapy. The patient successfully completed 6 cycles of therapy. In addition, no significant adverse events occurred in this patient during maintenance therapy. This regimen was well tolerated and provides a promising therapeutic strategy for the treatment of primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma.
In conclusion, we found that a combination of chidamide and a traditional chemotherapy regimen is a safe and effective treatment for primary cutaneous aggressive epidermotropic CD8+ cytotoxic cell T-cell lymphoma, but its long-term efficacy requires further evaluation. For patients with primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma refractory to traditional therapy (e.g., CHOP), we recommend early combination therapy with chidamide. This study also had limitations: Due to the rarity of primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma, analysis of the results obtained in this report requires caution. Whether the good efficacy in this patient was the result of synergy between traditional chemotherapy and chidamide or a separate effect of chidamide cannot be determined. In the future, we will continue to collect data to assess the efficacy and safety of chidamide in combination with CHOPE in patients with primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma.
We sincerely thank the chief editor and all review experts for reviewing this paper.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ferreira GSA S-Editor: Liu JH L-Editor: Webster JR P-Editor: Liu JH
| 1. | Guitart J, Martinez-Escala ME, Subtil A, Duvic M, Pulitzer MP, Olsen EA, Kim E, Rook AH, Samimi SS, Wood GS, Girardi M, Junkins-Hopkins J, Ivan DS, Selim MA, Sable KA, Virmani P, Pincus LB, Tetzlaff MT, Kim J, Kim YH. Primary cutaneous aggressive epidermotropic cytotoxic T-cell lymphomas: reappraisal of a provisional entity in the 2016 WHO classification of cutaneous lymphomas. Mod Pathol. 2017;30:761-772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 2. | Nofal A, Abdel-Mawla MY, Assaf M, Salah E. Primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma: proposed diagnostic criteria and therapeutic evaluation. J Am Acad Dermatol. 2012;67:748-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Berti E, Tomasini D, Vermeer MH, Meijer CJ, Alessi E, Willemze R. Primary cutaneous CD8-positive epidermotropic cytotoxic T cell lymphomas. A distinct clinicopathological entity with an aggressive clinical behavior. Am J Pathol. 1999;155:483-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 282] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 4. | Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, Ralfkiaer E, Chimenti S, Diaz-Perez JL, Duncan LM, Grange F, Harris NL, Kempf W, Kerl H, Kurrer M, Knobler R, Pimpinelli N, Sander C, Santucci M, Sterry W, Vermeer MH, Wechsler J, Whittaker S, Meijer CJ. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2714] [Cited by in RCA: 2576] [Article Influence: 128.8] [Reference Citation Analysis (2)] |
| 5. | Sundram U. Cutaneous Lymphoproliferative Disorders: What's New in the Revised 4th Edition of the World Health Organization (WHO) Classification of Lymphoid Neoplasms. Adv Anat Pathol. 2019;26:93-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Slater DN. The new World Health Organization-European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas: a practical marriage of two giants. Br J Dermatol. 2005;153:874-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Shi Y, Dong M, Hong X, Zhang W, Feng J, Zhu J, Yu L, Ke X, Huang H, Shen Z, Fan Y, Li W, Zhao X, Qi J, Zhou D, Ning Z, Lu X. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann Oncol. 2015;26:1766-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 285] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 8. | Yan Z, Yao S, Liu Y, Zhang J, Li P, Wang H, Chu J, Zhao S, Yao Z. Durable Response to Sintilimab and Chidamide in a Patient With Pegaspargase- and Immunotherapy-Resistant NK/T-Cell Lymphoma: Case Report and Literature Review. Front Oncol. 2020;10:608304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Guan W, Jing Y, Dou L, Wang M, Xiao Y, Yu L. Chidamide in combination with chemotherapy in refractory and relapsed T lymphoblastic lymphoma/leukemia. Leuk Lymphoma. 2020;61:855-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Jiang X, Jiang L, Cheng J, Chen F, Ni J, Yin C, Wang Q, Wang Z, Fang D, Yi Z, Yu G, Zhong Q, Carter BZ, Meng F. Inhibition of EZH2 by chidamide exerts antileukemia activity and increases chemosensitivity through Smo/Gli-1 pathway in acute myeloid leukemia. J Transl Med. 2021;19:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Moskowitz AJ, Horwitz SM. Targeting histone deacetylases in T-cell lymphoma. Leuk Lymphoma. 2017;58:1306-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |