Published online Feb 6, 2022. doi: 10.12998/wjcc.v10.i4.1311
Peer-review started: August 10, 2021
First decision: September 29, 2021
Revised: October 5, 2021
Accepted: December 22, 2021
Article in press: December 22, 2021
Published online: February 6, 2022
Processing time: 166 Days and 17.4 Hours
Persistent vegetative state (PVS) is a devastating and long-lasting clinical con
We report the case of an 11-year-old boy with PVS caused by severe intracerebral bleeding in the left hemisphere following anticoagulation treatment. The patient’s PVS severity showed no notable improvement after 2-mo neuroprotective treatment and rehabilitation, including nerve growth factor and baclofen, hyperbaric oxygen, and comprehensive bedside rehabilitation therapies. Daily inhalation treatment (4-6 h) of high-concentration hydrogen (H2) gas (66.6% H2 + 33.3% O2) was provided. Surprisingly, the patient’s orientation, consciousness, ability to speak, facial expressions, and locomotor function were significantly restored, along with improvements in essential general health status, after H2 gas inhalation treatment, which was consistent with stabilized neuropathology in the left hemisphere and increased Hounsfield unit values of computed tomography in the right hemisphere. The patient finally recovered to a near normal conscious state with a Coma Recovery Scale-Revised Score of 22 from his previous score of 3.
Phase 1 clinical trials are needed to explore the safety and efficacy of H2 gas inhalation in patients with PVS.
Core Tip: We report a case in which hydrogen (H2) gas inhalation promoted the recovery of an 11-year-old boy with persistent vegetative state (PVS) caused by severe intracerebral bleeding in the left hemisphere following anticoagulation treatment. The patient‘s PVS severity showed no notable improvement after a 2-mo routine neuroprotection treatment and rehabilitation. Surprisingly, the patient’s orientation, consciousness, ability to speak, facial expressions, and locomotor function were significantly restored, after high-concentration H2 gas inhalation treatment. This case indicates that inhalation of H2 may be an effective intervention candidate for patients with loss of consciousness.
- Citation: Huang Y, Xiao FM, Tang WJ, Qiao J, Wei HF, Xie YY, Wei YZ. Hydrogen inhalation promotes recovery of a patient in persistent vegetative state from intracerebral hemorrhage: A case report and literature review. World J Clin Cases 2022; 10(4): 1311-1319
- URL: https://www.wjgnet.com/2307-8960/full/v10/i4/1311.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i4.1311
Urgent development of novel therapies for intracerebral hemorrhage (ICH) is required due to the high mortality of ICH and the lack of effective therapies[1]. Molecular hydrogen (H2) is known to protect neurons against reactive oxygen species (ROS) induced by cerebral ischemia/reperfusion (I/R) injury[2,3]. Previous experimental studies have shown that H2 gas can also alleviate inflammation and apoptosis[4], in addition to reducing neuronal damage in several rat models of diseases by suppressing the expression of S100 calcium-binding protein B, phosphorylation of c-Jun N-terminal kinase, and reactive astrogliosis[5-7]. H2 gas inhalation selectively reduces hydroxyl radical and peroxynitrite levels in vitro and exerts an antioxidant effect, reflected by decreased brain concentrations of 4-hydroxynonenal (a specific marker for lipid peroxidation), and 8-hydroxyguanosine (a nucleic acid oxidation marker) in a rat middle cerebral artery occlusion model[2]. Clinical studies have also indicated the effectiveness of H2 gas in the treatment of hepatic, renal, cardiac, and pulmonary diseases, including chronic obstructive pulmonary disease and coronavirus disease 2019[8-10]. H2 gas inhalation or H2-rich saline treatment has beneficial effects on early brain injury after subarachnoid hemorrhage[11,12], delayed brain injury in subarachnoid hemorrhage, and unilateral common carotid artery occlusion with the endovascular perforation method[13]. Here, we report the case of an 11-year-old boy treated by high-concentration H2 gas inhalation that helped with the recovery from persistent vegetative state (PVS) caused by ICH, which is the first clinical report of high-dose H2 gas therapy in a child in a PVS after ICH.
An 11-year-old boy treated with anticoagulation after aortic valve replacement surgery presented to the pediatric intensive care unit in our hospital following fever and abdominal pain for 2 d, and coma for 2 h on May 27, 2020.
An emergency brain surgical intervention was carried out immediately to relieve the intracranial pressure and, subsequently, reduce brain injury. Assisted by neuronavigation, both left ventricle and hematoma drains were established under general anesthesia. In addition, critical life support consisting of tracheostomy, intracranial pressure probe implantation, and mechanical ventilation was also established.
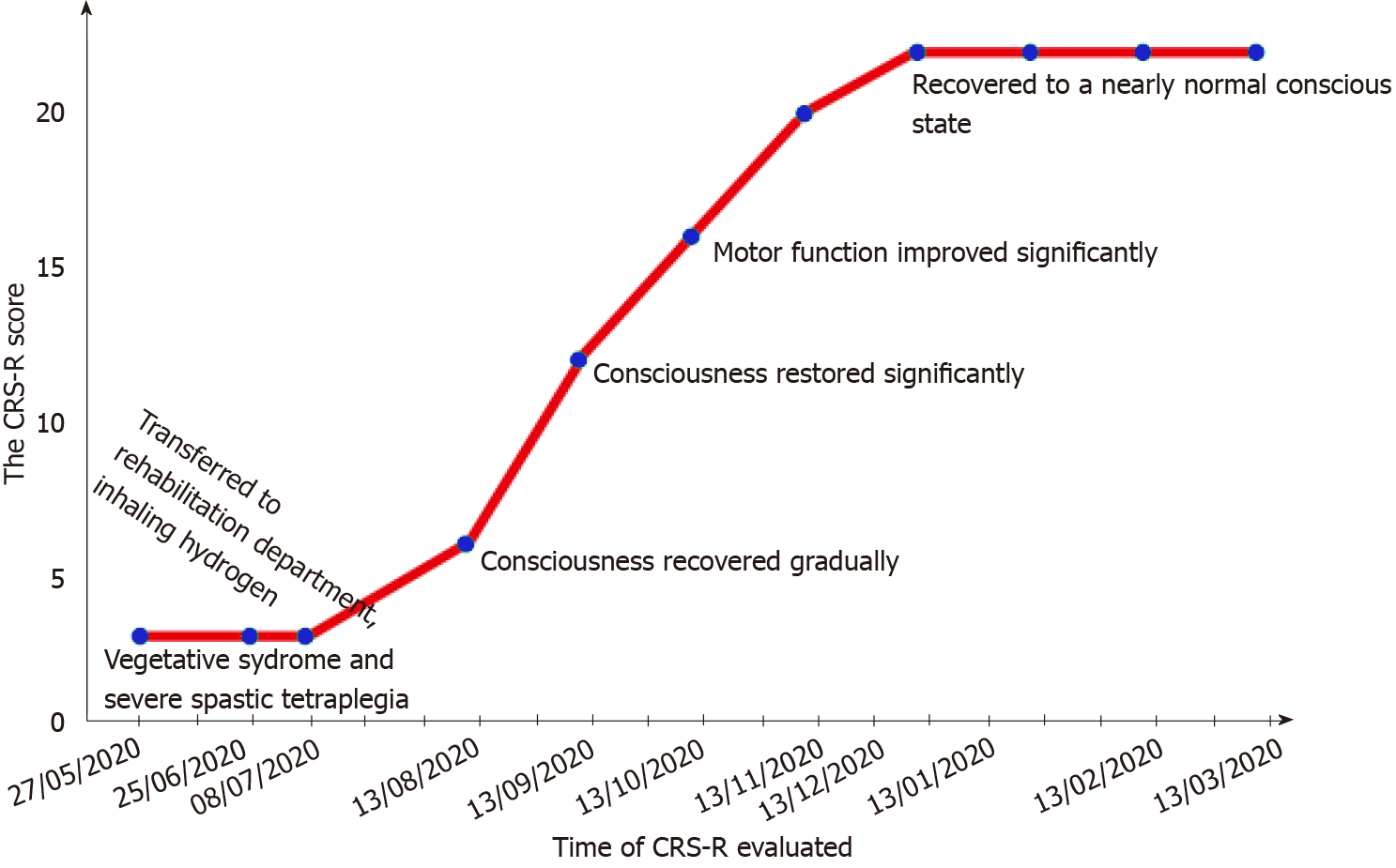
Approximately 6 wk (41 d) after surgery, the patient was still in a completely bedridden vegetative state (VS) with a Coma Recovery Scale-Revised (CRS-R) score[14] of 3 (auditory function: 0, visual function: 0, motor function: 1, verbal function: 0, communication: 0, and arousal: 2). Although his life support relied on nasal tube-feeding, the patient had normal heartbeat and breathing rates.
As the patient’s VS status did not show signs of improvement for more than 4 wk after brain surgery, he was transferred to the rehabilitation department of the same hospital and was diagnosed with PVS, and neuroprotective treatments and rehabilitation training were initiated. The neuroprotective treatments included nasal administration of nerve growth factor, baclofen, and hyperbaric oxygen. The functional rehabilitation therapies included comprehensive bedside rehabilitation therapies, such as anticonvulsive treatment, range-of-motion maintenance, and swallowing and feeding training. Unfortunately, despite these therapeutic interventions for 4 more weeks, his PVS symptoms and severity showed no improvement. Therefore, it was necessary to explore a new and safe therapeutic intervention with potential effects on the patient who had been in a VS for over 2 mo.
At the age of 3 years, the patient underwent repair of an atrial septal defect and ventricular septal defect due to complex congenital heart disease. In October 2018, the patient underwent aortic valve replacement surgery. He received warfarin anticoagulant therapy for nearly 2 years after aortic valve replacement.
The patient had no personal or family history.
The patient could occasionally open his eyes and yawn, but he had no response to pain stimulation, and could not distinguish between his family members and strangers. Moreover, he was unable to listen and follow instructions or speak. Furthermore, his body posture was abnormal, with bent elbows and ulnar deviation, wrist flexion, fists with high tonic metacarpophalangeal joints, and stiff, straightened lower limbs with inverted feet. His muscle tone was significantly high in the lower limbs with a modified Ashworth spasm scale score of 2. Additionally, the patient had no voluntary movement control and could not hold his head steady, sit down, stand alone, or walk. The patient, however, had normal reflexes, including biceps reflex +, triceps reflex +, cough reflex +, knee reflex +++, Achilles tendon reflex +++, and Babinski sign and ankle clonus +.
Blood analysis revealed mild leukocytosis of 8.35 × 109/L, with predominant neutrophils (67%), and normal hematocrit and platelet count. Prothrombin and partial thromboplastin times were normal, and D-dimer was slightly increased at 1.08 mg/L. Blood biochemistry analyses and urine analysis were normal. Electrocardiogram showed a sinus rhythm, frequent atrial premature beats, abnormal left atrium, large left ventricle, and complete left bundle branch block.
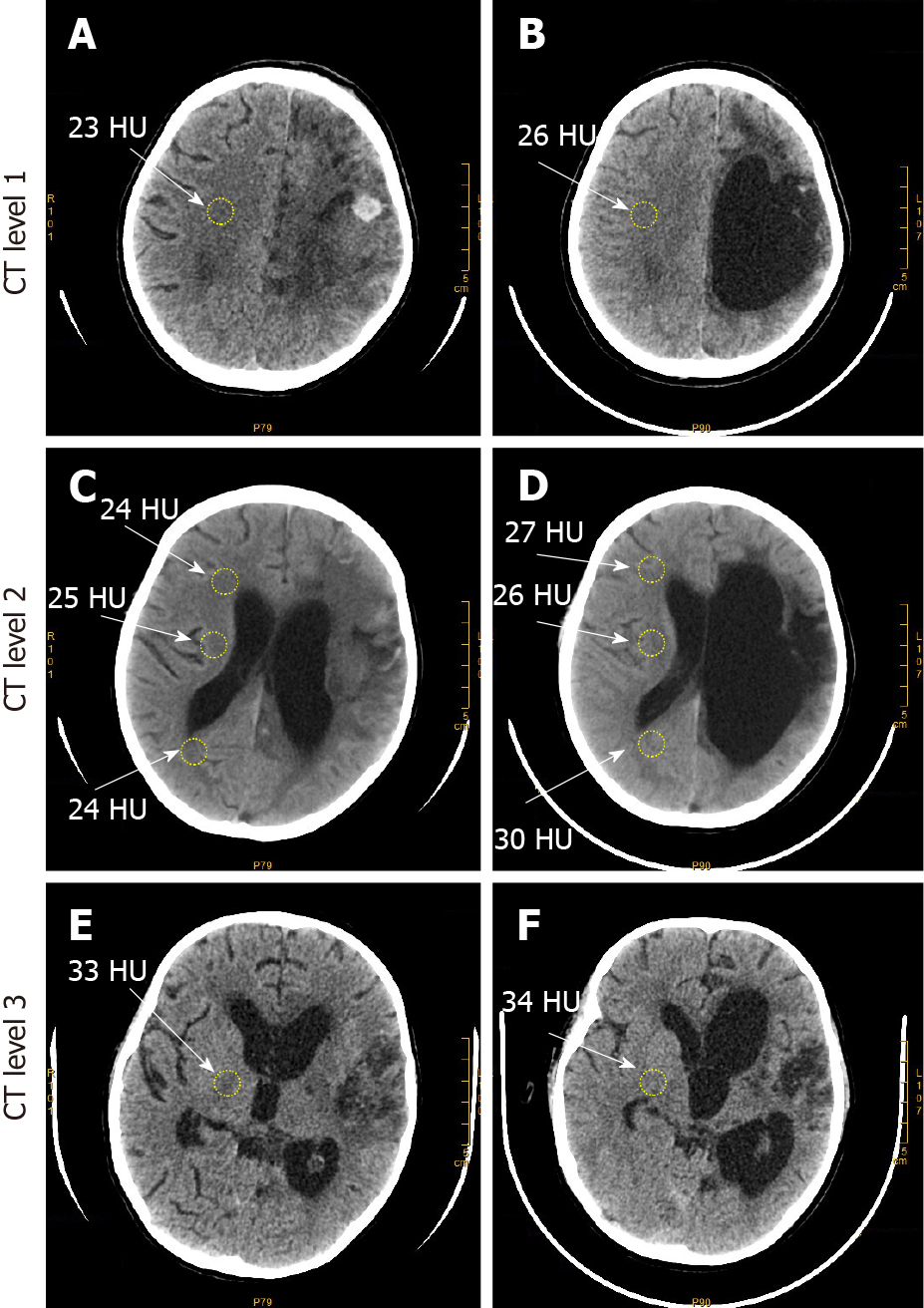
A computed tomography (CT) scan of the patient showed irregularly shaped and low-density CT images of the left frontal, parietal, and basal ganglia regions, which covered most of the left hemisphere (Figures 1A, 1C, and 1E). Similar low-density CT images were also observed in the posterior horn of the bilateral ventricles and the third and fourth ventricles near the sickle and sulci regions of the left brain. The left lateral ventricle was compressed and narrowed by the hematoma and cerebral edema compared to that of the right ventricle, and midline brain structures were also slightly shifted to the right.
PVS, coagulation dysfunction, ICH, brain hernia, and postsurgical syndrome after aortic valve replacement.
H2 has been used in the treatment of patients in critical situations such as traumatic brain injury and cerebral ischemia, and no side effects have been reported to date[15]. After a thorough discussion and explanation of the patient’s status with his family and with their permission, high-concentration H2 (66.6% H2 and 33.3% O2) inhalation treatment was administered. The treatment was given twice daily, for 2-3 h each time, for 5 mo. The initial H2 gas inhalation treatment started 2 mo after the patient developed PVS.
To our surprise, the patient gradually began to show signs of improvement, such as spontaneous eye opening and occasional flexion/extension of his left lower limb shortly after treatment. A CT scan after treatment showed that the hematoma in the left hemisphere was replaced by an irregular cavity filled and surrounded by degenerated brain parenchyma indicated by shadows of low density on CT images after H2 gas inhalation treatment, but the area with shadows of low density on CT images was reduced compared to that before treatment. The left lateral ventricle was markedly enlarged due to drainage of the left lateral ventricle and hematoma, as well as significant neuronal degeneration in the patient’s left brain (Figures 1B, 1D, and 1F). These shadows of low density on CT images in the left hemisphere may have been caused by cerebral edema and ICH, and reduction of the shadows of low density on CT images indicated that the brain hemorrhage and edema were stabilized by H2 gas inhalation treatment compared to that before treatment. Furthermore, the median CT number, i.e., the X-ray attenuation coefficient, was 26 Hounsfield units (HU), 27 HU, 26 HU, 30 HU, and 34 HU in the precentral gyrus (Figure 1B), corpus callosum-forceps minor, internal capsule, corpus callosum-forceps-major (Figure 1C), and putamen in the patient’s right hemisphere (Figure 1F), respectively, after H2 gas inhalation treatment. These values were increased as compared to 23 HU, 24 HU, 25 HU, 24 HU, and 33 HU in the precentral gyrus (Figure 1A), corpus callosum-forceps minor, internal capsule, corpus callosum-forceps-major (Figure 1C), and putamen in the patient’s right hemisphere (Figure 1E), respectively, before treatment. The increased CT numbers in the right hemisphere after treatment were possibly due to decreased cerebral edema and were critical to the recovery of brain function in the patient. Due to the significantly improved condition of the patient, the nasogastric tube was withdrawn, and he was switched from tube feeding to an oral liquid diet 1 mo after treatment.
In the 2 mo after the first administration of treatment, the patient’s orientation and consciousness, visual pursuit, and localization to noxious stimulation also gradually recovered (Figure 2). The patient could follow simple instructions, open his mouth when his lips were touched with a spoon, chew soft food, and voluntarily bend and straighten his left lower limb. Moreover, the patient was making steady improvement with longer treatments of H2 gas inhalation. Ninety days after the initiation of treatment, his motor function was significantly improved, and he was able to make reproducible movements following instructions and autonomously lift his left limbs. His ability to produce facial expressions was vastly improved compared to that before H2 gas inhalation. He could briefly communicate with others and speak words and phrases. Five months after initiation of treatment with H2 gas inhalation, the patient had recovered to a near normal state of consciousness with a CRS-R score of 22 (auditory function: 4, visual function: 5, motor function: 5, verbal function: 3, communication: 2, and arousal: 3) along with improved speech ability.
Furthermore, the patient had functional recovery (Table 1) and fine motor function improvements (Table 2) 6-7 mo after the initiation of treatment. The patient could understand simple instructions, identify items, and read numbers. He could make requests with a hand gesture, steadily hold his head straight, independently turn his body over to the right side, lift his hands up and reach his head, touch his eyes and nose with his hands, and make voluntary movements with his lower left limb.
| Baseline | 1 mo | 2 mo | 3 mo | 5 mo | 8 mo | |
| Lying & rolling | 0 | 0 | 0 | 9.8 | 29.41 | 25.49 |
| Sitting | 0 | 0 | 0 | 0 | 0 | 8.33 |
| Crawling & kneeling | 0 | 0 | 0 | 0 | 0 | 0 |
| Standing | 0 | 0 | 0 | 0 | 0 | 0 |
| Walking, running & jumping | 0 | 0 | 0 | 0 | 0 | 0 |
| Total score | 0 | 0 | 0 | 1.96 | 5.68 | 6.76 |
| 2020-11-08 | 2020-12-08 | 2021-03-08 | ||||
| Left | Right | Left | Right | Left | Right | |
| Visual tracking | 21 | 21 | 21 | 21 | 21 | 21 |
| Upper limb joint activity | 7 | 0 | 11 | 0 | 16 | 0 |
| Grasping ability | 9 | 0 | 15 | 0 | 20 | 0 |
| Operation ability | 12 | 0 | 17 | 0 | 16 | 0 |
| Hand-eye coordination | 17 | 0 | 28 | 0 | 27 | 0 |
| Total score | 51.64 | 29.36 | 59.38 | 29.36 | 61.70 | 29.36 |
In brief, these clinical observations suggested a possible beneficial role of high concentration H2 gas inhalation in consciousness recovery, muscle tone, and locomotor function in this patient with ICH-induced PVS.
The brain of the PVS patient presented in this case report suffered mechanical damage due to abnormally high cerebral pressure, inflammation, oxidative stress, and other unknown injuries[16-19]. The patient failed to respond to neuroprotective treatment along with other methods of rehabilitation but steadily recovered after administration of high-concentration H2 gas inhalation treatment. CT scans revealed that the patient’s left hemisphere was severely damaged with an enlarged left lateral ventricle and significantly atrophied cerebral parenchyma. However, the CT numbers in the right hemisphere were notably increased after treatment. Other treatment effects included consciousness recovery, significantly alleviated motor and cognitive functional deficits, improved speech and facial expressions, and improvements in general health. The possible underlying mechanisms of H2 gas inhalation in this PVS patient may be closely related to its antioxidative and anti-inflammatory effects.
ICH is devastating and life-threatening, and is associated with severe disability and a high mortality rate, accounting for 10% to 15% of deaths caused by stroke[20]. The initial mechanisms of injury after ICH include mechanical destruction by accidental and abnormally increased intracerebral pressure, hematoma expansion, and/or herniation caused by the hematoma itself[21]. Subsequent inflammation, oxidative stress, and impairment in blood flow around the hematoma contribute to edema formation, delayed cell death, and neurological deficits[17]. For example, excessive generation of ROS causes peroxidation of lipid-rich structures of the blood-brain barrier (BBB), resulting in life-threatening BBB disruption and vasogenic cerebral edema[22]. Increased oxidative stress-induced injury occurs in almost all types of brain cells (including neurons, astrocytes, and microglia) and is also closely related to ICH-induced inflammation[19,23]. Therefore, attenuation of early brain injury by targeting oxidative stress and inflammation is a feasible intervention strategy in ICH. Previous studies have also revealed that antioxidative and anti-inflammatory agents can reduce brain atrophy and recover striatal function and memory after ICH[16,24,25].
Since Ohsawa et al[2] reported that H2 gas has antioxidant and anti-apoptotic properties that protect the brain against I/R injury and stroke by selectively neutralizing hydroxyl radicals, H2 gas has reached the biomedical research forefront as a therapeutic medical gas. Accumulated clinical and experimental biomedical evidence in a variety of models of different diseases has suggested that molecular H2, admi
Another mechanism underlying brain injury is its secondary inflammatory res
H2 can protect tissues and cells from a variety of diseases. For instance, H2 gas inhalation can protect lung function by ameliorating airway inflammation in a murine allergic airway inflammation model[33]. Molecular H2 can also protect the heart from cardiotoxicity and hepatotoxicity induced by doxorubicin by inhibiting inflammation and apoptosis[34]. H2 also reduces inflammatory responses after exercise by de
It is worth noting that not all PVS patients were responsive to molecular H2 treatment in our clinical research. We tried high-concentration H2 inhalation in patients with acute necrotizing encephalopathy, but there was no significant therapeutic effect regarding the recovery of consciousness in some patients after several weeks of high-concentration H2 inhalation. Considering that the pathophy
In summary, a patient with PVS caused by ICH did not respond to routine neuronal rehabilitation treatment but recovered consciousness and locomotor function and restored his speech and emotional expression abilities following the administration of high-concentration H2 inhalation treatment for 5 mo. Although the exact underlying mechanisms remain unclear, molecular H2 may protect the brain from ICH due to its antioxidative stress and anti-neuroinflammatory properties.
Phase 1 clinical trials are needed to determine the safety and efficacy of H2 gas inhalation in PVS.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Chinese Association for Laboratory Animal Sciences, S360202401S; Chinese Neuronscience Society, S4209217208M.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chiu CD, Ozair A S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
| 1. | Hostettler IC, Seiffge DJ, Werring DJ. Intracerebral hemorrhage: an update on diagnosis and treatment. Expert Rev Neurother. 2019;19:679-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 2. | Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1346] [Cited by in RCA: 1690] [Article Influence: 93.9] [Reference Citation Analysis (1)] |
| 3. | Ohta S. Direct Targets and Subsequent Pathways for Molecular Hydrogen to Exert Multiple Functions: Focusing on Interventions in Radical Reactions. Curr Pharm Des. 2021;27:595-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 4. | Camara R, Matei N, Camara J, Enkhjargal B, Tang J, Zhang JH. Hydrogen gas therapy improves survival rate and neurological deficits in subarachnoid hemorrhage rats: a pilot study. Med Gas Res. 2019;9:74-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Liu FT, Xu SM, Xiang ZH, Li XN, Li J, Yuan HB, Sun XJ. Molecular hydrogen suppresses reactive astrogliosis related to oxidative injury during spinal cord injury in rats. CNS Neurosci Ther. 2014;20:778-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Huo TT, Zeng Y, Liu XN, Sun L, Han HZ, Chen HG, Lu ZH, Huang Y, Nie H, Dong HL, Xie KL, Xiong LZ. Hydrogen-rich saline improves survival and neurological outcome after cardiac arrest and cardiopulmonary resuscitation in rats. Anesth Analg. 2014;119:368-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Wang C, Li J, Liu Q, Yang R, Zhang JH, Cao YP, Sun XJ. Hydrogen-rich saline reduces oxidative stress and inflammation by inhibit of JNK and NF-κB activation in a rat model of amyloid-beta-induced Alzheimer's disease. Neurosci Lett. 2011;491:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Lu W, Li D, Hu J, Mei H, Shu J, Long Z, Yuan L, Guan R, Li Y, Xu J, Wang T, Yao H, Zhong N, Zheng Z. Hydrogen gas inhalation protects against cigarette smoke-induced COPD development in mice. J Thorac Dis. 2018;10:3232-3243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Bo Chen, Heng Zhai, Hongyuan Hu, Ping Zhou, Fang Zhang, Liang Li, Youzhen Wei. Nivolumab Immunotherapy Plus Hydrogen Inhalation for Treatment of KRAS-Mutant Pulmonary Sarcomatoid Carcinoma: A Case Report. Nano LIFE. 2021;11:2140003. [DOI] [Full Text] |
| 10. | Guan WJ, Wei CH, Chen AL, Sun XC, Guo GY, Zou X, Shi JD, Lai PZ, Zheng ZG, Zhong NS. Erratum to hydrogen/oxygen mixed gas inhalation improves disease severity and dyspnea in patients with Coronavirus disease 2019 in a recent multicenter, open-label clinical trial. J Thorac Dis. 2020;12:4591-4592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Zhan Y, Chen C, Suzuki H, Hu Q, Zhi X, Zhang JH. Hydrogen gas ameliorates oxidative stress in early brain injury after subarachnoid hemorrhage in rats. Crit Care Med. 2012;40:1291-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Hong Y, Guo S, Chen S, Sun C, Zhang J, Sun X. Beneficial effect of hydrogen-rich saline on cerebral vasospasm after experimental subarachnoid hemorrhage in rats. J Neurosci Res. 2012;90:1670-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Kumagai K, Toyooka T, Takeuchi S, Otani N, Wada K, Tomiyama A, Mori K. Hydrogen gas inhalation improves delayed brain injury by alleviating early brain injury after experimental subarachnoid hemorrhage. Sci Rep. 2020;10:12319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004;85:2020-2029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1043] [Cited by in RCA: 1255] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 15. | Ono H, Nishijima Y, Adachi N, Sakamoto M, Kudo Y, Kaneko K, Nakao A, Imaoka T. A basic study on molecular hydrogen (H2) inhalation in acute cerebral ischemia patients for safety check with physiological parameters and measurement of blood H2 Level. Med Gas Res. 2012;2:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Lekic T, Hartman R, Rojas H, Manaenko A, Chen W, Ayer R, Tang J, Zhang JH. Protective effect of melatonin upon neuropathology, striatal function, and memory ability after intracerebral hemorrhage in rats. J Neurotrauma. 2010;27:627-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Choi KS, Kim HJ, Do SH, Hwang SJ, Yi HJ. Neuroprotective effects of hydrogen inhalation in an experimental rat intracerebral hemorrhage model. Brain Res Bull. 2018;142:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Chen CH, Manaenko A, Zhan Y, Liu WW, Ostrowki RP, Tang J, Zhang JH. Hydrogen gas reduced acute hyperglycemia-enhanced hemorrhagic transformation in a focal ischemia rat model. Neuroscience. 2010;169:402-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Manaenko A, Lekic T, Ma Q, Zhang JH, Tang J. Hydrogen inhalation ameliorated mast cell-mediated brain injury after intracerebral hemorrhage in mice. Crit Care Med. 2013;41:1266-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Sacco S, Marini C, Toni D, Olivieri L, Carolei A. Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke. 2009;40:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 398] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 21. | Xue M, Del Bigio MR. Intracerebral injection of autologous whole blood in rats: time course of inflammation and cell death. Neurosci Lett. 2000;283:230-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 199] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke. 2011;42:1781-1786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 682] [Cited by in RCA: 655] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 23. | Shao A, Wu H, Hong Y, Tu S, Sun X, Wu Q, Zhao Q, Zhang J, Sheng J. Hydrogen-Rich Saline Attenuated Subarachnoid Hemorrhage-Induced Early Brain Injury in Rats by Suppressing Inflammatory Response: Possible Involvement of NF-κB Pathway and NLRP3 Inflammasome. Mol Neurobiol. 2016;53:3462-3476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 24. | Fujii M, Yan J, Rolland WB, Soejima Y, Caner B, Zhang JH. Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl Stroke Res. 2013;4:432-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 415] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 25. | Yang F, Wang Z, Wei X, Han H, Meng X, Zhang Y, Shi W, Li F, Xin T, Pang Q, Yi F. NLRP3 deficiency ameliorates neurovascular damage in experimental ischemic stroke. J Cereb Blood Flow Metab. 2014;34:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 354] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 26. | Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang QW. Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol. 2014;115:25-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 475] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 27. | Zhu H, Wang Z, Yu J, Yang X, He F, Liu Z, Che F, Chen X, Ren H, Hong M, Wang J. Role and mechanisms of cytokines in the secondary brain injury after intracerebral hemorrhage. Prog Neurobiol. 2019;178:101610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 223] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 28. | Lan X, Han X, Liu X, Wang J. Inflammatory responses after intracerebral hemorrhage: From cellular function to therapeutic targets. J Cereb Blood Flow Metab. 2019;39:184-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 29. | Tschoe C, Bushnell CD, Duncan PW, Alexander-Miller MA, Wolfe SQ. Neuroinflammation after Intracerebral Hemorrhage and Potential Therapeutic Targets. J Stroke. 2020;22:29-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 292] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 30. | Ren H, Han R, Chen X, Liu X, Wan J, Wang L, Yang X, Wang J. Potential therapeutic targets for intracerebral hemorrhage-associated inflammation: An update. J Cereb Blood Flow Metab. 2020;40:1752-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 31. | Shi E, Shi K, Qiu S, Sheth KN, Lawton MT, Ducruet AF. Chronic inflammation, cognitive impairment, and distal brain region alteration following intracerebral hemorrhage. FASEB J. 2019;33:9616-9626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 32. | Wang Z, Zhou F, Dou Y, Tian X, Liu C, Li H, Shen H, Chen G. Melatonin Alleviates Intracerebral Hemorrhage-Induced Secondary Brain Injury in Rats via Suppressing Apoptosis, Inflammation, Oxidative Stress, DNA Damage, and Mitochondria Injury. Transl Stroke Res. 2018;9:74-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 241] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 33. | Zhang N, Deng C, Zhang X, Zhang J, Bai C. Inhalation of hydrogen gas attenuates airway inflammation and oxidative stress in allergic asthmatic mice. Asthma Res Pract. 2018;4:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Gao Y, Yang H, Fan Y, Li L, Fang J, Yang W. Hydrogen-Rich Saline Attenuates Cardiac and Hepatic Injury in Doxorubicin Rat Model by Inhibiting Inflammation and Apoptosis. Mediators Inflamm. 2016;2016:1320365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Nogueira JE, Passaglia P, Mota CMD, Santos BM, Batalhão ME, Carnio EC, Branco LGS. Molecular hydrogen reduces acute exercise-induced inflammatory and oxidative stress status. Free Radic Biol Med. 2018;129:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 36. | Ning K, Liu WW, Huang JL, Lu HT, Sun XJ. Effects of hydrogen on polarization of macrophages and microglia in a stroke model. Med Gas Res. 2018;8:154-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Meng J, Yu P, Jiang H, Yuan T, Liu N, Tong J, Chen H, Bao N, Zhao J. Molecular hydrogen decelerates rheumatoid arthritis progression through inhibition of oxidative stress. Am J Transl Res. 2016;8:4472-4477. [PubMed] |
| 38. | Takeuchi S, Nagatani K, Otani N, Wada K, Mori K. Hydrogen does not Exert Neuroprotective Effects or Improve Functional Outcomes in Rats After Intracerebral Hemorrhage. Turk Neurosurg. 2016;26:854-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |