Published online Feb 6, 2022. doi: 10.12998/wjcc.v10.i4.1278
Peer-review started: August 6, 2021
First decision: November 6, 2021
Revised: November 16, 2021
Accepted: December 25, 2021
Article in press: December 25, 2021
Published online: February 6, 2022
Processing time: 171 Days and 5.1 Hours
Castleman’s disease (CD) is a lymphatic proliferative disorder of unknown cause and is rarely seen clinically. It has been divided into unicentric and multicentric types. Unicentric CD (UCD) occurs as a solitary enlarged mass and mediastinal lymph nodes are the most common site. Surgical excision has proven to be curative for UCD. Multicentric CD (MCD) appears as a systemic disease with peripheral lymphadenopathy. MCD had a poor response to surgery and mon
A 44-year-old woman presented with a pancreatic mass during routine physical examination. She had no obvious symptoms, such as fever, abdominal pain, abdominal distension, or jaundice. Ultrasound examination indicated a hypoechoic mass between the body of the pancreas, left lobe of the liver and stomach. It had a clear boundary, irregular shape, uneven echo, and no obvious blood flow signals. To clarify the diagnosis, contrast-enhanced ultrasound examination was performed, which showed a benign pancreatic lesion. Neuroendocrine or solid pseudopapillary tumor was a possible diagnosis. The patient underwent further contrast-enhanced computed tomography and contrast-enhanced magnetic resonance imaging, which were suggestive of solid pseudopapillary tumor or neuroendocrine tumor. All the examinations failed to give a definitive diagnosis, and the patient underwent surgery. The final pathological and immunohistochemical results showed that the mass was CD.
This case highlights when lymphadenopathy is encountered clinically, CD should be considered and a biopsy should be performed.
Core Tip: Castleman’s disease (CD) is a lymphoproliferative disorder of unknown cause. We present a rare case of unicentric CD. A 44-year-old woman presented with a pancreatic mass during routine physical examination. The patient had no obvious symptoms. None of the three enhanced images gave a clear diagnosis. The patient underwent surgery. The final pathological and immunohistochemical results showed that the mass was CD. This case highlights when lymphadenopathy is encountered clinically, CD should be considered and a biopsy performed. It is especially important to rule out diseases with known causes first.
- Citation: Zhai HY, Zhu XY, Zhou GM, Zhu L, Guo DD, Zhang H. Unicentric Castleman disease was misdiagnosed as pancreatic mass: A case report. World J Clin Cases 2022; 10(4): 1278-1285
- URL: https://www.wjgnet.com/2307-8960/full/v10/i4/1278.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i4.1278
Castleman’s disease (CD) is a lymphoproliferative disorder of unknown cause and is rarely seen clinically. The pathological features are obvious proliferation of lymph follicles, blood vessels and plasma cells. CD is divided into two main types: Unicentric CD (UCD) and multicentric CD (MCD). UCD occurs as a solitary enlarged mass and mediastinal lymph nodes are the most commonly affected. Surgical excision has proven to be curative for UCD. MCD appears as a systemic disease with peripheral lymphadenopathy. MCD has a poor response to surgery and monoclonal antibodies with rituximab have become a research hotspot. Here, we present a 40-year-old woman with characteristic clinical, radiological and histological findings of UCD, demonstrating an incidental retroperitoneal mass misdiagnosed as pancreatic tumor.
A 40-year-old woman presented to the ultrasound department of our hospital complaining of a pancreatic mass found by physical examination. She came to our hospital to clarify the diagnosis. She had no obvious conscious symptoms, such as fever, abdominal pain, abdominal distension, or jaundice.
The patient had no obvious conscious symptoms, such as fever, abdominal pain, abdominal distension, or jaundice.
The patient had no previous medical history.
The patient had no personal or family medical history.
The patient’s temperature was 36.6 °C, heart rate 73 bpm, respiratory rate 16 breaths/min, blood pressure 120/70 mmHg and oxygen saturation in room air 98%. Clinical abdominal examination did not reveal any positive signs. Our clinical considerations were benign or malignant tumor of the pancreas.
Blood analysis showed normal prothrombin and partial thromboplastin times and D-dimer level. Urinalysis was normal for a-fetoprotein, ferritin, carcinoembryonic antigen, carbohydrate antigen-199, sugar chain antigen as well as human epididymal epithelial secretory protein.
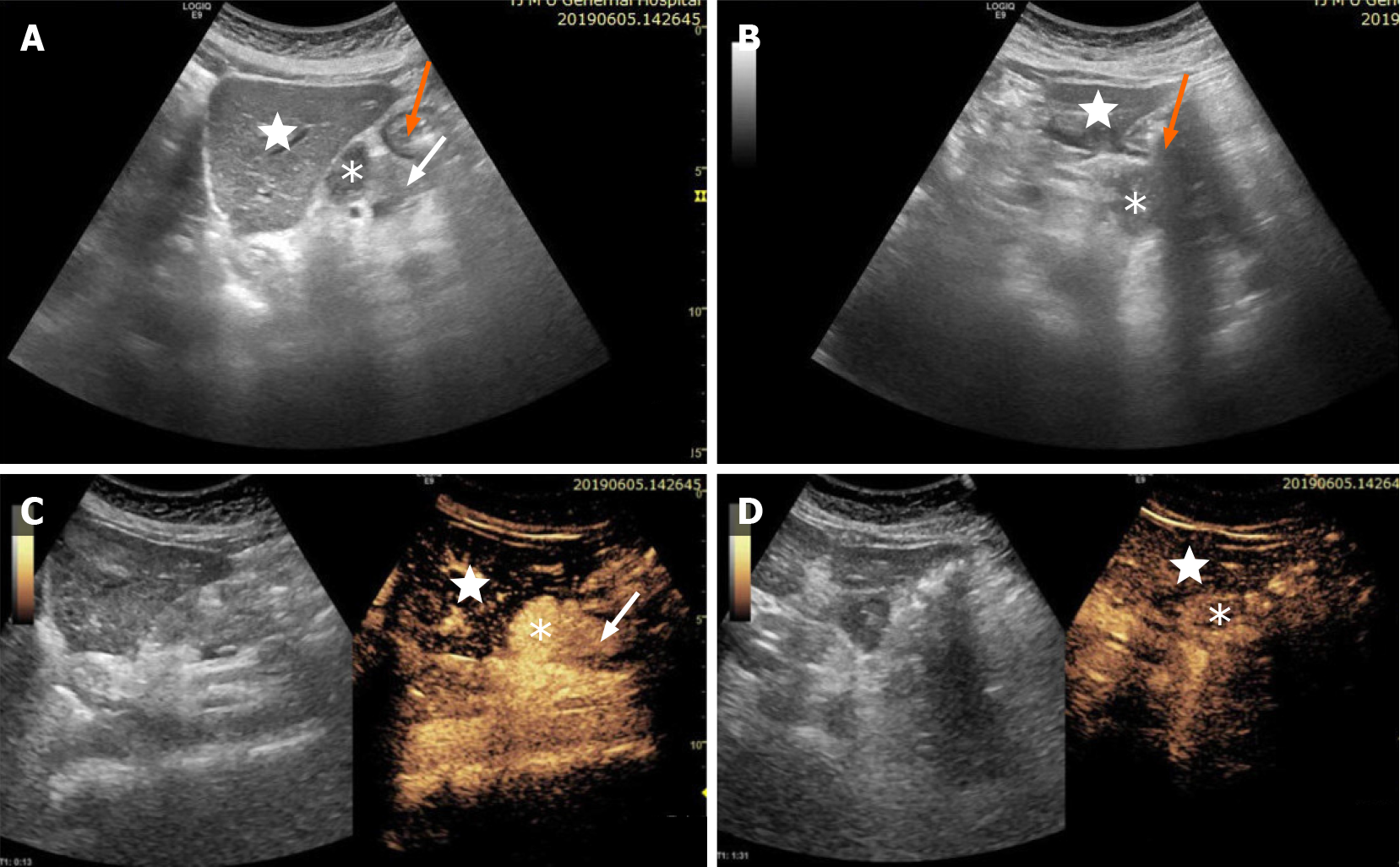
Initial imaging with conventional ultrasound (US) examination, revealed a 3.2 cm × 2.5 cm hypoechoic mass between the body of the pancreas, left lobe of the liver and stomach, with clear boundaries, irregular shape, uneven echoes, and no obvious blood flow signals. In order to make a diagnosis, the patient underwent contrast-enhanced US (CEUS) examination. CEUS showed hyperenhancement of the lesion in the arterial phase, and slightly high enhancement in the venous phase. CEUS suggested differential diagnosis of benign pancreatic tumor, neuroendocrine tumor or solid pseudopapillary tumor, but the possibility of tumor in other organs could not entirely be excluded (Figure 1).
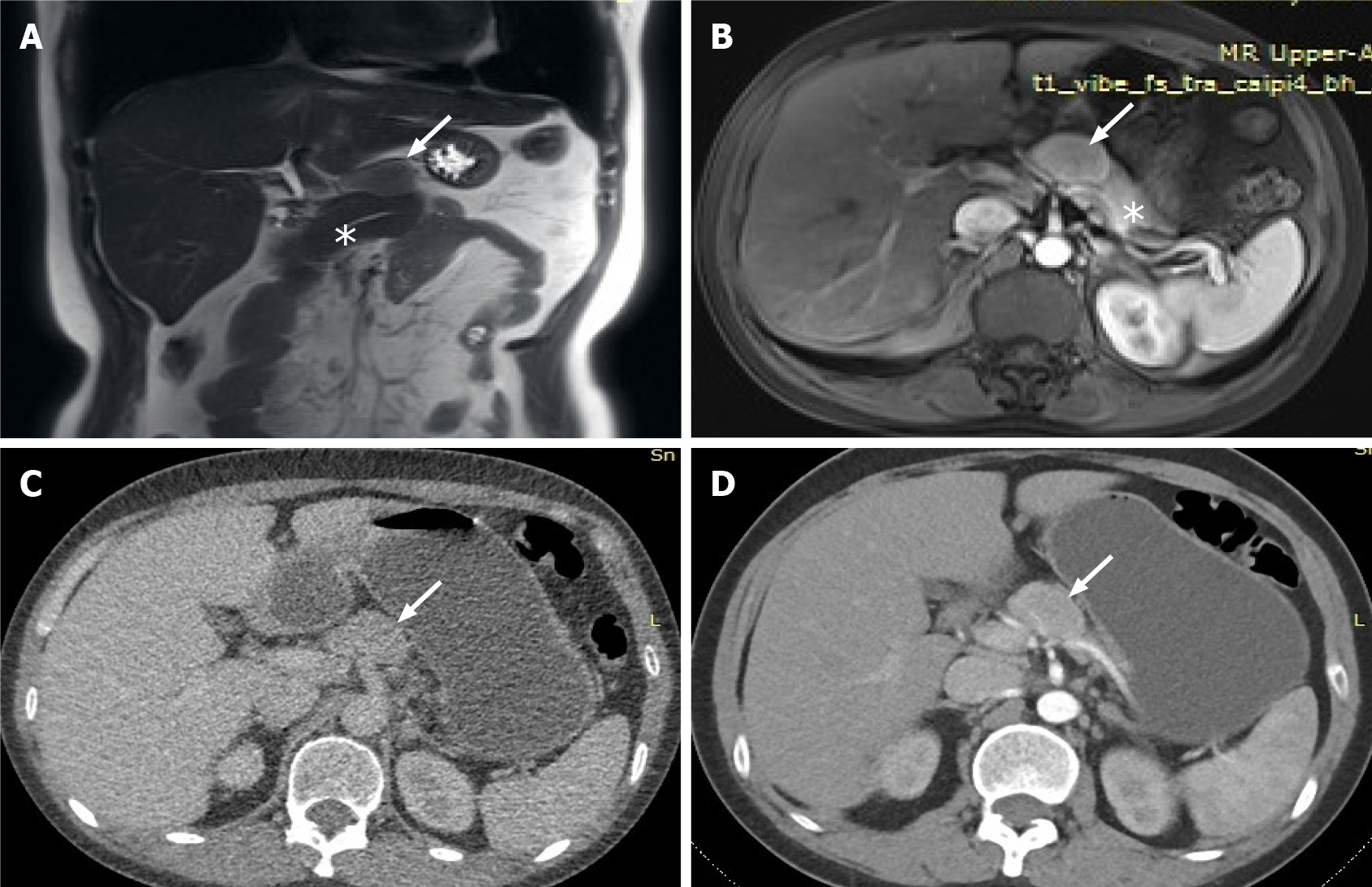
The lesion was further evaluated by computed tomography (CT) and magnetic resonance imaging (MRI). CT revealed a mixed density nodule located above the pancreatic body that was convex and shallowly lobulated, showing uneven progressive enhancement, and the degree of enhancement in each stage was lower than that of pancreatic parenchyma (Figure 2). MRI revealed an isointense lesion located above the pancreatic body that was clearly separated from the normal pancreas. The edge was enhanced in the arterial phase, and the degree of internal enhancement in each phase was lower than that of the pancreatic parenchyma. As most pancreatic adenocarcinomas have poor blood supply, they usually display low enhancement on arterial phase of enhanced images. However, the lesion in this case showed rich blood supply on CEUS, enhanced MRI, and enhanced CT, so pancreatic adenocarcinoma was ruled out. Therefore, we believed that the abnormal enhancement of the upper part of the pancreatic body was a solid pseudopapillary tumor or a neuroendocrine tumor.
All the examinations failed to give a definitive diagnosis; therefore, the patient underwent surgery. During the surgery, we observed a 3 cm × 2 cm mass above the pancreatic body, with a clear boundary and tough texture, located anterior to the bifurcation of the splenic artery and the common hepatic artery, pushing the upper edge of the pancreas interior to it. Careful separation of the adhesion between the tumor and the surrounding tissues along the surface of the tumor ensured that the tumor was well demarcated from the pancreas.
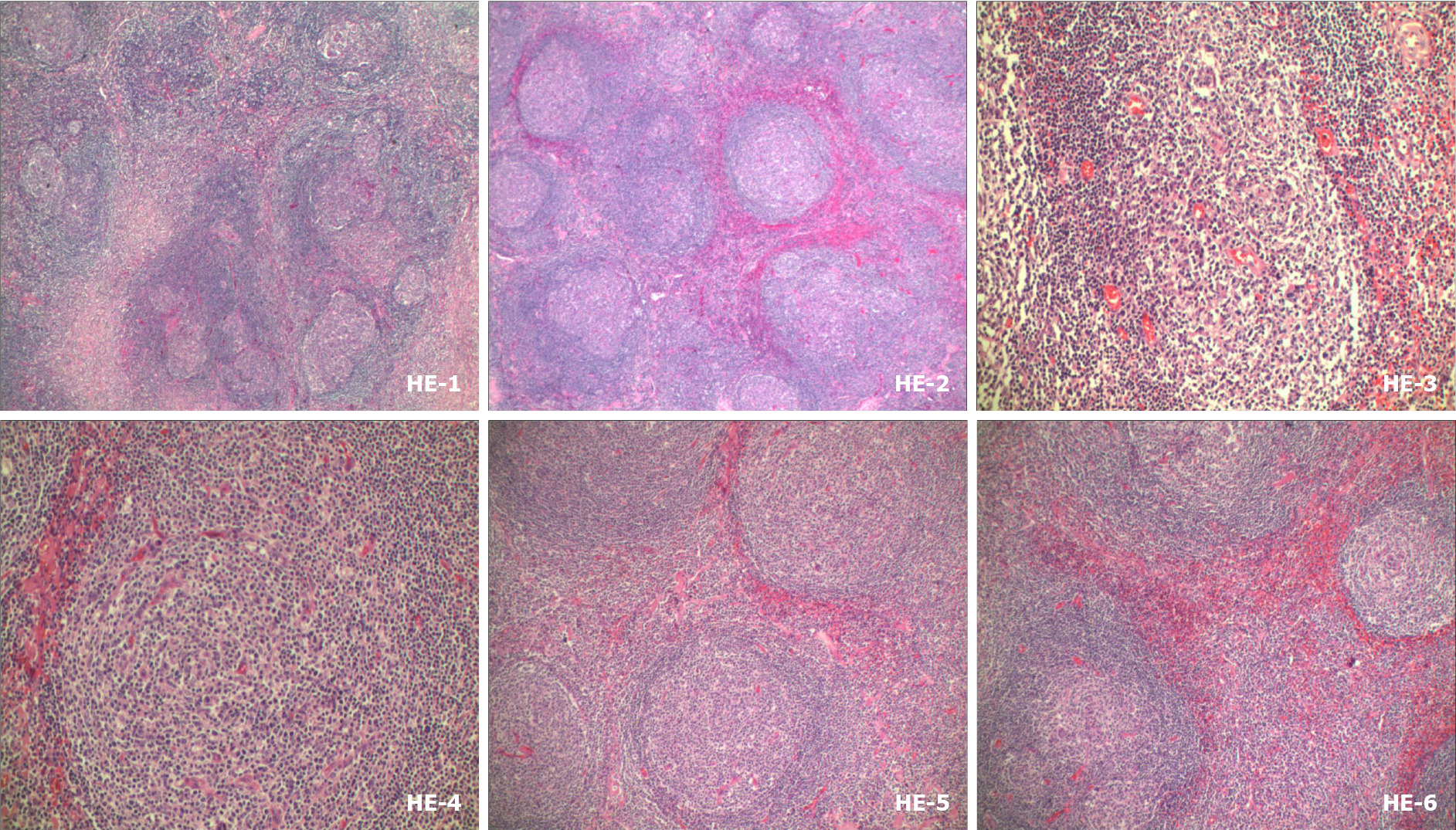
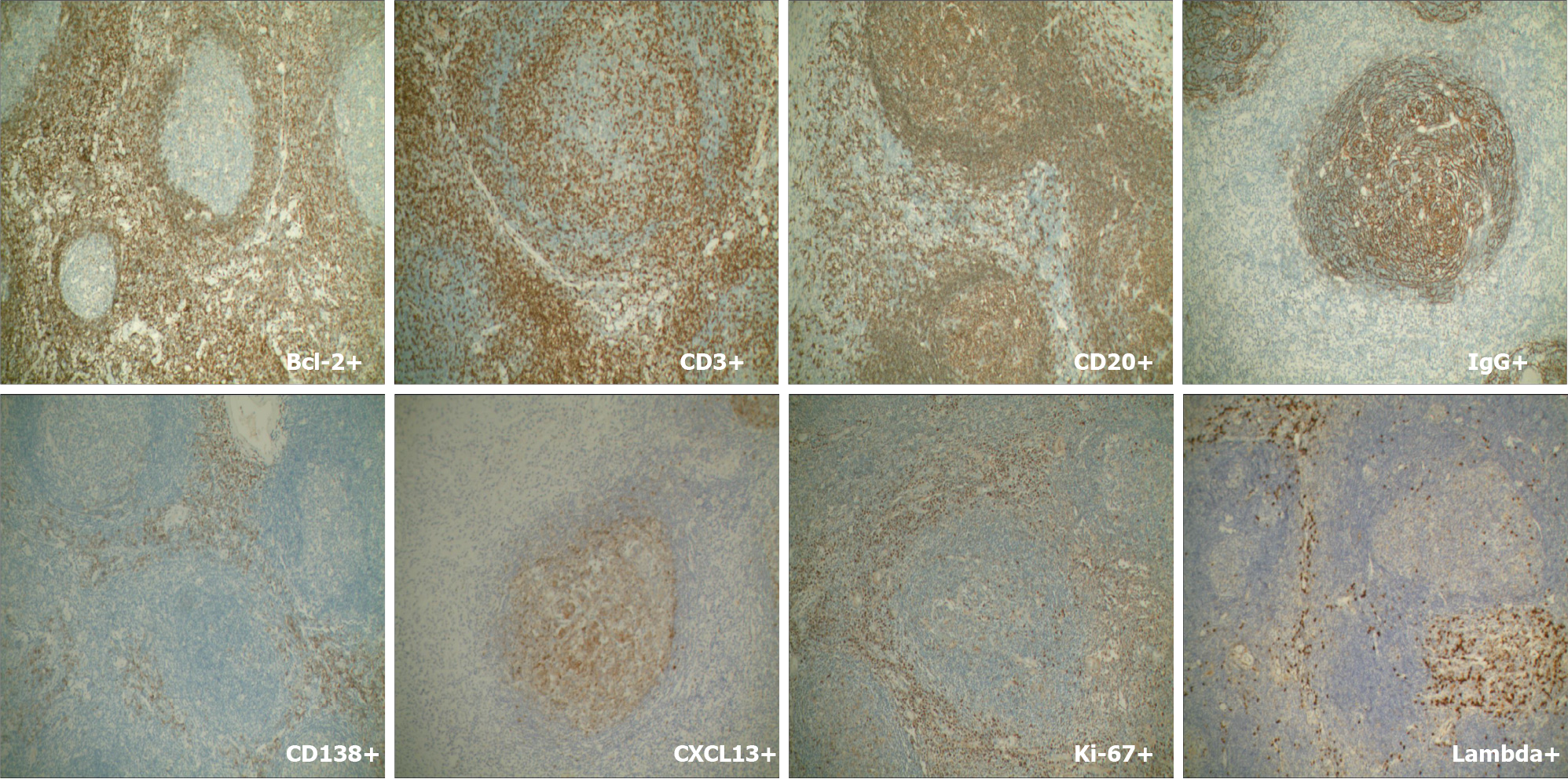
Mass resection was performed and histological examination revealed lymphoid tissue proliferation, with lymphoid follicular hyperplasia, mantle hyperplasia, interfollicular plasma cell hyperplasia, and local vascular hyperplasia (Figure 3). Plasma cells showed strong immunostaining for CD138 and IgG. IgG4 was occasionally positive and the expression rate was < 40%. No restrictive expression of kappa and lambda was observed. B and T cells were positive for CD20 and CD30. CD21 and CD23 showed the follicular dendritic cell network. Germinal center cells were positive for Bcl-2, and weakly positive for CD10 and Bcl-6. Cxc113 and cyclin D11 were positive in a few scattered cells. Ki-67 was highly expressed in germinal centers (Figure 4). These results gave the most likely diagnosis as CD.
The patient underwent surgery to remove the mass located above the pancreatic body.
The patient had an uneventful postoperative clinical course. Two months after surgical removal of the mass, the patient was asymptomatic, and a new MRI scan showed complete removal of the tumor.
CD is a lymphoproliferative disorder of unknown cause and is rarely seen clinically. The pathological features are obvious proliferation of lymph follicles, blood vessels and plasma cells. Clinically, it is characterized by significant enlargement of deep or superficial lymph nodes. Since its first recognition in 1956[1], understanding of CD has improved after segregation of UCD and MCD, showing distinctive prognosis and treatment approaches.
The incidence and prevalence of CD has become difficult to evaluate due to its rare occurrence in the general population, and it affects both sexes equally[2]. UCD is usually found to have a peak incidence in the second to fourth decades of life[3]. In most cases, UCD occurs as an isolated mediastinal lymph node enlargement. Patients present with a single lymph node with painless enlargement and slow growth, ranging from several centimeters to approximately 20 cm in diameter. Mediastinal lymph nodes are the most commonly affected, followed by cervical, axillary and abdominal lymph nodes. Occasionally, they are found in external tissues such as larynx, vulva, pericardium, subcutaneous muscle, lung, and orbit, as well as intracranially. Most patients have no systemic symptoms and a few complain of cough, dyspnea or other constitutional symptoms. Patients can survive for a long time after tumor resection, which is a benign course of disease.
MCD is rarer than UCD and tends to present later in the sixth and seventh decades. MCD has a constellation of systemic symptoms with peripheral lymphadenopathy involving multiple compartments throughout the neck, chest, abdomen and pelvis. It also has an association with human immunodeficiency virus and human herpesvirus-8 infections[4,5]. In a few patients, multiple neuropathy, organ enlargement (liver and spleen), endocrine disease, serum immunoglobulin and skin disease were found simultaneously. MCD often presents with an invasive venereal course that is accompanied with infection.
Frizzera et al[6] proposed the diagnostic criteria of CD (Table 1). It should be pointed out that it may be difficult to make a definite and accurate clinical pathological diagnosis based on histopathology or clinical manifestations alone, and the two must be combined, especially for MCD.
| Unicentric | Multicentric |
| Swollen lymph nodes in a single site | Significant swollen lymph node and involvement of multiple peripheral lymph nodes |
| Characteristic hyperplastic histopathological changes and exclude possible primary disease | With characteristic hyperplastic histopathological changes |
| No systemic symptoms | Multiple system involvement performance |
| Long-term survival after tumor removal | Rule out known possible causes |
The differential diagnosis of CD includes lymphoma and any other causes of lymphadenopathy. Initial investigations encompasses a CT scan that reveals the location, extent and number of lymph nodes involved, which are then visualized with homogeneously intense contrast enhancement[7]. MRI and positron emission tomography/CT[8] can be used to clarify the involvement of any soft tissues. Due to the diversity of the clinical and pathological manifestations of CD, sometimes it is difficult to distinguish from scrofula, malignant lymphoma, mediastinal neurogenic tumor (ectopic paraganglioma), ectopic gland tumor, connective tissue disease, blood vessels and immune cell lymphadenopathy[9,10]. When patients present with lymph node enlargement, we should think of CD, and excisional biopsy is usually required to establish the final diagnosis. Combining medical history, physical examination, laboratory examination and pathological examination, it is important to initially rule out diseases of known cause.
In the present case, the patient was initially misdiagnosed with a pancreatic tumor by enhanced CT, enhanced MRI and CEUS. Based on CEUS, we highly suspected that the mass might be derived from the pancreas, and/or it might be a tumor from other parts of the body. We initially differentiated the diagnosis between benign and malignant lesions. The pancreatic adenocarcinoma usually present hypoenhancement on arterial phase of CEUS. Patients with focal pancreatitis have symptoms of upper abdominal pain and digestive systems, and the enhancement of focal pancreatitis was similar to normal pancreatic parenchymas, and most of them were overall isoen
Surgical excision has proven to be curative for UCD. Just like the patient in our manuscript, in some reports[13-15], no recurrence was found either clinically or radiologically that underwent surgical excision. Moreover, surgical excision was seen to decrease the mortality rates (17.6% to 3.8%) in UCD in a systematic review of 404 published cases[2,16]. In contrast, MCD had a poor response to surgery and worse prognosis when compared to UCD. MCD does not have a proven treatment regimen. Multicentric type is ineffective after surgery, the prognosis is poor, and the median survival time is about 14-30 mo; Severe infections, multiple organ failure, and transformation to malignant tumors are the main causes of death in such patients. However, monoclonal antibodies, anti-IL-6 receptor monoclonal antibodies or chemotherapy with rituximab has have become a research hotspot[17-19].
CD is a lymphoproliferative disorder and is rarely seen clinically. The pathological features are obvious proliferation of lymph follicles, blood vessels and plasma cells. CD divided into unicentric and multicentric types. UCD occurs as a solitary enlarged mass and shows a benign disease course. Surgical excision has proven to be curative. MCD presents as a systemic disease with peripheral lymphadenopathy and has a poor response to surgery. When patients with lymph node enlargement are encountered clinically, we should consider CD, and excisional biopsy is usually required. It is particularly important to first rule out diseases that are known causes of lymphadenopathy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Corvino A, Pota V S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | INADA K, HAMAZAKI M. Localized mediastinal lymph-node hyperplasia resembling thymoma; a case report. Ann Surg. 1958;147:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Pasha H, Siddiqui MI, Wasif M. Castleman disease of the neck: A case report. J Pak Med Assoc. 2020;70:354-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Keller AR, Hochholzer L, Castleman B. Hyaline-vascular and plasma-cell types of giant lymph node hyperplasia of the mediastinum and other locations. Cancer. 1972;29:670-683. [PubMed] |
| 4. | Dargent JL, Lespagnard L, Sirtaine N, Cantinieaux B, Li R, Hermans P. Plasmablastic microlymphoma occurring in human herpesvirus 8 (HHV-8)-positive multicentric Castleman's disease and featuring a follicular growth pattern. APMIS. 2007;115:869-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Kawano M, Hara S, Yachie A, Inoue D, Sato Y, Fajgenbaum DC. HHV-8-negative multicentric Castleman disease patients with serological, histopathological and imaging features of IgG4-related disease. Rheumatology (Oxford). 2021;60:e3-e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Frizzera G, Peterson BA, Bayrd ED, Goldman A. A systemic lymphoproliferative disorder with morphologic features of Castleman's disease: clinical findings and clinicopathologic correlations in 15 patients. J Clin Oncol. 1985;3:1202-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 267] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Sumi M, Ohki M, Nakamura T. Comparison of sonography and CT for differentiating benign from malignant cervical lymph nodes in patients with squamous cell carcinoma of the head and neck. AJR Am J Roentgenol. 2001;176:1019-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Verçosa AFA, Flamini MEDM, Loureiro LVM, Flamini RC. 68Ga-DOTATATE and 18F-FDG in Castleman Disease. Clin Nucl Med. 2020;45:868-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Ekwere TA, Eziagu UB. Multicentric Plasma-Cell Type Castleman Disease Masquerading As Hodgkin Lymphoma: A Case Report. J Lab Physicians. 2020;12:225-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Glick L, Xu H, Han TM, HooKim K, Vogel A, Lallas CD. Castleman Disease: An Uncommon Mass in the Retroperitoneum. Urology. 2020;136:e12-e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | D'Onofrio M, Canestrini S, De Robertis R, Crosara S, Demozzi E, Ciaravino V, Pozzi Mucelli R. CEUS of the pancreas: Still research or the standard of care. Eur J Radiol. 2015;84:1644-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Maurea S, Corvino A, Imbriaco M, Avitabile G, Mainenti P, Camera L, Galizia G, Salvatore M. Simultaneous non-functioning neuroendocrine carcinoma of the pancreas and extra-hepatic cholangiocarcinoma. A case of early diagnosis and favorable post-surgical outcome. JOP. 2011;12:255-258. [PubMed] |
| 13. | Buesing K, Perry D, Reyes C, Abdessalam S. Castleman disease: surgical cure in pediatric patients. J Pediatr Surg. 2009;44:e5-e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Lim KE, Tsai KT, Yue CT, Hsu YY. Surgical Treatment of Castleman Disease Using Cardiopulmonary Bypass. Acta Cardiol Sin. 2014;30:169-172. [PubMed] |
| 15. | Beckham TH, Yang JC, Chau KW, Noy A, Yahalom J. Excellent Outcomes with Surgery or Radiotherapy in the Management of Castleman Disease Including a Case of Oligocentric Disease. Clin Lymphoma Myeloma Leuk. 2020;20:685-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Talat N, Belgaumkar AP, Schulte KM. Surgery in Castleman's disease: a systematic review of 404 published cases. Ann Surg. 2012;255:677-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 233] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 17. | van Rhee F, Oksenhendler E, Srkalovic G, Voorhees P, Lim M, Dispenzieri A, Ide M, Parente S, Schey S, Streetly M, Wong R, Wu D, Maillard I, Brandstadter J, Munshi N, Bowne W, Elenitoba-Johnson KS, Fössa A, Lechowicz MJ, Chandrakasan S, Pierson SK, Greenway A, Nasta S, Yoshizaki K, Kurzrock R, Uldrick TS, Casper C, Chadburn A, Fajgenbaum DC. International evidence-based consensus diagnostic and treatment guidelines for unicentric Castleman disease. Blood Adv. 2020;4:6039-6050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 126] [Article Influence: 31.5] [Reference Citation Analysis (1)] |
| 18. | Koga T, Hagimori N, Takemori S, Morimoto S, Sumiyoshi R, Shimizu T, Hosogaya N, Fukushima C, Yamamoto H, Kawakami A. Randomized, double-blind, placebo-controlled, parallel-group trial of sirolimus for tocilizumab-resistant idiopathic multicentric Castleman disease: Study protocol for clinical trial. Medicine (Baltimore). 2020;99:e20710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Ramaswami R, Lurain K, Peer CJ, Serquiña A, Wang V, Widell A, Goncalves P, Steinberg SM, Marshall V, George J, Figg WD, Whitby D, Ziegelbauer J, Uldrick TS, Yarchoan R. Tocilizumab in patients with symptomatic Kaposi sarcoma herpesvirus-associated multicentric Castleman disease. Blood. 2020;135:2316-2319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |