Published online Feb 6, 2022. doi: 10.12998/wjcc.v10.i4.1242
Peer-review started: September 26, 2021
First decision: October 18, 2021
Revised: October 26, 2021
Accepted: December 25, 2021
Article in press: December 25, 2021
Published online: February 6, 2022
Processing time: 119 Days and 19.3 Hours
Paediatric supraglottic airway devices (SGAs) are widely used in routine anaesthesia and serve as primary or back-up devices for difficult airway management. The inflatable Ambu laryngeal masks and non-inflatable i-gel are two improvements of SGAs based on classic laryngeal masks. The clinical performance and safety of these two devices in paediatric patients are still unclear and warrant further investigation.
To perform a systematic review and meta-analysis on the clinical performance and safety of Ambu laryngeal masks and i-gel in anaesthetised paediatric patients.
MEDLINE, Embase, Web of Science and Cochrane Central Register of Controlled Trials were searched from inception dates to April 2020. We identified published randomised controlled trials (RCTs) in which the intervention involved the use of Ambu laryngeal masks and i-gel in anaesthetised paediatric patients (age < 18 years). We assessed the oropharyngeal leak pressure (OLP) as the primary outcome. The secondary outcomes were insertion time, success rate of insertion on the first attempt, and incidence of adverse events.
After searching for all relevant trials published up to April 2020, data from seven RCTs with a total of 667 paediatric patients (323 and 344 participants in the i-gel and Ambu groups, respectively) were evaluated. The mean OLP in anaesthetised paediatric patients was lower in the Ambu group [21.82 cmH2O for Ambu vs 23.98 cmH2O for i-gel, P = 0.003, 95% confidence interval (CI): -3.58 to -0.75, I2 = 68%, Mantel-Haenszel random model]. We did not find any clear evidence of differences between the devices in terms of insertion time, success rate of insertion, and incidence of adverse events except for blood staining (risk ratio 5.86, 95%CI: 1.76 to 19.46, P = 0.004, I2 = 0, fixed-effect model).
The i-gel airway may provide a better seal and is therefore probably more suitable than the Ambu laryngeal mask airway in anaesthetised paediatric patients. However, the evidence is insufficient to allow making firm conclusions or to guide clinical practice, owing to the small number of relevant published studies.
Core Tip: The inflatable Ambu laryngeal masks and non-inflatable i-gel are two improvements of supraglottic airway devices based on classic laryngeal masks. The clinical performance and safety of these two devices in paediatric patients are still unclear and warrant further investigation. We performed a systematic review and meta-analysis on the clinical performance and safety of Ambu laryngeal masks and i-gel in anaesthetised paediatric patients. The results of this study showed that the i-gel airway may provide a better seal with a lower risk of adverse events and is therefore probably more suitable than the Ambu laryngeal mask airway in anaesthetised paediatric patients.
- Citation: Bao D, Yu Y, Xiong W, Wang YX, Liang Y, Li L, Liu B, Jin X. Comparison of the clinical performance of i-gel and Ambu laryngeal masks in anaesthetised paediatric patients: A meta-analysis. World J Clin Cases 2022; 10(4): 1242-1254
- URL: https://www.wjgnet.com/2307-8960/full/v10/i4/1242.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i4.1242
Supraglottic airway devices (SGAs) have gained widespread acceptance for use in routine anaesthesia and emergency airway management in children since the 1980s, owing to advantages such as easy insertion, decreased use of neuromuscular blocking agents, hemodynamic stability, and low risk of postoperative airway complications compared with tracheal intubation[1-3]. To solve the deficiencies of classic laryngeal masks, including airway leak, gastric insufflation, and risk of aspiration with positive pressure ventilation[4,5], the design of the perfect paediatric SGA has undergone a long and productive evolution leading to i-gel and Ambu laryngeal masks, which are two improvements based on classic laryngeal masks.
The i-gel airway (Intersurgical Ltd., Wokingham, United Kingdom), a representative disposable second-generation SGA, has been available in small sizes since 2010. Made from a soft medical-grade thermoplastic elastomer with a non-inflatable cuff, i-gel was designed to create an anatomical seal of the pharyngeal, laryngeal, and peri-laryngeal structures while avoiding compression trauma. Moreover, its built-in drainage channel allows for gastric catheter placement to facilitate the efflux of gastric fluids. Studies in children have reported its easy insertion, high oropharyngeal leak pressure (OLP), and few postoperative adverse effects[6,7]. However, as some studies have shown that its straighter design makes it prone to sliding out and becoming displaced, it should be cautiously used especially in very small children[8,9].
Compared with the non-inflatable mask i-gel, the inflatable mask Ambu Aura (Ambu A/S, Ballerup, Denmark) family of SGAs has a variety of types, such as AuraGain, AuraOnce (single use, preformed shaft), Aura40 (preformed shaft, reusable), AuraStraight (straight shaft), AuraFlex (flexible shaft), and Aura-i[10]. AuraGain is a newly developed disposable SGA with an inflatable cuff and a curved body. Its wide airway tube allows for a conduit for tracheal intubation. In addition, it has a second port providing gastric access for draining gastric content and air. AuraOnce is constructed from a single-piece polyvinyl chloride mould with the cuff and tube forming a 90° angle, which is designed to approximate the airway anatomy and is thus difficult to displace. The clinical safety and efficacy of both Ambu AuraOnce and Ambu AuraGain in paediatric and adult use have already been demonstrated[10-13].
Several studies have compared the efficacy and safety of i-gel and Ambu laryngeal masks in paediatric patients[14-18]; however, the results have been inconsistent. To our knowledge, no previous systematic review has been sufficiently comprehensive to draw a clinically meaningful conclusion about the use of the two devices in paediatric patients[19]. To address this deficiency, we conducted an updated systematic review and meta-analysis to compare the clinical performance and safety of the non-inflatable mask i-gel and the inflatable mask Ambu Aura.
This meta-analysis was performed following the recommendations in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement[20]. The meta-analysis was registered at PROSPERO (registration No. CRD42020168555).
Two reviewers (Li L and Xiong W) independently searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (PubMed), Web of Science, and Embase to evaluate all potentially eligible studies using the Medical Subject Headings and text words related to ‘Ambu’, ‘Aura’, ‘i-gel’, and ‘paediatric’, from the inception dates to April 20, 2020. The reference lists of the retrieved full texts were also tracked. Furthermore, original randomised controlled trials (RCTs) included in relevant systematic reviews or meta-analyses, as well as ongoing studies in ClinicalTrials.gov, metaRegister of Controlled Trials, and other national trial registries were also identified. Any disagreement was resolved with the corresponding author of the study (Jin X) through a discussion and consensus process (see Supplementary material).
Published RCTs in which the intervention involved the use of Ambu laryngeal masks and i-gel in anaesthetised paediatric patients (age < 18 years) were included. We excluded manikin studies and animal studies, which are susceptible to bias. We also excluded trials that compared the two devices in cases of difficult intubation, tracheostomy procedures, or cardiopulmonary resuscitation. We did not impose language restrictions.
Two reviewers (Wang YX and Liang Y) independently extracted the following data: lead author, publication year, type of surgery, airway size, participant characteristics (age, weight, sample size), risk of bias, and outcome indicators. The primary outcome of our study was OLP, which is the most commonly used quantitative indicator of seal in SGAs. We extracted the data recorded 10 min after SGA insertion to ensure consistency in the pooled analysis. The secondary outcomes included insertion time and success rate of insertion on the first attempt, which are important potential advantages of SGAs. We also aimed to assess adverse events that may reflect irritation to the vocal cords, such as coughing or laryngospasm.
Two reviewers (Bao D and Xang YX) used the Cochrane method to assess the quality of data reporting according to Review Manager software (version 5.1; The Cochrane Collaboration, Oxford, United Kingdom), considering seven different criteria: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), incomplete outcome data (attrition bias), selective outcome reporting, and other biases. The methodology of each trial was independently assessed by two authors and graded as having ‘high’, ‘low’, or ‘unclear’ risk of bias. Any disagreements were resolved with the corresponding author of the study (Jin X) through a discussion and consensus process.
The pooled risk ratio (RR) or the mean difference (MD) and the corresponding 95% confidence interval (CI) were calculated for each outcome using Review Manager software (version 5.3, The Cochrane Collaboration). We assessed the heterogeneity of the included studies based on both clinical diversity (e.g., measurement methods) and methodological diversity (risk of bias assessment). We considered an I2 statistic value of > 50% to indicate considerable heterogeneity, mandating further subgroup analyses according to mean age and Ambu subtype. We also performed sensitivity analyses to evaluate the effect of a single study on the overall estimate by sequentially excluding each study. A funnel plot analysis was performed to qualitatively report bias or assess publication bias when > 10 studies were included[21].
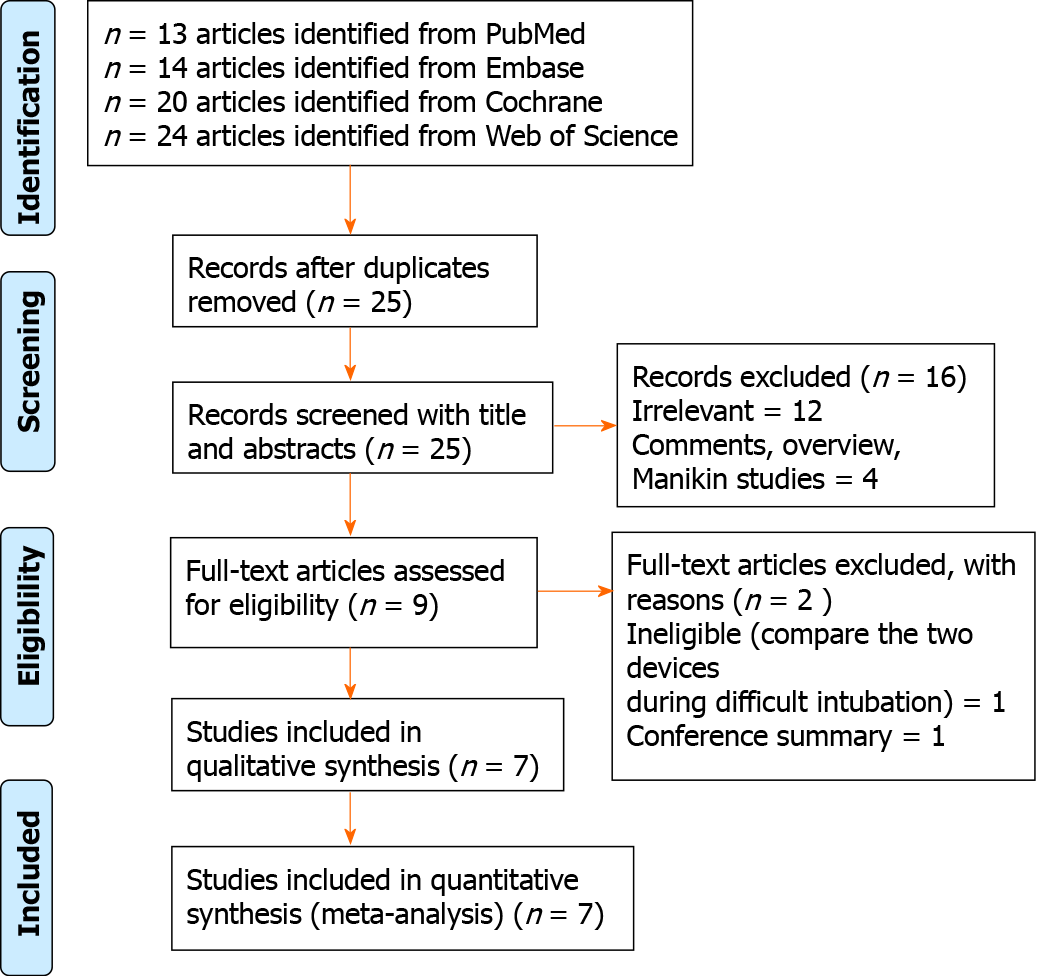
The database search identified 25 potentially relevant records after excluding 46 duplicates. On the basis of the titles, abstracts, and full texts, 18 records were removed, of which 5 were found to be comments, overview, manikin studies, and conference summary, and 1 article compared two devices in the setting of difficult intubation. Finally, seven eligible trials involving 667 paediatric patients in total (323 patients in the Ambu group and 344 patients in the i-gel group) were included in this meta-analysis[9,13-18]. A flowchart for identification is shown in Figure 1.
The seven included RCTs were published between 2011 and 2019 and were conducted in five different countries (China, Japan, Republic of Korea, Sweden, and Saudi Arabia). The sample size of the included trials ranged from 59 to 208. The patients in six studies underwent elective surgery, and three-dimensional magnetic resonance imaging of the head and neck was performed in one study. None of the studies administered neuromuscular blocking agents before laryngeal mask insertion, except for one trial[14]. Among the seven included RCTs, two studies did not report any funding sources[9,22]; one was not funded[13]; and the other four were sponsored by King Saud University[17,18], Asan Medical Center[15], or Seoul National University Hospital[14]. Further descriptions of the included trials are presented in Table 1.
| Ref. | Airway (intervention) | n | Airway size | Age (yr or mo) | Weight (kg) | OLP measurement method | Type of surgery | Ventilation | NBD | Depth of anesthesia for laryngeal mask placement and the proficiency of anesthesiologists | Primary outcomes |
| Theiler et al[9] (2011) | Aura once | 102 | Size 1.5 (5-9.9 kg); Size 2 (10-19.9 kg); Size 2.5 (20-29.9 kg); Size 3 (30-50 kg) | 6.2 ± 4.0 yr | 24.7 ± 11.6 | Manometric stability | Elective day surgery under general anaesthesia (urology, orthopaedics, visceral, dermatology) | Mechanical | No | Absence of motor and cardiovascular responses to the jaw thrust maneuver; anesthesiology staff at the University Hospital Bern | OLP |
| I-gel | 106 | Size 1.5 (5-9.9 kg); Size 2 (10-24.9 kg); Size 2.5 (25-34.9 kg); Size 3 (35-50 kg) | 6.3 ± 3.7 yr | 24.7 ± 11.2 | |||||||
| Gu et al[22] (2016) | AuraOnce | 32 | Size 2 | 29.28 ± 11.32 mo | 13.78 ± 2.55 | NR | Elective hypospadias repair surgery | Mechanical | No | After the eyelash reflex disappeared and the mandibularjoint loosened; NR | OLP and respiratory dynamic data |
| I-gel | 32 | 26.72 ± 12.16 mo | 13.95 ± 2.87 | ||||||||
| Alzahem et al[17] (2017) | Auraonce | 48 | NR | 32.3 ± 38 mo | 13.2 ± 8.3 | Noise detection | Elective surgery | Mechanical | No | Lack of a motor response to jaw thrust; had more than 20 years’ experience in the specialty and more than 1000 successful insertions of these SGADs | OLP |
| I-gel | 64 | 30.6 ± 37.4 mo | 12.7± 8.2 | ||||||||
| Aqil et al[18] (2017) | Auraonce | 30 | Size 1.5/2/2.5/3 | 4.62 ± 2.85 yr | 18.28 ± 7.23 | Noisedetection | 3D-MRI of the head and neck | Spontaneous breathing | No | NR | |
| I-gel | 29 | 4.76 ± 3.18 yr | 17.66 ± 7.47 | ||||||||
| Lee et al[14] (2019) | Auragain | 29 | Size 1.5 (5-10 kg); Size 2 (10-20 kg); Size 2.5 (20-30 kg) | 1.5 (0.75-5) yr | 12.2 (9.4-21.8) | Stethoscopic noise | Elective surgery | Mechanical | Yes | Muscle relaxation with rocuroniumand mask ventilation for 90 s; experienced anaesthesiologists | Safety margin |
| I-gel | 30 | Size 1.5 (5-12 kg); Size 2 (10-25 kg); Size 2.5 (25-35 kg) | 3 (0.75-6) yr | 13.9 (10.2-23.4) | |||||||
| Kim et al[15] (2019) | Auragain | 34 | Size 1.5 (5-9.9 kg); Size 2 (10-20 kg) | 23.5 ± 17.8 mo | 11.6 ± 3.3 | Manometric stability | Upper-/lower-extremity surgery under general anaesthesia | Mechanical | No | Absence of motor and cardiovascular responses to the jaw thrust maneuver; skilled and vastly experienced at inserting supraglottic airway devices | Requirement for additional airway manoeuvres |
| I-gel | 33 | 15.6 ± 11.5 mo | 10.5 ± 2.4 | ||||||||
| Mihara et al[19] (2019) | Auragain | 48 | Size 1.5/2.0/2.5 | 42 (14-66) mo | 14.4 ± 5.0 | Manometric stability | Elective surgery with an expected surgery time of < 2 h | Mechanical | No | Lack of a motor response to jaw thrust; had experience of SGA insertion of more than 20 times | OLP |
| I-gel | 50 | 42 (14-66) mo | 13.7 ± 5.4 |
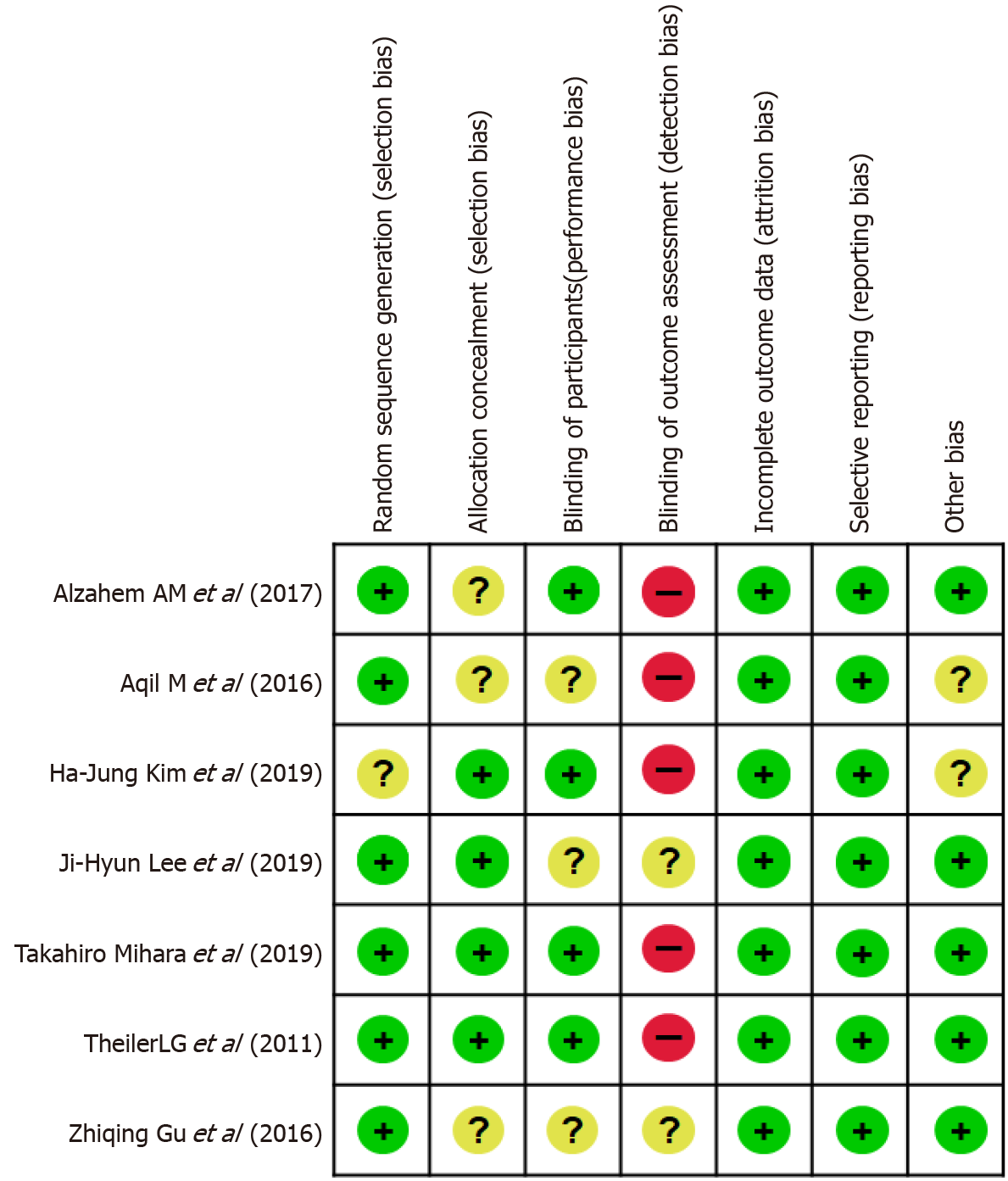
Six of the seven studies mentioned the specific methods used for random sequence generation, and four studies[9,13-15] performed allocation concealment using sealed or opaque envelopes. The assessment of postoperative adverse events in three studies was performed by a blinded investigator[13,17] or investigators who were not involved in the clinical procedure[9]. One study did not set blinding[15]. One study did not evaluate blinding[18]. The other two studies did not mention the specific method of blinding[14,22]. Three studies[9,13,15] used objective methods (manometric stability) of obtaining the OLP. However, it was obviously not possible in any study to blind the operator involved in airway management or the assessors of leak pressure. Funding sources were not stated in three studies[15,16,18], and it was not apparent whether any commercial sponsors were involved. The other studies had no obvious commercial involvements. The risk of bias is summarised in Figure 2. Aqil et al[18] reported randomisation, but did not describe the methods of allocation concealment and participant blinding. A sensitivity analysis was performed to determine the impact of their study on the results.
OLP, insertion time, success rate of insertion on the first attempt, and adverse events with the Ambu laryngeal mask and i-gel in anaesthetised paediatric patients were evaluated in this review.
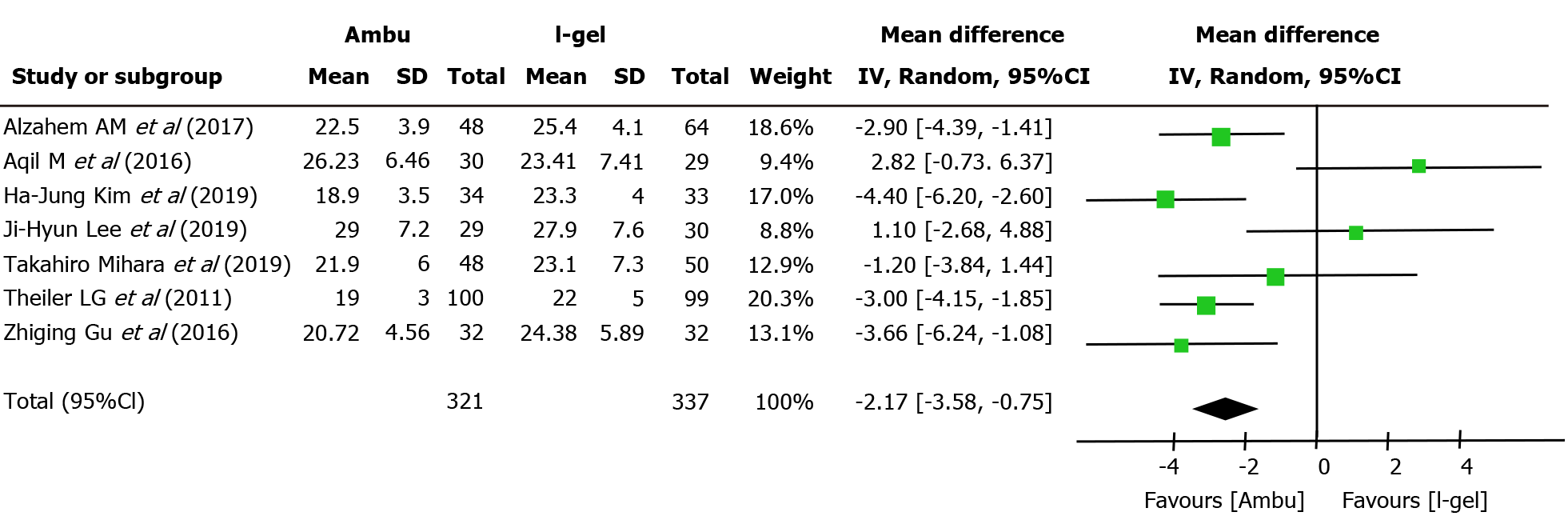
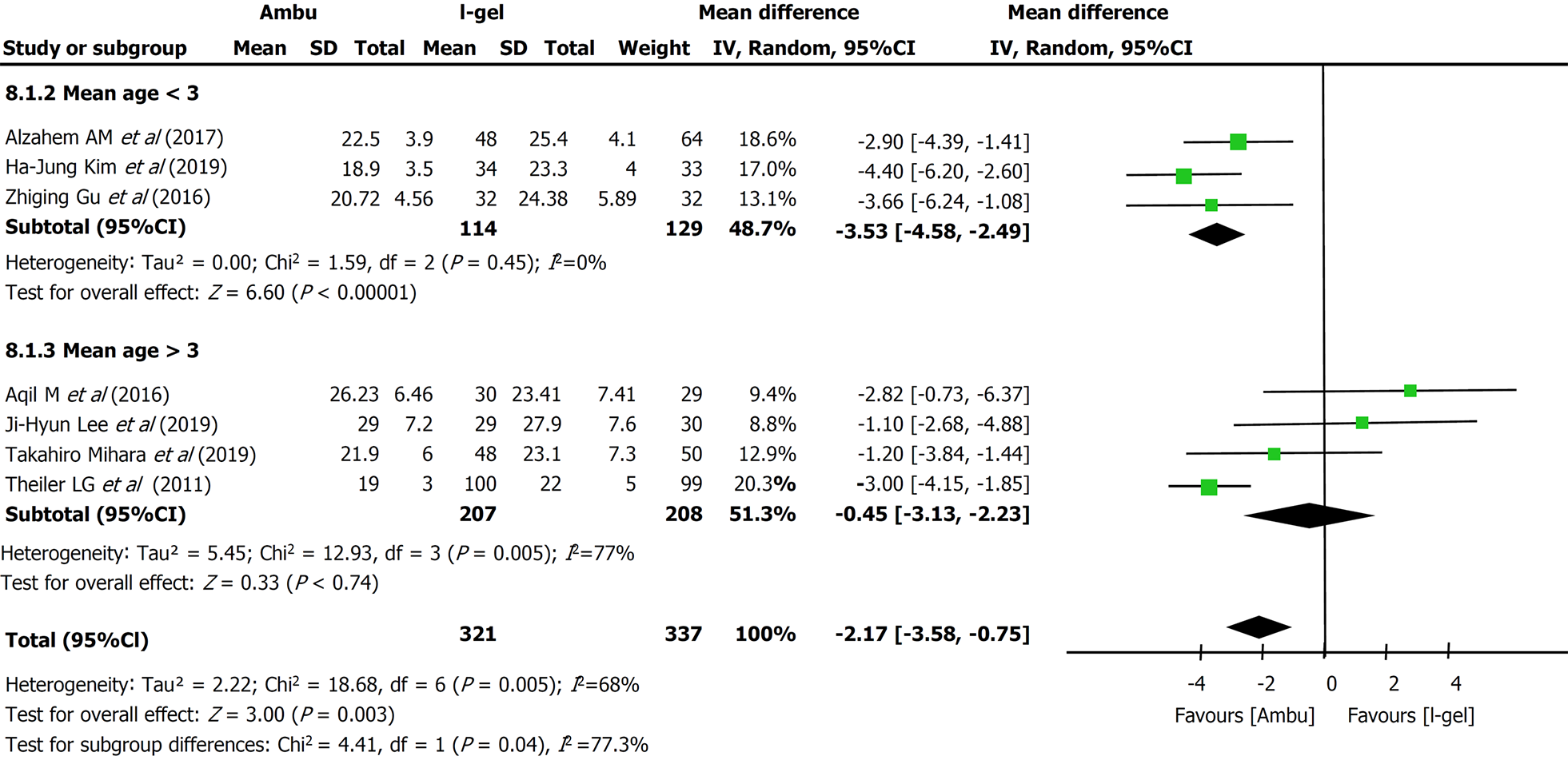
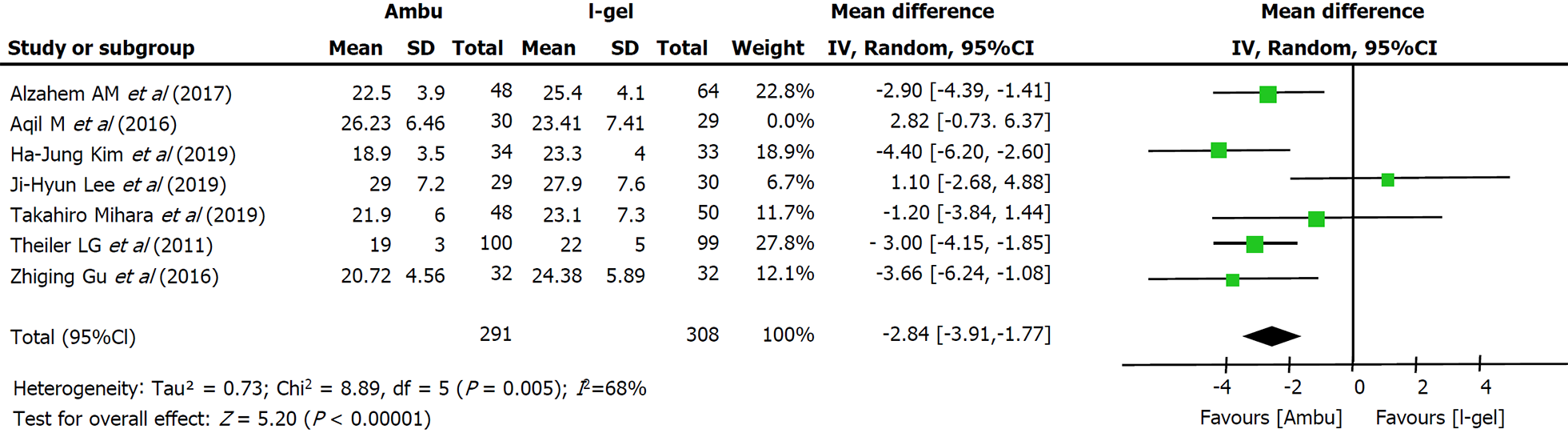
Primary outcome: All seven studies assessed the OLP of the two devices. The intracuff pressures were maintained at 20-40 cmH2O[13-15,22] or 60 cmH2O[9,17,18], and a fresh gas flow of 3 L/min was maintained to determine the OLP. The methods used to quantify OLP included audible noise detection[17,18], stethoscopic noise[14], and manometric stability[9,13,15]; however, one study did not describe the methodological details[22]. Excluding two studies[14,18], five studies individually showed higher mean leak pressures in the i-gel group. Overall, the combined results of all seven studies revealed that the mean leak pressure was higher in the i-gel group than in the Ambu group, with substantial heterogeneity (21.82 cmH2O for Ambu vs 23.98 cmH2O for i-gel, P = 0.003, 95%CI: -3.58 to -0.75, I2 = 68%, Mantel-Haenszel random model) (Figure 3). A subgroup analysis according to the mean age of the study participants (Figure 4) was performed to assess the impact of age, and the combined OLP from studies with participants whose mean age was < 3 years was significantly higher for i-gel (MD -3.53 cmH2O, 95%CI: -4.58 to -2.49, P < 0.00001, I2 = 0%). Pooled analysis from the other four studies in which the mean age was ≥ 3 years showed no significance between the two devices (MD -0.45 cmH2O, 95%CI: -3.12 to -2.23, P = 0.74, I2 = 77%). Another subgroup analysis according to the Ambu subtype was performed, and the pooled results revealed significant differences with a still high heterogeneity (AuraGain: MD -4.03 cmH2O, 95%CI: -7.37 to -0.72, P = 0.02, I2 = 77%; AmbuOnce: MD -2.24 cmH2O, 95%CI: -4.03 to -0.45, P = 0.02, I2 = 75%). The sensitivity analysis (Figure 5) suggested that the results were relatively stable, except when Aqil et al[18]’s study was excluded, which resulted in a lower heterogeneity (from 68% to 44%).
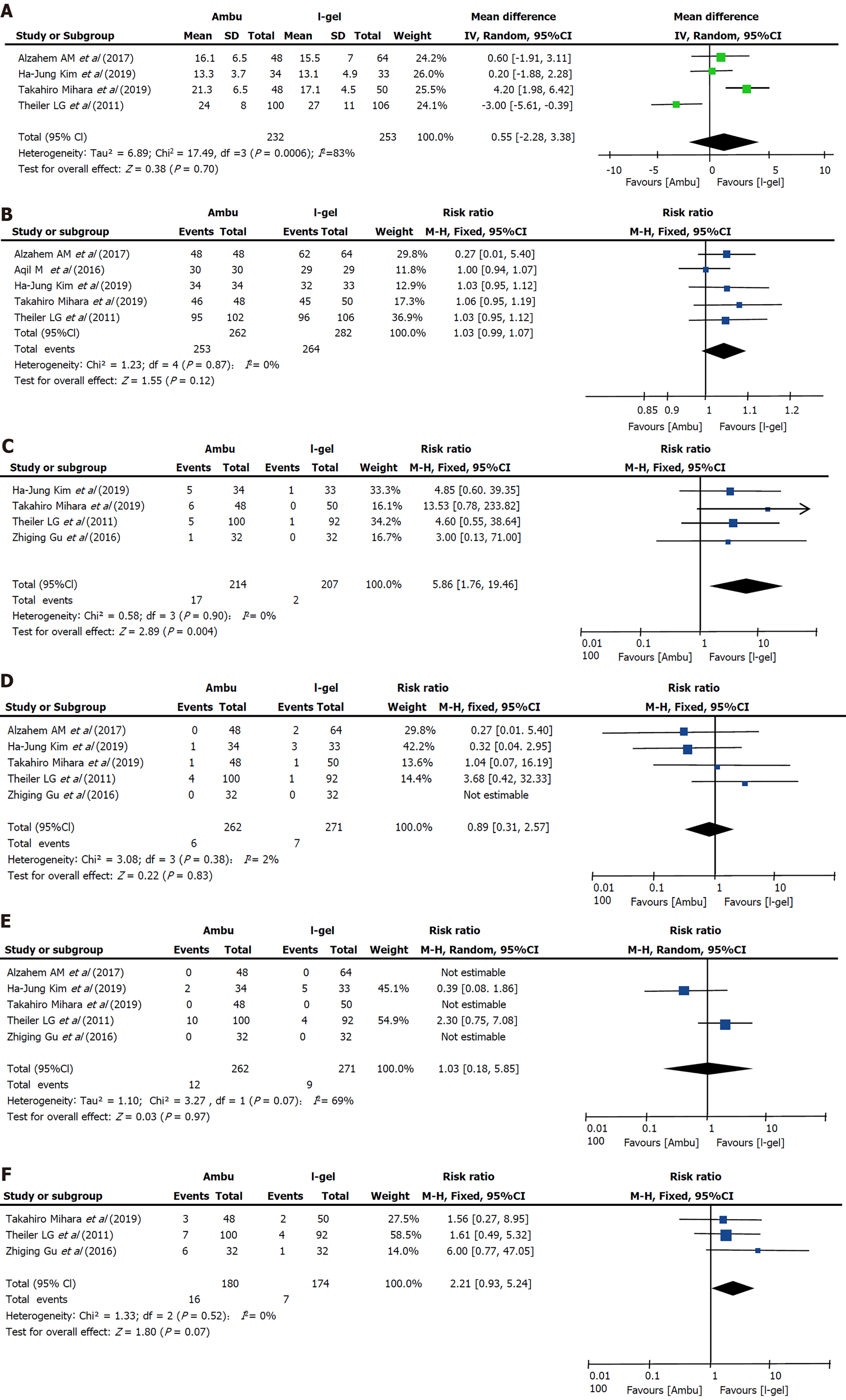
Insertion time: Data on insertion time were obtained from four trials[9,15,17,19] including 485 patients, and no clear evidence of differences was seen between the two devices (i-gel: 18.052 s vs Ambu: 18.602 s, P = 0.70, 95%CI: -2.28 to 3.38, I2 = 83%, Mantel-Haenszel random model) (Figure 6A). The most common depth of anesthesia for laryngeal mask placement was the lack of a motor response to jaw thrust[9,15,17,19] while one study did not describe it[18], and one described it as “muscle relaxation with rocuronium and mask ventilation for 90 s”[14]. The proficiency of anesthesiologists in these four studies is significantly different, from “who had experience of SGA insertion of more than 20 times”[19] to “who had more than 20 years' experience in the specialty and more than 1000 successful insertions of these SGADs”[17]. Insertion time was defined as the time from the moment the mask was removed and the SGA was picked up to the moment that stable capnography was traced on the monitor[13,15,17] or the achievement of sufficient ventilation[9]. We performed subgroup analysis to assess the effect of the Ambu type and patient age; however, the results were not altered and a large heterogeneity was still observed.
Success of insertion on first attempt: Five studies (n = 544; 262 in the Ambu group and 282 in the i-gel group)[9,15,17-19] reported successful insertion on the first attempt. One study[18] reported no instances of failed airway insertion for the two devices, whereas the average success rates in the other four studies were 94.5% for Ambu and 91.2% for i-gel. Although all four studies individually found that the success rate with i-gel was lower than that with Ambu, there was no evidence for differences in the success rate on the first attempt (RR 1.03, 95%CI: 0.99 to 1.07, P = 0.12, I2 = 0%) (Figure 6B).
Blood staining on the removed device: Four studies[9,13,15,22] that included 421 patients compared the incidence of blood staining on the removed device. Overall, blood staining occurred in 17 participants (7.9%) in the Ambu group and 2 participants (0.96%) in the i-gel group. A statistically significant reduction was found with i-gel (RR 5.86, 95%CI: 1.76 to 19.46, P = 0.004) (Figure 6C). The heterogeneity was low (I2 = 0%).
Desaturation: Among five studies evaluating the occurrence of desaturation, four studies[9,13,17,22] assessed desaturation as pulse oximetry saturation (SpO2) < 90% and one study did not specify a quantitative standard[15]. No evidence for a difference in desaturation between the two devices was found (RR 0.89, 95%CI: 0.31 to 2.57, P = 0.83, I2 = 2%) (Figure 6D).
Bronchospasm: Of the five studies[9,13,15,17,22] that evaluated bronchospasm, two studies[9,15] reported its occurrence. The overall incidence was 5.6% (12 of 214 participants) in the Ambu group and 4.3% (9 of 207 participants) in the i-gel group, and no clinically important differences were found between the two devices (RR 1.03, 95%CI: 0.18 to 5.85, P = 0.97, I2 = 69%) (Figure 6E). Notably, the incidence of laryn
Three studies[9,13,22] reported this outcome. Overall, coughing occurred in 16 participants (8.89%) in the Ambu group and in 7 participants (4.02%) in the i-gel group. The total incidence of coughing was 4.87% higher in the Ambu group; however, whether the difference is reasonable or not is uncertain (RR 2.21, 95%CI: 0.93 to 5.24, P = 0.07, I2 = 0%) (Figure 6F).
No cases of aspiration of gastric fluid were reported in any of the studies.
The principal finding of our meta-analysis was that i-gel provides a higher OLP than Ambu laryngeal masks with a low incidence of adverse events in anaesthetised paediatric patients, and we considered that i-gel may be superior to the Ambu laryngeal masks; however, the generalizability of the overall results is limited owing to the small number of published studies.
OLP is the most commonly used quantitative indicator of seal in SGAs. It indicates the degree of airway protection, successful SGA placement, and the feasibility of positive pressure ventilation[23]. Seven studies with 658 participants revealed a statistically higher (by 2.17 cm) OLP with i-gel. It showed that although I-gel laryngeal mask does not contain cuffs and cannot adjust the cuff pressure to achieve the purpose of sealing the airway as Ambu, the gel material of its cover achieve small amplitude shaping based on the children’s oropharyngeal structure to achieve better sealing effects. Higher oropharyngeal leak pressure results in better sealing of the hypopharynx, which may be beneficial in clinical settings requiring increased airway pressure and important for patients with aspiration and reflux risks. However, moderate to high heterogeneity (I2 = 68%) was suspected when we pooled the results, which was probably due to the clinical diversity of the OLP measurements. When exploring the heterogeneity, the hypotheses that the mean patient age and the subtype of Ambu were the causes of heterogeneity were not supported by the subgroup analyses, whereas there was a significant reduction in heterogeneity after sensitivity testing. The reasons for the high heterogeneity generated by Aqil et al[18]’s study may include the following two aspects: first, the risk of bias in this study was set from ‘unclear’ to ‘high’ at least once, indicating that the overall quality of evidence was very low, which resulted in potential methodological sources of heterogeneity among the evaluated studies. Second, spontaneous breathing mode was applied in Aqil et al[18]’s trial, whereas mechanical ventilation was required for elective surgery in the other trials, resulting in greater clinical heterogeneity. We downgraded this outcome from high quality to moderate quality because of the risk of bias with imprecision (small sample size). Previous meta-analyses yielded similar results, showing that the OLP with i-gel was higher than that with other laryngeal mask airways in children[22]. Although it cannot be sealed by cuff inflation, its shape, contour, and softness precisely fit with the anatomy and account for a better sealing effect of the pharyngeal, laryngeal, and peri-laryngeal structures.
A high rate of insertion success on the first attempt was reported in our meta analysis, and that insertion of the devices takes only about 18 s for both devices, demonstrating their effectiveness in anaesthetised paediatric patients and especially in emergency situations such as failure to intubate and ventilate. Although the non-inflatable cuff of i-gel can help save time, the final time was similar because additional airway intervention is required during i-gel insertion whereas the curved airway tube of Ambu may facilitate its insertion. The different definitions of insertion time, the use of muscle relaxants, the depth of anesthesia and the experience level of the anesthesiologist who inserted the laryngeal mask may be the sources of high heterogeneity. Notably, Theiler et al[9]’s study showed that the pediatric i-gel has a straighter ventilating tube than the adult model, which correlates with the tendency for the device to slide out. Kim et al[15]’s study also point out that the large-sized mask of i-gel is a disadvantage with respect to dislodgement. Therefore, it is necessary to choose the appropriately size and secure it with tape when applying i-gel in small children.
Most adverse events were infrequent and did not differ between the two devices, except for the higher incidence of blood staining on Ambu with significant differences. The significantly lower incidence of blood staining on i-gel in our results indicated a lower incidence of oral or pharyngeal mucosal injuries during the insertion or removal of the device. This factor might become the dominant advantages of i-gel laryngeal mask in pediatric anesthesia and indicated that awareness of compression damage of the throat induced by Ambu should be concerned. Previous comparative analyses[19,24] revealed that the risk of blood staining on i-gel was significantly lower than that on other SGAs. This may be because of its unique soft gel-like cuff and certain shape, which allow it to function in harmony with the anatomy, thus reducing compression and displacement trauma. In addition, the cuffs of Ambu is made of poly vinyl chloride, which are more likely to induce sore throat in pediatric patients. However, the study by Mihara et al[13] showed that there was no direct relationship with postoperative sore throat, and the clinical impact was unclear.
Insignificant differences were observed between the two devices in terms of the incidence of laryngospasm. However, as the depth of anaesthesia at extubation was different for each trial, the validity of combining different studies within this outcome is unquestionable. Both devices are efficient in protecting the airway from aspiration, and no cases of aspiration of gastric fluid were reported in any study.
This review had several limitations. First, although subgroup and sensitivity analyses were performed, there are many potential clinical and methodological sources of heterogeneity among the evaluated studies, including different methods of measurement of outcomes, use of neuromuscular blocking agents, proficiency of practitioners, different ventilation methods, and depth of anaesthesia. Second, publication bias could not be visually assessed using a funnel plot because the number of studies was too few to obtain valid results. Consequently, it is not yet possible to draw firm conclusions based on single-centre studies with limited available data.
In conclusion, we compared the clinical performance and safety of two types of SGAs in paediatric patients, and performed subgroup and sensitivity analyses to identify the sources of heterogeneity, including quality assessment. Both devices are suitable for airway management during general anaesthesia, with sufficient OLP, ease of insertion, and few adverse events. The results of the current meta-analysis suggest that i-gel is a better SGA in terms of superior OLP with a low risk of adverse events, which provided clinical evidence for the application of laryngeal mask in anaesthetised paediatric patients. However, it should be used with caution in paediatric patients. Further high-quality clinical studies are required to confirm our results.
The inflatable Ambu laryngeal masks and non-inflatable i-gel are two widely used paediatric supraglottic airway devices (SGAs) in routine anaesthesia and served as primary or back-up devices for difficult airway management. However, the clinical performance and safety of the two devices in paediatric patients are still unclear and warrant further investigation.
In this study, we aimed to perform a systematic review and meta-analysis on the clinical performance and safety of Ambu laryngeal masks and i-gel in anaesthetised paediatric patients. The results of this study may provide clinical evidence for the application of laryngeal mask in anaesthetised paediatric patients.
To perform a systematic review and meta-analysis on the clinical performance and safety of Ambu laryngeal masks and i-gel in anaesthetised paediatric patients.
We identified published randomised controlled trials (RCTs) in which the intervention involved the use of Ambu laryngeal masks and i-gel in anaesthetised paediatric patients (age < 18 years) in MEDLINE, Embase, Web of Science, Cochrane Central Register of Controlled Trials from the inception dates to April 20, 2020 . We assessed the oropharyngeal leak pressure (OLP) as the primary outcome. The secondary outcomes were insertion time, success rate of insertion on the first attempt, and incidence of adverse events.
Data from seven RCTs with a total of 667 paediatric patients were evaluated and showed that the mean OLP and the incidence of adverse events was lower in the non-inflatable i-gel group in anaesthetised paediatric patients.
The non-inflatable i-gel airway may provide a better seal with a low risk of adverse events and is therefore probably more suitable than the inflatable Ambu laryngeal mask airway in anaesthetised paediatric patients. However, the evidence is insufficient to allow making firm conclusions or to guide clinical practice, owing to the small number of relevant published studies.
Further high-quality clinical studies of the application of laryngeal masks in anaesthetised paediatric patients are required to confirm our results.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anesthesiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Poddighe D, Toida C S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | White MC, Cook TM, Stoddart PA. A critique of elective pediatric supraglottic airway devices. Paediatr Anaesth. 2009;19 Suppl 1:55-65. [PubMed] |
| 2. | Goel D, Shah D, Hinder M, Tracy M. Laryngeal mask airway use during neonatal resuscitation: a survey of practice across newborn intensive care units and neonatal retrieval services in Australian New Zealand Neonatal Network. J Paediatr Child Health. 2020;56:1346-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Luce V, Harkouk H, Brasher C, Michelet D, Hilly J, Maesani M, Diallo T, Mangalsuren N, Nivoche Y, Dahmani S. Supraglottic airway devices vs tracheal intubation in children: a quantitative meta-analysis of respiratory complications. Paediatr Anaesth. 2014;24:1088-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Keller C, Brimacombe J, Bittersohl J, Lirk P, von Goedecke A. Aspiration and the laryngeal mask airway: three cases and a review of the literature. Br J Anaesth. 2004;93:579-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Griffin RM, Hatcher IS. Aspiration pneumonia and the laryngeal mask airway. Anaesthesia. 1990;45:1039-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Keijzer C, Buitelaar DR, Efthymiou KM, Srámek M, ten Cate J, Ronday M, Stoppa T, Huitink JM, Schutte PF. A comparison of postoperative throat and neck complaints after the use of the i-gel and the La Premiere disposable laryngeal mask: a double-blinded, randomized, controlled trial. Anesth Analg. 2009;109:1092-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Richez B, Saltel L, Banchereau F, Torrielli R, Cros AM. A new single use supraglottic airway device with a noninflatable cuff and an esophageal vent: an observational study of the i-gel. Anesth Analg. 2008;106:1137-1139, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Hughes C, Place K, Berg S, Mason D. A clinical evaluation of the I-gel ™ supraglottic airway device in children. Paediatr Anaesth. 2012;22:765-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Theiler LG, Kleine-Brueggeney M, Luepold B, Stucki F, Seiler S, Urwyler N, Greif R. Performance of the pediatric-sized i-gel compared with the Ambu AuraOnce laryngeal mask in anesthetized and ventilated children. Anesthesiology. 2011;115:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Goyal R. Small is the new big: An overview of newer supraglottic airways for children. J Anaesthesiol Clin Pharmacol. 2015;31:440-449. [PubMed] |
| 11. | Stögermüller B, Ofner S, Ziegler B, Keller C, Moser B, Gasteiger L. Ambu® Aura Gain™ vs Ambu® Aura Once™ in children: a randomized, crossover study assessing oropharyngeal leak pressure and fibreoptic position. Can J Anaesth. 2019;66:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | López AM, Valero R, Bovaira P, Pons M, Sala-Blanch X, Anglada T. A clinical evaluation of four disposable laryngeal masks in adult patients. J Clin Anesth. 2008;20:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Mihara T, Nakayama R, Ka K, Goto T. Comparison of the clinical performance of i-gel and Ambu AuraGain in children: A randomised noninferiority clinical trial. Eur J Anaesthesiol. 2019;36:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Lee JH, Park S, Jang YE, Kim EH, Kim HS, Kim JT. The distance between the glottis and the cuff of a tracheal tube placed through three supraglottic airway devices in children: A randomised controlled trial. Eur J Anaesthesiol. 2019;36:721-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Kim HJ, Park HS, Kim SY, Ro YJ, Yang HS, Koh WU. A Randomized Controlled Trial Comparing Ambu AuraGain and i-gel in Young Pediatric Patients. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Yang GZ, Xue FS, Li HX, Liu YY. Comparing i-gel and Ambu AuraOnce laryngeal mask airway in pediatric patients. Saudi Med J. 2017;38:1262-1263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Alzahem AM, Aqil M, Alzahrani TA, Aljazaeri AH. Ambu AuraOnce vs i-gel laryngeal mask airway in infants and children undergoing surgical procedures. A randomized controlled trial. Saudi Med J. 2017;38:482-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Aqil M, Delvi B, Abujamea A, Alzahrani T, Alzahem A, Mansoor S, Aljazaeri A. Spatial relationship of i-gel® and Ambu® AuraOnceTM on pediatric airway: a randomized comparison based on three dimensional magnetic resonance imaging. Minerva Anestesiol. 2017;83:23-32. [PubMed] |
| 19. | Mihara T, Asakura A, Owada G, Yokoi A, Ka K, Goto T. A network meta-analysis of the clinical properties of various types of supraglottic airway device in children. Anaesthesia. 2017;72:1251-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13355] [Article Influence: 834.7] [Reference Citation Analysis (0)] |
| 21. | Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997;315:1533-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1364] [Cited by in RCA: 1448] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 22. | Gu Z, Jin Q, Liu J, Chen L. Observation of ventilation effects of I-gel™, Supreme™ and Ambu AuraOnce™ with respiratory dynamics monitoring in small children. J Clin Monit Comput. 2017;31:1035-1041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Seet E, Rajeev S, Firoz T, Yousaf F, Wong J, Wong DT, Chung F. Safety and efficacy of laryngeal mask airway Supreme vs laryngeal mask airway ProSeal: a randomized controlled trial. Eur J Anaesthesiol. 2010;27:602-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |