Published online Feb 6, 2022. doi: 10.12998/wjcc.v10.i4.1164
Peer-review started: September 15, 2021
First decision: October 18, 2021
Revised: October 27, 2021
Accepted: December 22, 2021
Article in press: December 22, 2021
Published online: February 6, 2022
Processing time: 130 Days and 20.5 Hours
Lung cancer is one of the deadliest cancers in the world with the highest incidence and mortality rate among all cancers. Non-small cell lung cancer (NSCLC) accounts for approximately 80% of primary lung cancer. However, efficacy and safety of the current regimens for NSCLC is unsatisfactory. Therefore, there has been an increasing urgency for development of potential therapeutic therapies for NSCLC.
To investigate the therapeutic outcomes and safety of continuous intravenous infusion of recombinant human endostatin (Rh-endostain) using an infusion pump in retreated advanced NSCLC.
Patients with retreated advanced NSCLC who were admitted to Zhejiang Provincial People's Hospital from October 2017 to April 2019 were recruited. These patients received continuous intravenous infusion of Rh-endostain using an infusion pump. Objective response rate (ORR), clinical benefit rate (CBR), median progression-free survival (mPFS), and incidences of adverse events (AEs) were analyzed after treatment.
A total of 45 patients with retreated advanced NSCLC were included, and all of them were evaluated. In these patients, ORR was 22.2%, CBR was 84.4%, and mPFS was 5.3 mo. The following AEs were observed, decreased hemoglobin (34 cases, 75.6%), nausea/vomiting (32 cases, 71.1%), elevated transaminase (24 cases, 53.3%), leukopenia (16 cases, 35.6%), thrombocytopenia (14 cases, 31.1%), and constipation (1 case, 3.4%). None of the patients had leukopenia, nausea /vomiting, and constipation of grade III and above.
The patients showed improved adherence to 5-d continuous intravenous infusion of Rh-endostain using an infusion pump. Favorable efficacy and safety of this treatment regimen were achieved in retreated advanced NSCLC.
Core Tip: Lung cancer is one of the malignancies with the highest incidence and mortality worldwide. However, the efficacy and safety of the current regimens is unsatisfactory. Therefore, the development and upgrade of potential therapies that are more effective and less toxic is warranted. This is a retrospective study to investigate the efficacy and safety of 5-d continuous intravenous infusion of Recombinant human endostatin (Rh-endostain) in advanced non-small cell lung cancer (NSCLC) patients. Our results revealed that 5-day continuous intravenous infusion of Rh-endostain using infusion pump improved patient adherence and showed favorable efficacy and safety, which brought significant clinical benefits to advanced NSCLC patients.
- Citation: Qin ZQ, Yang SF, Chen Y, Hong CJ, Zhao TW, Yuan GR, Yang L, Gao L, Wang X, Lu LQ. Continuous intravenous infusion of recombinant human endostatin using infusion pump plus chemotherapy in non-small cell lung cancer. World J Clin Cases 2022; 10(4): 1164-1171
- URL: https://www.wjgnet.com/2307-8960/full/v10/i4/1164.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i4.1164
Lung cancer is one of the malignancies with the highest incidence and mortality worldwide[1]. By pathological typing, lung cancer is divided into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC, accounting for about 80%)[2]. Approximately 60%-70% of NSCLC patients are diagnosed at a late stage. The median survival of stage IV patients is less than nine months. Chemotherapy regimens for NSCLC mainly include platinum-containing double-drug chemotherapy or gemcitabine and docetaxel monotherapy[3]. However, the efficacy and safety of the current regimens is unsatisfactory. Therefore, the development and upgrade of treatments that are more effective, better tolerated, and less toxic is urgently warranted.
In 1971, Professor Folkman from the Harvard Medical School first proposed that the growth and spread of malignancies depended on tumor angiogenesis[4,5]. Vascular endothelial growth factor (VEGF) and its receptor are important factors in tumor angiogenesis. A synergistic effect may result from the combined use of antiangiogenic drugs and chemotherapy. Recombinant human endostatin (Rh-endostain, Endostar) was approved by the National Medical Products Administration in September 2005 for the treatment of NSCLC. Previous report revealed that Rh-endostain could inhibit tumor angiogenesis, the proliferation and migration of endothelial cells by down-regulating various angiogenic factors, such as VEGF[6-8]. Besides, Rh-endostain could also regulate tumor microenvironment normalization, thereby promoting the proliferation of endothelial cells and podocytes and increasing the blood supply. As a result, tumor cells have an increased sensitivity to chemotherapy and radiotherapy[9,10]. In a clinical study of Rh-endostain plus chemotherapy for the treatment of advanced NSCLC, the patients' prognosis was noticeably improved, and the anti-tumor effects of this regimen were demonstrated. Rh-endostain was usually administered by intermittent intravenous infusion, 3-4 times per day for 14 consecutive days. However, patients might have poor adherence to this dosing regimen, which remained to be optimized. Several clinical studies were conducted based on stability tests of the continuous intravenous infusion of Rh-endostain using an infusion pump[11-13]. In brief, the results showed that this administration regimen of Rh-endostain was convenient and guaranteed patient adherence. In addition, this administration regimen was conducive to maintaining the steady-state concentration of Rh-endostain in the blood, which was widely accepted and used clinically[14-19].
The present study observed the efficacy and safety of 5-d continuous intravenous infusion of Rh-endostain in advanced NSCLC patients, which may provide further valuable clinical data for the treatment of advanced NSCLC.
The medical records of 45 NSCLC patients who were treated at Zhejiang Provincial People's Hospital from October 2017 to April 2019 were retrospectively analyzed. Eligibility of the patients was assessed using the following inclusion criteria: (1) NSCLC confirmed by pathohistology or cytology; (2) Retreated advanced NSCLC (stage IV according to the American Joint Committee on Cancer staging system); (3) Eastern Cooperative Oncology Group performance status score, 0-2; (4) Measurable and evaluated lesions without contraindications; and (5) Data on the following examinations were available: routine blood and urine tests, liver and kidney function tests, cardiac enzyme profile, and electrocardiogram, computed tomography scan of the chest, abdomen and brain, and whole-body bone scan after two cycles of treatment. The patients were excluded if any of the following exclusion criteria were met: (1) Women who were pregnant or lactating; (2) Hemorrhagic tendency, history of thrombosis, or currently taking anticoagulant medication; (3) Abnormal organ functions and unable to tolerate the side effects of Rh-endostain and chemotherapy; and (4) The presence of other malignancies. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of Zhejiang Provincial People’s Hospital (People’s Hospital of Hangzhou Medical College) (2021QT290). Individual consent for this retrospective analysis was waived.
Rh-endostain (Shandong Simcere-Medgenn Bio-pharmaceutical Co., Ltd., 15 mg/bottle) was administered concomitantly with chemotherapy. Rh-endostain was given by continuous intravenous infusion using an infusion pump at a dose of 210 mg for 5 consecutive days. Each treatment cycle lasted 21 d (q21d). The chemotherapy regimens included the following: (1) AP regimen: Pemetrexed 500 mg d1 + carboplatin Area under roc curve (AUC) = 5-6 (or cisplatin 75 mg/m2) d1 q21d; (2) GP regimen: Gemcitabine 1000-1250 mg/m2 d1 + cisplatin 75 mg/m2 (or carboplatin AUC = 5-6) d1 q21d; (3) Pemetrexed monotherapy: Pemetrexed, 500 mg/m2 d1 q21d; and (4) Docetaxel monotherapy: Docetaxel, 60-75 mg/m2 d1 q21d. Tumors were assessed as planned until disease progression.
Treatment efficacy was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) criteria[20]. The efficacy indicators were as follows: complete response (CR, defined as disappearance of all target lesions, no new lesions, and return of tumor markers to normal, for at least 4 wk), partial response (PR, defined as the sum of the decrease in the maximum diameters of the target lesions by more than 30%, for at least 4 wk), stable disease (SD, defined as the sum of the decrease in maximum diameters of the target lesions, yet not reaching the standard of PR or being increased yetnot reaching the standard of progressed disease), and progressed disease (PD, defined as the sum of the increase in the maximum diameter of the target focus by at least 20%, or the appearance of new lesions). Objective response rate (ORR) = (CR+PR)/(total number of cases in each group), and clinical benefit rate (CBR) were determined. Progression-free survival (PFS) was defined as the time from the first administration to disease progression confirmed by objective evidence or death due to any cause. Adverse events (AEs) were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE version 5.0), and were recorded.
Data analysis were performed using SPSS 22.0 software. The ORR and CBR in patients with different pathological types of NSCLC who received different treatment regimens were compared using the χ2 test. P < 0.05 indicated a significant difference. All tests were two-sided. The survival curve was estimated using the Kaplan–Meier method.
A total of 45 NSCLC patients who were treated at Zhejiang Provincial People's Hospital from October 2017 to April 2019 were retrospectively analyzed in this study, and baseline characteristics were shown in Table 1. The median age was 65 years (Interquartile range: 35-83 years). Besides, 27 of the NSCLC patients in this study were male, with the rest 18 of the patients being female. Among them, 10 NSCLC patients (22.2%) were Squamous cell carcinoma, with the rest of them being Adenocarcinoma type. Besides, the chemotherapy drugs combined with Rh-endostain included AP (n = 2), GP (n = 2), Pemetrexed monotherapy (n = 16) and Docetaxel monotherapy (n = 11).
| Features | Basic information |
| Age | 65 yr (35-83 yr) |
| Gender | |
| Male | 27 |
| Female | 18 |
| Pathological type | |
| Squamous cell carcinoma | 10 |
| Adenocarcinoma | 35 |
| Chemotherapy regimen | |
| AP | 13 |
| GP | 5 |
| Pemetrexed monotherapy | 16 |
| Docetaxel monotherapy | 11 |
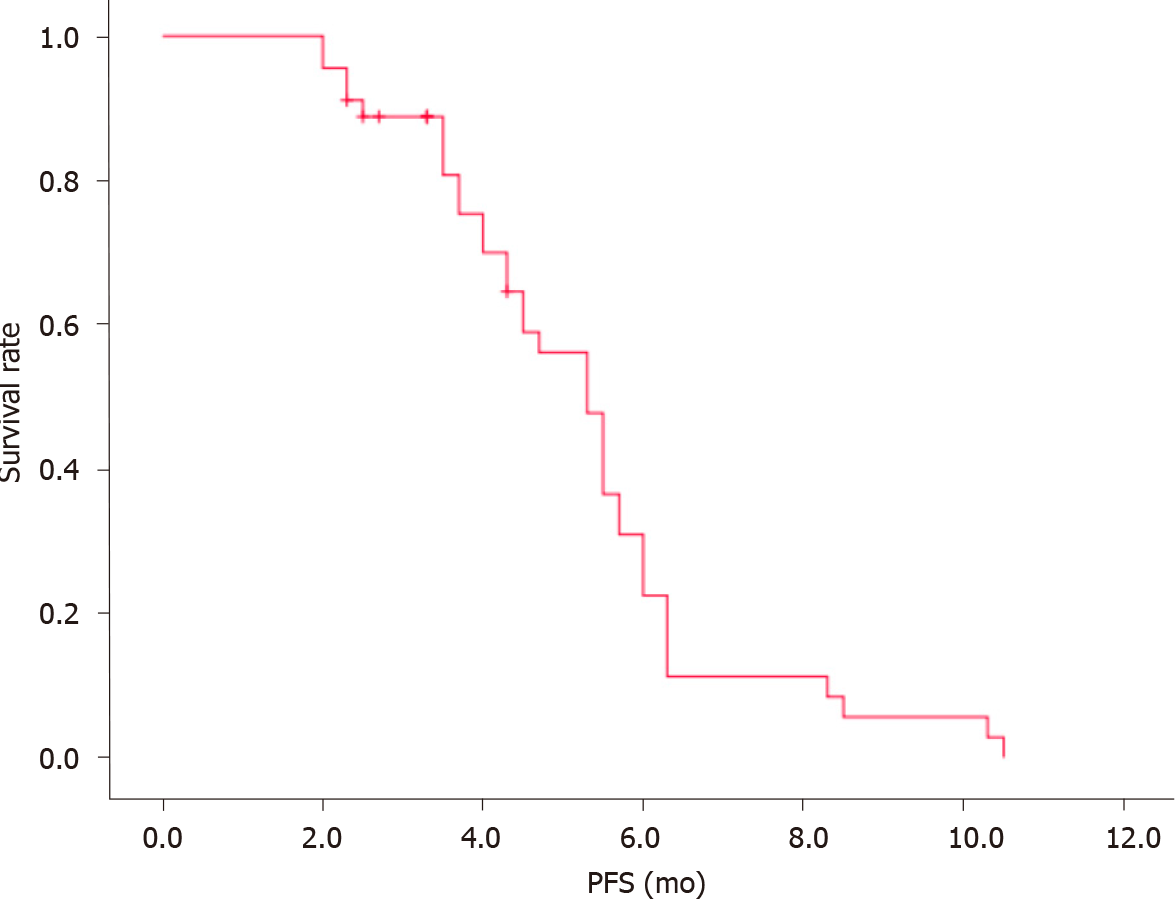
The clinical data of all 45 enrolled patients were evaluated. There were no cases of CR, 10 cases of PR, 28 cases of SD, and 7 cases of PD. ORR was 22.2%, CBR was 84.4%, and median progression-free survival (mPFS) was 5.3 mo (Figure 1). The patients were also stratified by pathological type and chemotherapy regimens. No significant differences were observed in patients with different types of NSCLC who received different treatments (P > 0.05). Further details were provided in Table 2.
| Clinicopathological features | Case | CR | PR | SD | PD | ORR | CBR | P value |
| Pathological type | 0.848 | |||||||
| Squamous cell carcinoma | 10 | 0 | 2 | 6 | 2 | 20.0% | 80.0% | |
| Adenocarcinoma | 35 | 0 | 8 | 22 | 5 | 22.9% | 85.7% | |
| Chemotherapy regimen | 0.411 | |||||||
| Dual-drug chemotherapy + Rh-endostain | 18 | 0 | 5 | 11 | 2 | 27.8% | 88.9% | |
| Single-drug chemotherapy + Rh-endostain | 27 | 0 | 5 | 17 | 5 | 18.5% | 81.5% |
The following AEs were observed as follows decreased hemoglobin (34 cases, 75.6%), nausea/vomiting (32 cases, 71.1%), elevated transaminase (24 cases, 53.3%), leuko
| AEs | Any grade | Grade III and above |
| Decreased hemoglobin | 34 (75.6) | 5 (11.1) |
| Leukopenia | 16 (35.6) | 0 (0) |
| Thrombocytopenia | 14 (31.1) | 1 (2.2) |
| Elevated transaminase | 24 (53.3) | 7 (15.6) |
| Nausea/vomiting | 32 (71.1) | 0 (0) |
| Constipation | 1 (3.4) | 0 |
Several studies evaluated the efficacy and safety of Rh-endostain plus platinum-containing double-drug chemotherapy. However, there were only limited data on the combination of Rh-endostain plus monodrug chemotherapy or platinum-containing double-drug chemotherapy as the second-line regimen and below in advanced NSCLC patients. Patients generally showed lower adherence to intravenous drip infusion in previous studies[21,22]. In the present study, the efficacy and safety of Rh-endostain administered by continuous intravenous infusion for five days using an infusion pump in retreated advanced NSCLC were assessed.
Our study was observational in nature. All the enrolled patients had stage IV NSCLC in which no driver genes were identified. The chemotherapy regimens used were primarily the platinum-containing double-drug regimen and monodrug therapy (monodrug therapy was favored as a later-line treatment or for patients in a poor general condition (PS ≥2), such as gemcitabine and docetaxel monotherapy). The above chemotherapy regimens combined with rh-endostain, as a targeted antiangiogenic agent, can normalize tumor vessels, sensitize tumor cells to chemotherapy, and improve patient prognosis. Our results showed that in the 45 enrolled patients, ORR was 22.2%, CBR was 84.4%, and mPFS was 5.3 mo. The incidences of hematological and non-hematological toxicities of grade III and above were low. No Rh-endostain-related cardiac functional abnormalities, as reported previously, occurred in our study. Furthermore, the efficacy was compared in patients with different patho
Some previous studies have reported similar findings. For example, five days of intravenous infusion of Rh-endostain using an infusion pump as first-line treatment achieved similar efficacy to a continuous intravenous drip in advanced NSCLC patients (PFS: 6.0 mo vs 3.8 mo, P = 0.10). In addition, the incidences of AEs did not increase[23]. The use of an infusion pump improved the adherence of patients to rh-endostain treatment. The short-term efficacy and tolerance of Rh-endostain using the above treatment regimen with concurrent radiochemotherapy in unresectable stage Ⅲ NSCLC were satisfactory[24]. Rh-endostain administered by continuous intravenous infusion using an infusion pump plus concurrent radiochemotherapy was associated with a low incidence of AEs in advanced NSCLC patients. These patients also reported a higher level of comfort and demonstrated better adherence. Therefore, the quality of medical care and nursing was improved[25].
Limitations in this retrospective analysis should never be neglected. For one thing, this was a retrospective study with a small sample size, and prospective clinical randomized controlled trials will be conducted for further validation of the efficacy and safety of continuous intravenous infusion of Rh-endostatin combined with chemotherapy in retreated advanced NSCLC. For another, heterogeneity of patients enrolled in this study should be further considered to reduce potential selective bias.
Taken together, 5-d continuous intravenous infusion of Rh-endostain using an infusion pump improved patient adherence, bringing significant clinical benefits to the patients. Further clinical studies were warranted to further confirm the efficacy and safety of this regimen, in order to improve the prognosis of patients with advanced NSCLC.
To date, current available treatment options for non-small cell lung cancer (NSCLC) are associated with significant limitations in safety and efficacy. Therefore, develo
This study mainly evaluated the efficacy and safety of continuous intravenous infusion of recombinant human endostatin (Rh-endostain) using an infusion pump in patients with retreated advanced NSCLC.
This study aimed to investigate the efficacy and safety of continuous intravenous infusion of Rh-endostain in retreated advanced NSCLC patients.
Forty-five patients from Zhejiang Provincial People's Hospital received continuous intravenous infusion of Rh-endostain using an infusion pump. Objective response rate (ORR), clinical benefit rate (CBR), median progression-free survival (mPFS), and adverse events were analyzed after treatment.
In these 45 patients, ORR was 22.2%, CBR was 84.4%, and mPFS was 5.3 mo. The following AEs were observed as follows, decreased hemoglobin (34 cases, 75.6%), nausea/vomiting (32 cases, 71.1%), elevated transaminase (24 cases, 53.3%), leuko
Five-day continuous intravenous infusion of Rh-endostain using an infusion pump improved patient adherence, and brought about favorable efficacy and safety in retreated advanced NSCLC.
Prospective clinical randomized controlled trials will be conducted for further validation of the efficacy and safety of continuous intravenous infusion of Rh-endostatin combined with chemotherapy in retreated advanced NSCLC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang J, Weinberg F S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Zheng RS, Sun KX, Zhang SW, Zeng HM, Zou XN, Chen R, Gu XY, Wei WW, He J. [Report of cancer epidemiology in China, 2015]. Zhonghua Zhong Liu Za Zhi. 2019;41:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 276] [Reference Citation Analysis (0)] |
| 2. | Di JZ, Peng JY, Wang ZG. Prevalence, clinicopathological characteristics, treatment, and prognosis of intestinal metastasis of primary lung cancer: a comprehensive review. Surg Oncol. 2014;23:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Lv Y, Cao Z, Pan J, Gong E, Zheng H, Cai X. Pemetrexed-based first-line chemotherapy had particularly prominent objective response rate for advanced NSCLC: A network meta-analysis. Open Med (Wars). 2021;16:183-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Gasparini G. Remembering Judah Moses Folkma. Int J Biol Markers. 2008;23:63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Sitohy B, Nagy JA, Dvorak HF. Anti-VEGF/VEGFR therapy for cancer: reassessing the target. Cancer Res. 2012;72:1909-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 277] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 6. | Dhanabal M, Ramchandran R, Waterman MJ, Lu H, Knebelmann B, Segal M, Sukhatme VP. Endostatin induces endothelial cell apoptosis. J Biol Chem. 1999;274:11721-11726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 424] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 7. | Jia Y, Liu M, Huang W, Wang Z, He Y, Wu J, Ren S, Ju Y, Geng R, Li Z. Recombinant human endostatin endostar inhibits tumor growth and metastasis in a mouse xenograft model of colon cancer. Pathol Oncol Res. 2012;18:315-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Yu M, Han Y, Zhuo H, Zhang S. Endostar, a Modified Endostatin Induces Vascular Normalization to Improve Chemotherapy Efficacy Through Suppression of Src Signaling Pathway. Cancer Biother Radiopharm. 2018;33:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Bedford L, Lowe J, Dick LR, Mayer RJ, Brownell JE. Ubiquitin-like protein conjugation and the ubiquitin-proteasome system as drug targets. Nat Rev Drug Discov. 2011;10:29-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 454] [Cited by in RCA: 459] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 10. | Yang Y, Kitagaki J, Wang H, Hou DX, Perantoni AO. Targeting the ubiquitin-proteasome system for cancer therapy. Cancer Sci. 2009;100:24-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Tong Y, Zhong K, Tian H, Gao X, Xu X, Yin X, Yao W. Characterization of a monoPEG20000-Endostar. Int J Biol Macromol. 2010;46:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Yu XC, Shou J, Zhou Q. [Stability Study of Endostatin in PVC, Non-PVC Infusion Bags and Drug Infusion Pumps]. Zhongguo Xiandai Yingyong Yaoxue. 2013;30:1299-1302. |
| 13. | Jiang LP, Zou C, Yuan X, Luo W, Wen Y, Chen Y. N-terminal modification increases the stability of the recombinant human endostatin in vitro. Biotechnol Appl Biochem. 2009;54:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Hu DJ, Hu XW, Wang Y. [Clinical observation of recombinant human endostatin durative transfusion combined with window period chemotherapy in advanced lung squamous cell carcinoma]. Linchuang Feike Zazhi. 2018;23:1030-1034. |
| 15. | Li L, Tao L, Lang J, Zhang J, Li B, Ke X. [Study on Concomitant Radiotherapy and Chemotherapy Combinating with Endostatin for IIIB and IV Stage Non-small Cell Lung Cancer]. Zhongliu Yufang Yu Zhiliao. 2017;30:265-270. [DOI] [Full Text] |
| 16. | Yao D, Shen H, Huang J, Yuan Y, Dai H. Influence of different drug delivery methods for Endostar combined with a gemcitabine/cisplatin regimen in locally advanced or metastatic lung squamous cell carcinoma: A retrospective observational study. Medicine (Baltimore). 2018;97:e11822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Zhai Y, Ma H, Hui Z, Zhao L, Li D, Liang J, Wang X, Xu L, Chen B, Tang Y, Wu R, Xu Y, Pang Q, Chen M, Wang L. HELPER study: A phase II trial of continuous infusion of endostar combined with concurrent etoposide plus cisplatin and radiotherapy for treatment of unresectable stage III non-small-cell lung cancer. Radiother Oncol. 2019;131:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Ma HL, Hui ZG, Zhao LJ, Xu YJ, Zhai YR, Wu RY, Pang QS, Zhu GY, Li DM, Tang Yu. [Continuous intravenous pumping (CIP) of recombinant human endostatin (Endostar) combined with concurrent radiochemotherapy in patients with unresectable stage III non-small-cell lung cancer: preliminary data of a prospective multicenter phase II clinical trial]. Zhonghua Fangshe Zhongliuxue Zazhi. 2016;25:114-119. [DOI] [Full Text] |
| 19. | Shen JQ, Shao QY, Ye YJ. [Adverse reactions and management in endostar combined with concurrent chemoradiotherapy for inoperable stage III non-small cell lung cancer. Chinese Journal of General Practice]. Zhonghua Quanke Yixue. 2017;15:1805-1807. [DOI] [Full Text] |
| 20. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21455] [Article Influence: 1340.9] [Reference Citation Analysis (1)] |
| 21. | Hu W, Fang J, Nie J, Dai L, Zhang J, Chen X, Ma X, Tian G, Wu D, Han S, Han J, Wang Y, Long J. Efficacy and safety of extended use of platinum-based doublet chemotherapy plus endostatin in patients with advanced nonsmall cell lung cancer. Medicine (Baltimore). 2016;95:e4183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Zhou S, Zuo L, He X, Pi J, Jin J, Shi Y. Efficacy and safety of rh-endostatin (Endostar) combined with pemetrexed/cisplatin followed by rh-endostatin plus pemetrexed maintenance in non-small cell lung cancer: A retrospective comparison with standard chemotherapy. Thorac Cancer. 2018;9:1354-1360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Cheng Y, Nie L, Liu Y, Jin Z, Wang X, Hu Z. Comparison of Endostar continuous versus intermittent intravenous infusion in combination with first-line chemotherapy in patients with advanced non-small cell lung cancer. Thorac Cancer. 2019;10:1576-1580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Honglian M, Zhouguang H, Fang P, Lujun Z, Dongming L, Yujin X, Yong B, Liming X, Yirui Z, Xiao H, Jin W, Yue K, Lvhua W, Ming C. Different administration routes of recombinant human endostatin combined with concurrent chemoradiotherapy might lead to different efficacy and safety profile in unresectable stage III non-small cell lung cancer: Updated follow-up results from two phase II trials. Thorac Cancer. 2020;11:898-906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Lv Y, Jiang R, Ma C, Li J, Wang B, Sun L, Mu N. [Clinical Observation of Recombinant Human Vascular Endostatin Durative Transfusion Combined with Window Period Arterial Infusion Chemotherapy in the Treatment of Advanced Lung Squamous Carcinoma]. Zhongguo Fei Ai Za Zhi. 2015;18:500-504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |