Published online Dec 26, 2022. doi: 10.12998/wjcc.v10.i36.13381
Peer-review started: September 6, 2022
First decision: September 26, 2022
Revised: October 17, 2022
Accepted: November 28, 2022
Article in press: November 28, 2022
Published online: December 26, 2022
Processing time: 106 Days and 6.1 Hours
Acute fibrinous and organizing pneumonia (AFOP) is a rare, noninfective lung disease, histologically characterized by a patchy distribution of intra-alveolar fibrin “balls” and organizing pneumonia. The clinical manifestations of AFOP are nonspecific. Diagnosis depends on pathology. Surgical lung biopsy is optimal for tissue sampling to diagnose AFOP. However, many patients have no tolerance to the operation, including mentally and physically. There is still no standard therapy for AFOP and the methods remain controversial. Therefore, further clinical attention and discussion are warranted.
A 53-year-old woman presented with fever, cough and dyspnea for 15 d. Anti-infective therapy was ineffective. Chest computed tomography showed bilateral patchy consolidation, especially in the lower lobes. We performed both ultr
Percutaneous needle biopsy combined with transbronchial lung biopsies may be a good choice in the absence of surgical biopsy. Methylprednisolone alone is eff
Core Tip: We describe the case of a 53-year-old woman with fever, cough and dyspnea for 15 d. Chest computed tomography showed rapidly progressive bilateral patchy consolidation especially in the lower lobes. Anti-infective therapy was ineffective. We performed ultrasound-guided transbronchial lung biopsy of the posterior basal segment of the left lung and ultrasound-guided percutaneous fine needle puncture of the right lung nodule. Both samples supported the diagnosis of acute fibrinous and organizing pneumonia (AFOP). Methylprednisolone alone is effective and safe in the treatment of idiopathic AFOP.
- Citation: Liu WJ, Zhou S, Li YX. Two methods of lung biopsy for histological confirmation of acute fibrinous and organizing pneumonia: A case report. World J Clin Cases 2022; 10(36): 13381-13387
- URL: https://www.wjgnet.com/2307-8960/full/v10/i36/13381.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i36.13381
Acute fibrinous and organizing pneumonia (AFOP), first described by Beasley et al[1] in 2002, is a rare histological form of interstitial pneumonia by the American Thoracic Society and the European Respiratory Society[2]. AFOP has been receiving increasing clinical attention in recent years. AFOP is typically characterized histologically by the presence of intra-alveolar fibrin “balls” and organizing pneumonia in a patchy distribution[1]. The most common symptoms are dyspnea, cough and fever, which often lead to misdiagnosis and delayed diagnosis. According to the literature, most cases were initially misdiagnosed as pneumonia and lung tumors[3,4]. Beasley et al[1] described a mean time from onset of symptoms to diagnosis of 19 d, and Gomes et al[4] reported a mean time of 43.9 d. A definitive diagnosis of AFOP requires histopathological evaluation[1,2]. Chen et al[3] suggested that surgical biopsy is optimal for tissue sampling to make an AFOP diagnosis. However, many patients showed no tolerance to the operation.
We report a case of a 53-year-old female patient with AFOP, who was diagnosed by pathology of percutaneous and transbronchial lung biopsies and successfully treated with steroid monotherapy. The patient was diagnosed 5 d after hospitalization, and discharged after 11 d of hospitalization.
A 53-year-old woman was referred to the First Affiliated Hospital of Dalian Medical University in April 2022 due to fever with cough and dyspnea for 15 d.
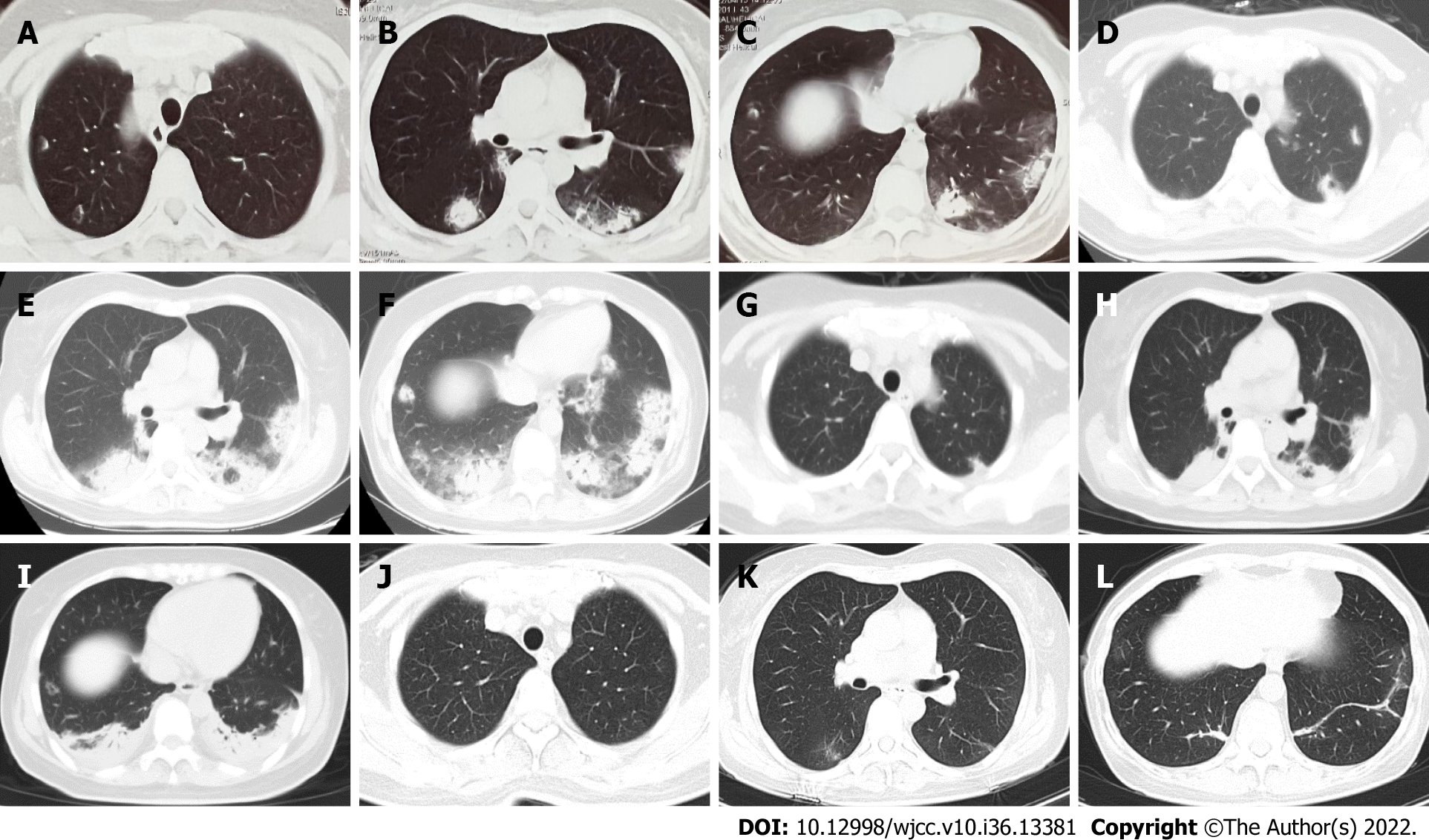
The patient had a fever without obvious precipitating causes for 15 d, and her body temperature was 38.5 °C, with cough, sputum and dyspnea. There was no chest pain, hemoptysis, or night sweats. Chest computed tomography (CT) at the local hospital suggested multiple exudative opacities in both lungs (Figure 1A–C). She received penicillin and macrolide antibiotics as treatment for 9 d. However, she had persistent fever, and her symptoms of dyspnea worsened.
She denied a history of previous illnesses or autoimmune system diseases such as joint swelling and pain, dry mouth and eyes, mouth sores, and rashes.
She was a nonsmoker. She denied a history of exposure to occupational dust or keeping pets. She had no special drug history. She also denied a family history of lung disease.
On examination the patient was alert, with a temperature of 38.2 ºC, pulse rate 94/min, respiratory rate 18/min, blood pressure 120/70 mmHg, and oxygen saturation with room air 92%. Chest auscultation revealed increased breath sounds with no crackles. The rest of the physical examination was unre
The patient’s laboratory test results are shown in Table 1.
| Laboratory examinations | Result |
| Blood gas | pH: 7.477, PO2: 71 mmHg, PCO2: 34.1 mmHg |
| Routine blood | WBC: 10.70 × 109, N%: 80.6%, HGB: 106 g/L, PLT: 583 × 109 |
| CEA, Cyfra21-1, NSE | Negative |
| Coagulation | PT: 12.4 s, APTT: 28.2 s, Fib: 8.77 g/L |
| Liver biochemistry | ALT: 18 U/L, AST: 12 U/L, Prealbumin: 78 mg/L, ALB: 29.4 g/L |
| BNP | Normal |
| Nucleic acid testing for COVID-19 | Negative |
| ESR | 96 mm/h (0-20 mm/h) |
| PCT | Normal |
| CRP | 143 mg/L (< 8.0 mg/L) |
| Serum HIV antibody | Negative |
| Tuberculosis-SPOT | Negative |
| (1,3)-beta-D-glucan assay | Negative |
| Galactomannan | Negative |
| CrAg | Negative |
| Bronchoalveolar lavage fluid | No bacteria, No aspergillus, No Tuberculosis, No Cryptococcus |
| Blood culture | Sterile |
| Respiratory pathogen profile detection | Negative |
| Lymphocyte subsets | CD4: 337 cells/μL, CD3: 590 cells/μL |
| Immunological test | |
| Antinuclear antibodies | Positive, titer 1:100 |
| nRNP/Sm | Weakly positive |
| Sm | Weakly positive |
| Ds-DNA | Negative |
| ENA | Negative |
| ANCA | Negative |
| ACA | Negative |
| Anti-CCP | Negative |
| Rheumatoid factor | Normal |
| IgE | 257 IU/mL (< 100) |
| Immunoglobulins and complement | Normal |
High-resolution CT (HRCT) of the thorax on admission revealed patchy, diffuse alveolar opacities and consolidation with bilateral and peripheral distributions, which was significantly more extensive than before (Figure 1D–F).
Twodimensional echocardiography and electrocardiography were normal. Blood and alveolar lavage fluid were both examined by next-generation sequencing (NGS), and no bacteria, fungi or viruses were found.
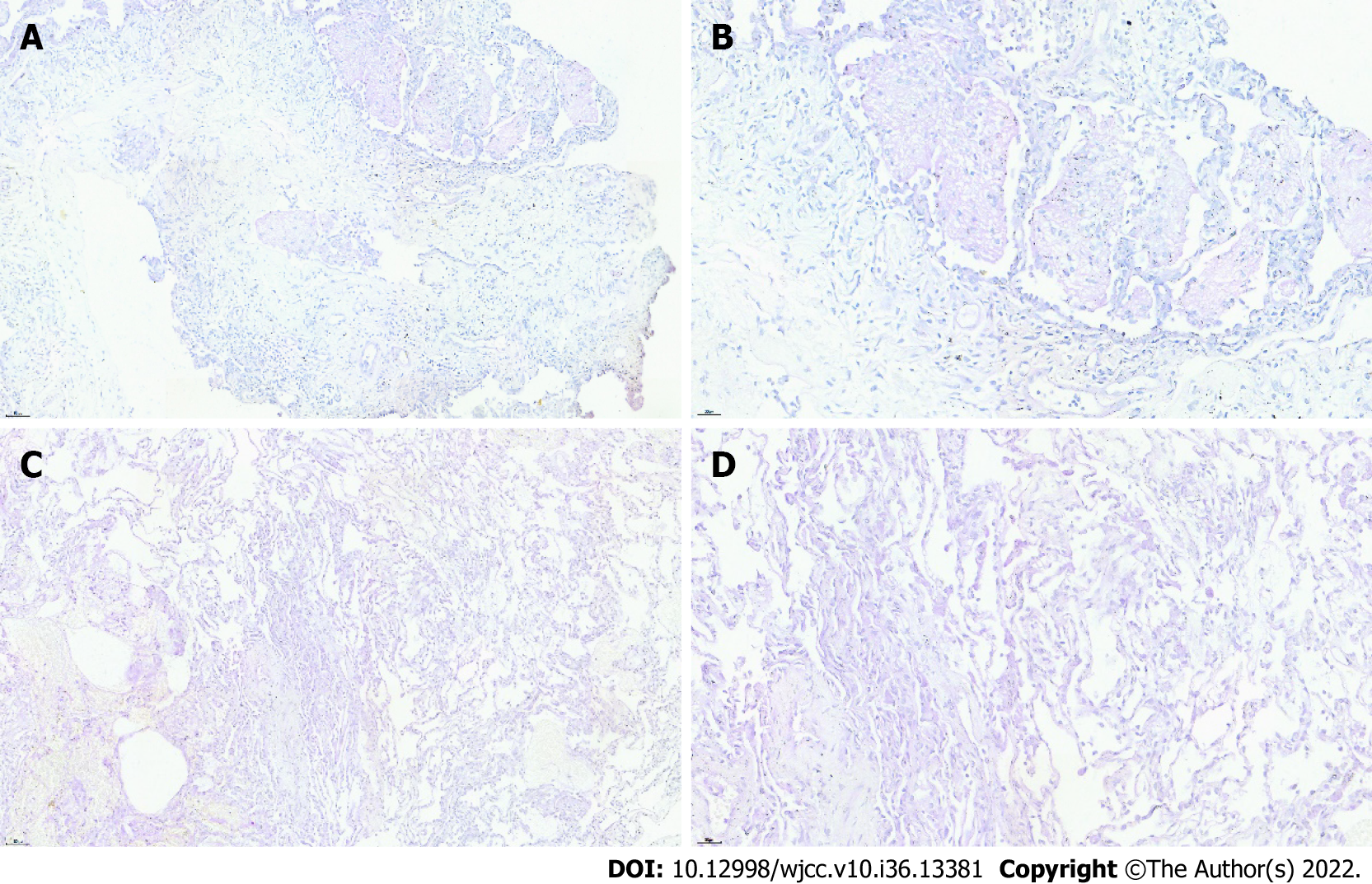
Ultrasound-guided right lung puncture biopsy revealed intra-alveolar fibrin in the form of “fibrin balls” without the formation of hyaline membranes (Figure 2A and B).
Bronchoscopy followed by bronchoalveolar lavage (BAL) was performed. The molecular diagnostic test for tuberculosis was negative; BAL galactomannan was unremarkable. BAL cultures were negative. Transbronchial lung biopsy was performed from the left lower lobe, and the corresponding report revealed AFOP (Figure 2C and D).
AFOP.
Based on the results of the lung biopsy, intravenous methylprednisolone 40 mg twice daily was initiated. The patient’s body temperature returned to normal on the first day of corticosteroid therapy. Her cough and shortness of breath improved significantly. The lung opacities decreased significantly after 3 d of corticosteroid treatment (Figure 1G–I). Then, methylprednisolone was decreased to 40 mg once daily. She was discharged home 3 d later, and continued oral methylprednisolone 40 mg/d for 10 d followed by 20 mg/d for 14 d, 16 mg/d for 14 d, 12 mg/d for 14 d, and 8 mg/d for 1 mo.
At follow-up of 1 mo after discharge, chest HRCT showed that most of the lesions had improved in absorption (Figure 1J–L). She is currently being treated with 4 mg methylprednisolone for 2 mo and on regular follow-up. She has no symptoms such as dyspnea, fever or cough, and no side effects of steroids such as obesity, hypertension, hyperglycemia or osteoporosis.
In 2002, Beasley et al[1] described a new histological pattern of lung injury named AFOP. The dominant histological pattern of AFOP is intra-alveolar fibrin deposition and OP without the presence of classical hyaline membranes and eosinophilia, differentiating the disease from diffuse alveolar damage (DAD), OP, and eosinophilic pneumonia[1,2]. Two forms of the disease are described: An acute form with a fulminant course and rapid progression to respiratory failure, and a subacute form with a better outcome[1,3]. AFOP can be idiopathic or associated with a variety of disease conditions, including infections, collagen vascular diseases, adverse drug or chemical reactions, hematological malignancy, altered immune status, inhalation disease, and occupational or environmental exposures[4-10]. Our patient was weakly positive for anti-Smith (Sm) antibody and nuclear ribonucleoprotein/Sm without any symptoms. Possibility of systemic lupus erythematosus should be considered. Therefore, it is necessary to monitor abnormal immune indicators and corresponding symptoms.
The clinical manifestations of AFOP are nonspecific. The most common symptoms are dyspnea, cough and fever. The most common radiological findings are diffuse, patchy opacities with both peripheral and bilateral distributions, and the lesions may be limited to the lung bases[1]. Chen et al[3] suggested that consolidation is manifested more frequently in patients with idiopathic AFOP, while converse ground-glass opacity is seen more frequently in patients with secondary AFOP.
As clinical characteristics associated with this disease are nonspecific, a definitive diagnosis of AFOP requires histopathological evaluation. The methods of lung biopsy often include percutaneous needle biopsy, endobronchial ultrasound-guided transbronchial lung biopsy, and surgical lung biopsy.
Chen et al[3] suggested that surgical biopsy is the best choice for tissue sampling for AFOP diagnosis, as it can minimize the missing areas of the hyaline membrane in DAD. However, our patient showed no tolerance to the operation. To increase the reliability of the biopsy, we performed both ultrasound-guided transbronchial lung biopsy of the posterior basal segment of the left lung and ultrasound-guided percutaneous fine needle puncture of the right lung nodule. Both samples supported the diagnosis of AFOP.
Most patients begin with respiratory symptoms such as fever and cough and often have elevated nonspecific inflammatory indicators such as C-reactive protein and erythrocyte sediment rate, and chest CT suggests a patch in both lungs, hence many AFOP patients are first diagnosed with community-acquired pneumonia (CAP)[4]. This leads to high use of antibiotics. AFOP needs to be differentiated from CAP because the treatment modalities are markedly different. The patient’s blood and BAL fluid were examined with NGS, and no definitive etiological evidence was found, confirming the final diagnosis of AFOP. It is important to exclude infection. Lu et al[11] suggested that NGS technology plays an important role in the diagnosis of infectious diseases, and has potential for exclusion of non
The most common therapy for AFOP is corticosteroids. Other options include immunosuppressants and antibiotics, depending on the cause of AFOP. Usually 0.5–1 mg/kg/d prednisone (or equivalent) is prescribed initially[12,13]. A maximal dose of methylprednisolone was reported to be up to 1 g/d[8]. Corticosteroids should be reduced after remission, and the total course of treatment should be mai
Percutaneous needle biopsy combined with transbronchial lung biopsies may be a good choice in the absence of surgical biopsy. Methylprednisolone alone is effective and relatively safe in the treatment of idiopathic AFOP.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bukhari SM, Pakistan; Sedaghattalab M, Iran S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Beasley MB, Franks TJ, Galvin JR, Gochuico B, Travis WD. Acute fibrinous and organizing pneumonia: a histological pattern of lung injury and possible variant of diffuse alveolar damage. Arch Pathol Lab Med. 2002;126:1064-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 209] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 2. | Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU, Behr J, Bouros D, Brown KK, Colby TV, Collard HR, Cordeiro CR, Cottin V, Crestani B, Drent M, Dudden RF, Egan J, Flaherty K, Hogaboam C, Inoue Y, Johkoh T, Kim DS, Kitaichi M, Loyd J, Martinez FJ, Myers J, Protzko S, Raghu G, Richeldi L, Sverzellati N, Swigris J, Valeyre D; ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2232] [Cited by in RCA: 2905] [Article Influence: 242.1] [Reference Citation Analysis (0)] |
| 3. | Chen H, Kuang Y, Huang X, Ye Z, Liu Y, Xie C, Tang KJ. Acute fibrinous and organizing pfneumonia: two case reports and literature review. Diagn Pathol. 2021;16:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 4. | Gomes R, Padrão E, Dabó H, Soares Pires F, Mota P, Melo N, Jesus JM, Cunha R, Guimarães S, Souto Moura C, Morais A. Acute fibrinous and organizing pneumonia: A report of 13 cases in a tertiary university hospital. Medicine (Baltimore). 2016;95:e4073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Prahalad S, Bohnsack JF, Maloney CG, Leslie KO. Fatal acute fibrinous and organizing pneumonia in a child with juvenile dermatomyositis. J Pediatr. 2005;146:289-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Heo JY, Song JY, Noh JY, Yong HS, Cheong HJ, Kim WJ. Acute fibrinous and organizing pneumonia in a patient with HIV infection and Pneumocystis jiroveci pneumonia. Respirology. 2010;15:1259-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Yokogawa N, Alcid DV. Acute fibrinous and organizing pneumonia as a rare presentation of abacavir hypersensitivity reaction. AIDS. 2007;21:2116-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Lee SM, Park JJ, Sung SH, Kim Y, Lee KE, Mun YC, Lee SN, Seong CM. Acute fibrinous and organizing pneumonia following hematopoietic stem cell transplantation. Korean J Intern Med. 2009;24:156-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Otto C, Huzly D, Kemna L, Hüttel A, Benk C, Rieg S, Ploenes T, Werner M, Kayser G. Acute fibrinous and organizing pneumonia associated with influenza A/H1N1 pneumonia after lung transplantation. BMC Pulm Med. 2013;13:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Kligerman SJ, Franks TJ, Galvin JR. From the radiologic pathology archives: organization and fibrosis as a response to lung injury in diffuse alveolar damage, organizing pneumonia, and acute fibrinous and organizing pneumonia. Radiographics. 2013;33:1951-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 11. | Lu J, Yin Q, Zha Y, Deng S, Huang J, Guo Z, Li Q. Acute fibrinous and organizing pneumonia: two case reports and literature review. BMC Pulm Med. 2019;19:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Ning YJ, Ding PS, Ke ZY, Zhang YB, Liu RY. Successful steroid treatment for acute fibrinous and organizing pneumonia: A case report. World J Clin Cases. 2018;6:1053-1058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Lu Y, Zheng W, Cao W, Yang X, Zhao L, Chen Y. Acute fibrinous and organizing pneumonia in a patient with Sjögren's syndrome and Legionella pneumonia: a case report and literature review. BMC Pulm Med. 2022;22:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |