Published online Dec 26, 2022. doi: 10.12998/wjcc.v10.i36.13349
Peer-review started: July 31, 2022
First decision: August 22, 2022
Revised: September 24, 2022
Accepted: November 30, 2022
Article in press: November 30, 2022
Published online: December 26, 2022
Processing time: 148 Days and 5.4 Hours
Pregnancy is a complex physiological process. Physiological leukocytosis occurs often and is mainly associated with increased neutrophil counts, especially in the third trimester of pregnancy. Non-congenital leukocytosis with white blood cell counts above 20 × 109/L lasting 13 wk during pregnancy is rare and has been reported occasionally. Herein, we present a case of pregnancy-induced leukocytosis.
We present the case of a 33-year-old Chinese woman at 27 wk of gestation who had a leukocytosis complication. No abnormalities were detected in the examinations before pregnancy or in the first trimester. From the third trimester of pregnancy, the patient began to suffer from asymptomatic leukocytosis. We administered antibiotics to treat the patient; however, the complication persisted until the patient underwent a cesarean section after 40+3 wk of gestation. One day after the cesarean section, the patient’s neutrophil count returned to normal. After 2 years of follow-up, we found that the patient and baby were healthy.
Pregnancy-induced leukocytosis seems to be associated with immunoregulation and pregnancy termination may be the most effective treatment approach for pregnancies complicated with malignant leukocytosis.
Core Tip: Physiological leukocytosis often occurs and is mainly associated with increased neutrophil levels. We present the case of a Chinese woman in her 27th wk of gestation who had the complication of leukocytosis with a white blood cell count above 20 × 109/L for 13 wk. One day after a cesarean section, the patient’s neutrophil levels returned to normal. After 2 years of follow-up, the patient and baby were found to be healthy. During pregnancy, asymptomatic leukocytosis appears to be related to immunoregulation and termination of pregnancy may be an effective treatment approach in pregnancies with malignant leukocytosis.
- Citation: Wang X, Zhang YY, Xu Y. Pregnancy-induced leukocytosis: A case report. World J Clin Cases 2022; 10(36): 13349-13355
- URL: https://www.wjgnet.com/2307-8960/full/v10/i36/13349.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i36.13349
Pregnancy is a complex physiological process[1]. The normal range of white blood cell (WBC) counts changes with age and pregnancy[2,3]. In pregnant women, local adaptation of the maternal immune system enables the successful coexistence of the mother and fetus/placenta[4]. Physiological leu
Non-congenital leukocytosis with WBC counts above 20 × 109/L for 13 wk during pregnancy is rare and has been reported occasionally. Herein, we present the case of gestation-induced leukocytosis.
A 33-year-old woman presented to the emergency department with a complaint of high blood pressure for 6 wk and leukocytosis for 13 wk.
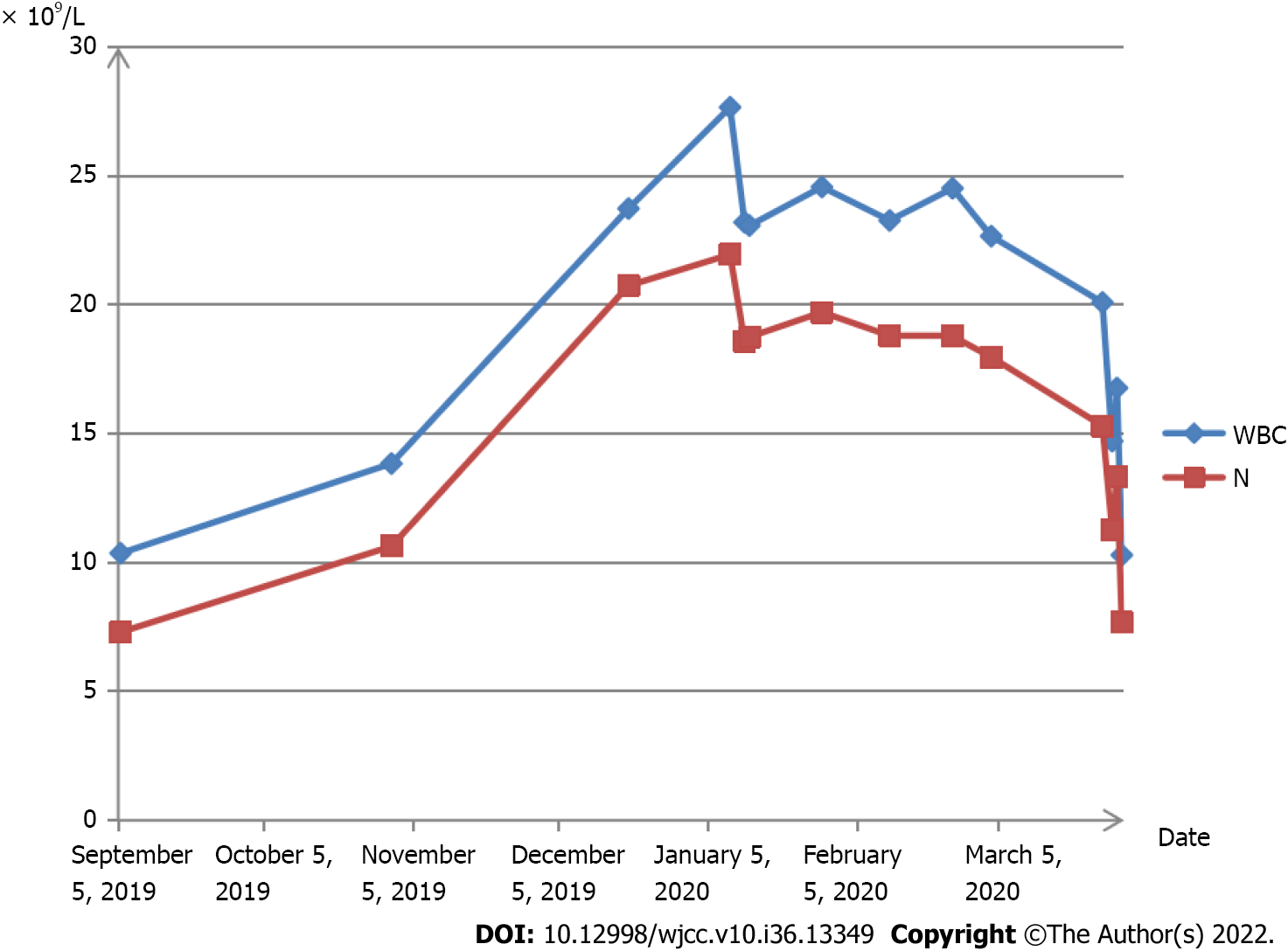
The patient had experienced leukocytosis for 13 wk at the time she presented to the emergency department. To prevent implantation failure after IVF, she took aspirin enteric-coated tablets 75 mg a day, 5 mg acetate orally once a day, and one vitamin complex tablet a day until 12 wk of gestation. During her pregnancy, repeated routine blood tests before 20 wk of gestation showed that the WBC and neutrophil counts were within the normal range. Ultrasonography suggested a post-placental hematoma with a diameter of approximately 20-30 mm before 20 wk of gestation, which disappeared thereafter. At 27 wk of gestation, the WBC rose to 23.73 × 109/L and the neutrophil count rose to 20.74 × 109/L (Figure 1).
The patient was diagnosed with polycystic ovarian syndrome and her partner was diagnosed with male factor infertility. The patient had no known allergies to food or medication. In addition, she denied any family history or history of sexually transmitted infections. The patient was an employee of an Internet company, did not smoke and was not exposed to second-hand smoking during pregnancy.
The patient had no specific personal and family history.
At the initial inspection, the patient had a blood pressure of 126/87 mmHg and a pulse rate of 68 beats per minute. The patient’s lungs were clear and she had normal heart sounds with no murmurs on auscultation.
At 27 wk of gestation, blood analysis revealed leukocytosis of 23.73 × 109/L, with predominantly neutrophils (87.4%) with normal hematocrit and platelet count, and the neutrophil count rose to 20.74 × 109/L (Figure 1). C-reactive protein count was 0.52 (< 0.8) mg/dL, erythrocyte sedimentation rate was 30 (0-20) mm/h, and procalcitonin (PCT) count was < 0.05 (< 0.5) ng/mL, which showed no sign of infection.
The manuscript is a case report and meets the requirements of biostatistics.
The final diagnosis of the case is asymptomatic leukocytosis.
The patient had no fever, and had a normal temperature. In addition, there was no presence of other symptoms, including no cough, expectoration, oral ulcers, or shivering. She was administered antibiotic treatment for 2 wk, which did not work. Afterward, the patient visited several other hospitals; during this time, routine blood tests showed a sustained high level of WBC and neutrophil counts. The patient visited the outpatient department of our institution because of leukocytosis. The C-reactive protein count was 0.52 mg/dL, the erythrocyte sedimentation rate was 30 mm/h, and the PCT count was < 0.05 ng/mL, which showed no sign of infection. Thereafter, the patient visited the outpatient hematology department. The patient refused a bone marrow biopsy. Peripheral blood smear showed that mature neutrophils accounted for 73.2%, and the count of immature granulocytes was 0.95 × 109/L, accounting for 3.7%. Tests at another hospital showed leukocytosis, but normal levels of red blood cells and megakaryocytes. The patient was hospitalized with an elevated blood pressure at 40+3 wk of gestation. On admission, the WBC count was 20.09 × 109/L, the neutrophil granulocyte count was 15.3 × 109/L, the blood platelet count was 343 × 109/L, and the hemoglobin concentration was 140 g/L. The next day, she underwent a cesarean section because of fetal distress. The surgery was successful.
On the first postoperative day, the WBC count was 14.71 × 109/L, the neutrophil granulocyte count was 11.26 × 109/L, the hemoglobin concentration was 124 g/L, and the platelet count was 304 × 109/L. The thyroid function tests were within the normal range; free thyroxine was 16.27 pmol/L and thyrotropin was 1.16 uIU/mL. Ultrasonography of the fetus, abdomen, lower limb arteries, and deep veins showed that all the tested areas were normal. Ultrasonography of the kidneys showed a right hydronephrosis with a renal pelvis approximately 1.1 cm wide. Tests for immunoglobulin M (IgM) against toxoplasma, IgM against rubella virus, and IgM against cytomegalovirus, herpes simplex type I virus, and herpes simplex type II virus were negative. Tests for hepatitis, human immunodeficiency virus, and Treponema pallidum were all negative. By 34 wk, blood pressure had risen to a range of 138/80 mmHg and 142/90 mmHg, and the patient was diagnosed with pregnancy-induced suspicious hypertension without medication. During 40+3 wk of gestation, she underwent a cesarean section because her blood pressure had increased to 143/90 mmHg. Six weeks postpartum, the patient’s blood pressure gradually returned to normal.
Postoperatively, neutrophil granulocytes returned to normal levels. The patient delivered a live, healthy, full-term baby via a cesarean section. After 2 years of follow-up, the patient and baby were found to be healthy.
Hematological diseases in pregnancy should be meticulously managed with multidisciplinary cooperation, including obstetrics and hematology. Distinguishing between reactive and malignant lymphocytosis is challenging and may vary with age and other demographics. Table 1 lists the most common etiologies[18]. The patient did not suffer from allergic reactions, malignancy, surgery, trauma, strenuous physical activity, or smoking; in addition, the patient had no fever, had normal temperature, experienced no other symptoms such as cough, expectoration, oral ulcers, or shivering. The patient visited the outpatient department for the complaint of an infection. The C-reactive protein count was 0.52 mg/dL, the erythrocyte sedimentation rate was 30 mm/h, and the PCT count was < 0.05 ng/mL, which showed no sign of infection. A peripheral blood smear showed that mature neutrophils accounted for 73.2%, and immature granulocytes count was 0.95 × 109/L, accounting for 3.7%. Tests at another hospital showed leukocytosis, but normal levels of red blood cells and megakaryocytes. Six weeks postpartum, the patient's blood pressure gradually returned to normal, which illustrated that it was not malignant. Molberg et al[19] found that the average WBC count in a laboring patient was 12.45 × 109/L, with a range of 4.4 × 109/L to 29.1 × 109/L. WBC counts in patients with postpartum complications were similar to that in patients without complications (12.9 × 109/L vs 12.3 × 109/L, P = 0.449)[19]. We describe a case of asymptomatic leukocytosis with WBC counts > 20 × 109/L during pregnancy. The patient did not suffer from leukocytosis until 27 wk of gestation; after cesarean section, WBC and granulocyte counts dropped to normal levels. Levothyroxine sodium is safe for pregnant women, and there is no evidence that its side effects include leukocytosis[20]. At the same time, there was no obvious evidence that hypothyroidism caused leukocytosis, and the patient had no history of using cytotoxic drugs or other medications that explicitly cause leukocytosis. Therefore, we believe that drug-induced leukocytosis was less likely the case.
| Infections | Lymphoproliferative disorders | Other hematological systemic disease | Drugs and drug hypersensitivity reactions | Stress | Asplenia |
| Viral infections | Chronic Lymphocytic Leukemia | MBL | Allopurinol | Cardiac conditions | |
| Bacterial Infections | Non-Hodgkin Lymphoma | Congenital B cell Lymphocytosis | Carbamazepine | Status epileptics | |
| Parasitic Infections | Adult T cell lymphoma/leukemia | Persistent B-cell polyclonal B-Lymphocytosis | Vancomycin | Epinephrine use | |
| Mycobacterial Tuberculosis | Large Granular Lymphocyte Leukemia | Sulfa drugs | |||
| Acute lymphoblastic lymphoma |
There are a few reports on leukocyte counts and differentials related to the severity of pregnancy-induced hypertension. Terrone et al[21] assessed the difference in leukocyte counts between normal pregnancies and pregnancies complicated by preeclampsia (PE). In a retrospective study of 240 women, women with severe PE had a significantly higher WBC count than those with mild PE and normal pregnancy controls [10.66 +/- 3.70 vs 9.47 +/- 2.59 and 8.55 +/- 1.93 (× 109/L) (P < 0.0001)]. The increase in the total WBC count was mainly due to an increase in the number of neutrophils [8.05 +/- 4.01 (severe) vs 6.69 +/- 2.23 (mild) and 5.90 +/- 1.79 (controls) (× 109/L) (P < 0.0001)]. Terrone et al[21] evaluated the total WBC count of 86 patients with severe PE with and without hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome of 91 patients. The WBC counts in patients with HELLP syndrome (12.5 +/- 0.442 × 109/L) were significantly higher than those in patients with severe PE (10.3 +/- 0.288 × 109/L). The patient was diagnosed with hypertension during pregnancy, without PE. Furthermore, the counts of WBCs were above 20 × 109/L. Leukocytosis may have had nothing to do with hypertension in this case.
There have been few reports on the relationship between leukocytosis and in vitro fertilization and embryo transfer (IVF). Ludwig et al[22] observed the effects of a luteinizing hormone-releasing hormone antagonist protocol (Cetrorelix) and the administration of recombinant follicle-stimulating hormone (FSH) on the development of leukocytosis compared to the administration of urinary human menopausal gonadotropin. Thirty patients underwent IVF/intracytoplasmic sperm injection treatment after controlled ovarian stimulation using a multiple dose protocol and the luteinizing hormone releasing hormone (LHRH) antagonist Cetrorelix, and no significant leukocytosis was discovered after controlled ovarian stimulation using the LHRH antagonist Cetrorelix and recFSH.
During pregnancy, the integration and balance of these immune factors produce an environment that allows the fetus to escape rejection by the maternal immune system. Multiple mechanisms influence the maternal immune system in accepting semiallogeneic fetal tissues during pregnancy[23]. Female sex hormones affect many immune pathways more often during pregnancy.
In summary, the etiology and mechanism of this phenomenon remain largely unknown. In addition, during pregnancy, asymptomatic leukocytosis seems to be related to immunosuppression induced by immunoregulation. The termination of pregnancy may be effective in pregnancies complicated with leukocytosis; however, further studies are needed to confirm this.
Thus, we conclude that leukocytosis seems to be associated with the pregnancy itself and is associated with immunoregulations. Although this study presents a case of leukocytosis without evidence of clinical infection, caution should be exercised when applying these data clinically. We suggest that pregnancy termination may be a therapeutic approach for pregnancies complicated with leukocytosis.
The authors are grateful to the patient in this study for her collaboration.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Ishida T, Japan; Jain N, Latvia; Mangla A, United States; Routray S, India; Tangsuwanaruk T, Thailand; Vyshka G, Albania S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Medicine DCCO Physiology of Pregnancy. Chapter 41. Hoboken, NJ: John Wiley & Sons Ltd; 2015. |
| 2. | Cheng CK, Chan J, Cembrowski GS, van Assendelft OW. Complete blood count reference interval diagrams derived from NHANES III: stratification by age, sex, and race. Lab Hematol. 2004;10:42-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 155] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 3. | Sanci M, Töz E, Ince O, Özcan A, Polater K, Inan AH, Beyan E, Akkaya E. Reference values for maternal total and differential leukocyte counts in different trimesters of pregnancy and the initial postpartum period in western Turkey. J Obstet Gynaecol. 2017;37:571-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 4. | Robertson SA. Immune regulation of conception and embryo implantation-all about quality control? J Reprod Immunol. 2010;85:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Wang H, Sun JL, Zhang ZL, Pei HH. Pregnancy complicated with agranulocytosis. Medicine (Baltimore). 2016;95:e5717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Lurie S, Rahamim E, Piper I, Golan A, Sadan O. Total and differential leukocyte counts percentiles in normal pregnancy. Eur J Obstet Gynecol Reprod Biol. 2008;136:16-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Cotran RS, Kumar V, Collins T. Robbins pathologic basis of disease. 6th ed. Philadelphia, PA: WB Saunders, 1999: 644–96. |
| 8. | Kehinde MO, Akinyanju OO. The pattern of leucocyte response to surgical trauma in the African Negro. Clin Lab Haematol. 1988;10:285-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Traiperm N, Gatterer H, Burtscher M. Plasma electrolyte and hematological changes after marathon running in adolescents. Med Sci Sports Exerc. 2013;45:1182-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Soper DE. Postpartum endometritis. Pathophysiology and prevention. J Reprod Med. 1988;33:97-100. [PubMed] |
| 11. | Yoon BH, Yang SH, Jun JK, Park KH, Kim CJ, Romero R. Maternal blood C-reactive protein, white blood cell count, and temperature in preterm labor: a comparison with amniotic fluid white blood cell count. Obstet Gynecol. 1996;87:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Mercelina-Roumans PE, Ubachs JM, van Wersch JW. Leucocyte count and leucocyte differential in smoking and non-smoking females during pregnancy. Eur J Obstet Gynecol Reprod Biol. 1994;55:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Reed WW, Diehl LF. Leukopenia, neutropenia, and reduced hemoglobin levels in healthy American blacks. Arch Intern Med. 1991;151:501-505. [PubMed] |
| 14. | Schwartz J, Weiss ST. Host and environmental factors influencing the peripheral blood leukocyte count. Am J Epidemiol. 1991;134:1402-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Petit E, Abergel A, Dedet B, Subtil D. [The role of infection in preterm birth]. J Gynecol Obstet Biol Reprod (Paris). 2012;41:14-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Gibbs RS. Diagnosis of intra-amniotic infection. Semin Perinatol. 1977;1:71-77. [PubMed] |
| 17. | Higgins RD, Saade G, Polin RA, Grobman WA, Buhimschi IA, Watterberg K, Silver RM, Raju TNK; Chorioamnionitis Workshop Participants. Evaluation and Management of Women and Newborns With a Maternal Diagnosis of Chorioamnionitis: Summary of a Workshop. Obstet Gynecol. 2016;127:426-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 347] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 18. | Hamad H, Mangla A. Lymphocytosis. 2022 Jul 18. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. [PubMed] |
| 19. | Molberg P, Johnson C, Brown TS. Leukocytosis in labor: what are its implications? Fam Pract Res J. 1994;14:229-236. [PubMed] |
| 20. | Mahler M, Ngo JT, Schulte-Pelkum J, Luettich T, Fritzler MJ. Limited reliability of the indirect immunofluorescence technique for the detection of anti-Rib-P antibodies. Arthritis Res Ther. 2008;10:R131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Terrone DA, Rinehart BK, May WL, Moore A, Magann EF, Martin JN Jr. Leukocytosis is proportional to HELLP syndrome severity: evidence for an inflammatory form of preeclampsia. South Med J. 2000;93:768-771. [PubMed] |
| 22. | Ludwig M, Strik D, Felberbaum R, Al-Hasani S, Diedrich K. No significant leukocytosis under controlled ovarian stimulation using the LHRH antagonist Cetrorelix and recFSH. Eur J Obstet Gynecol Reprod Biol. 2000;89:177-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Song D, Shi Y. Immune system modifications and feto-maternal immune tolerance. Chin Med J (Engl). 2014;127:3171-3180. [PubMed] |