Published online Dec 26, 2022. doi: 10.12998/wjcc.v10.i36.13304
Peer-review started: October 19, 2022
First decision: November 4, 2022
Revised: November 19, 2022
Accepted: November 28, 2022
Article in press: November 28, 2022
Published online: December 26, 2022
Processing time: 68 Days and 8.3 Hours
Cervical cancer is a gynecological malignancy common in middle-aged and older patients, with a high mortality rate. Spondin-2 is an extracellular matrix protein that involved in innate and acquired immune responses. Herein, we investigated the relationship between serum Spondin-2 expression, tumor invasion and infiltration, and immune response in patients with cervical cancer and provided a theoretical basis for clinical practice.
To investigate the relationship between serum Spondin-2 expression and cervical cancer-related indicators.
Overall, 147 patients with cervical cancer who were admitted to our institution between January 2019 and August 2019 were assigned to the cervical cancer group, and 92 patients with benign uterine lesions and 86 healthy individuals were assigned to the benign and control groups, respectively. In each group, serum Spondin-2 expression was measured, and the receiver operating characteristic (ROC) curve was determined. Patients with cervical cancer were classified into high or low Spondin-2 groups depending on the Spondin-2 threshold value used for diagnosing cervical cancer. Patient’s clinical data were collected to compare the clinicopathologic characteristics, immune cytokine levels, and prognosis of patients with varying Spondin-2 expression levels.
The expression level of serum Spondin-2 was significantly higher in the cervical cancer group than in the benign and control groups (P < 0.05). According to the ROC curve, the cutoff value of Spondin-2 used in the diagnosis of cervical carcinoma was 25.68 ± 7.11 μg/L. The proportion of patients with Federation of Gynecology and Obstetrics stage III, nerve invasion, vascular invasion, and lymph node metastasis was higher in the high Spondin-2 group than in the low Spondin-2 group (P < 0.05). Interleukin-5 (IL-5) and IL-4 Levels were higher in the high Spondin-2 group than in the low Spondin-2 group. In contrast, IL-2 and tumor necrosis factor-α levels were lower in the high Spondin-2 group than in the low Spondin-2 group (P < 0.05). After 3 years of follow-up, progression-free survival and overall survival were significantly shorter in the high Spondin-2 group than in the low Spondin-2 group (P < 0.05).
The expression of serum Spondin-2 is upregulated in patients with cervical carcinoma and is related to tumor invasion and infiltration, antitumor immune response, and prognosis.
Core Tip: We measured the expression of Spondin-2 in the sera of patients with cervical cancer, patients with benign uterine lesions, and healthy individuals. Spondin-2 expression was regulated in patients with cervical cancer and was related to tumor invasion and infiltration, antitumor immune response, and prognosis.
- Citation: Zhang LL, Lin S, Zhang Y, Yao DM, Du X. Serum Spondin-2 expression, tumor invasion, and antitumor immune response in patients with cervical cancer. World J Clin Cases 2022; 10(36): 13304-13312
- URL: https://www.wjgnet.com/2307-8960/full/v10/i36/13304.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i36.13304
Cervical cancer is a gynecological malignancy that is common in middle-aged and older patients; in recent years, it reportedly occurs in younger patients and seriously endangers patient health[1]. Relevant studies have indicated that cervical cancer is associated with high-risk human papillomavirus (HPV) infection and abnormal immune response, among other factors. Among these, an abnormal immune response may not be helpful in HPV clearance. It may interfere with the body’s normal antitumor response, leading to invasion and metastasis, which are important factors that cause high mortality[2,3]. Molecular markers that significantly affect the immune function in cervical cancer should be identified from the molecular biology perspective to guide patients’ treatment and increase their survival. Spondin-2 is an extracellular matrix protein that plays a role in innate and acquired immune responses. Its expression is currently associated with the development and spreading of several malignant tumors, including prostate cancer[4] and lung adenocarcinoma[5]. This study aimed to examine the association between serum Spondin-2 expression, tumor invasion and infiltration, and immune response in patients with cervical cancer to provide a theoretical basis for clinical practice.
We enrolled 147 patients with cervical cancer who were admitted to our hospital between January 2019 and August 2019 for this study. Inclusion criteria included patients: (1) Diagnosed with cervical cancer by postoperative pathological examination according to the clinical practice guidelines for cervical cancer proposed by the National Comprehensive Cancer Network in 2019[6]; (2) aged ≥18 years; (3) with serum samples obtained preoperatively; and (4) who signed an informed consent form. Exclusion criteria included patients: (1) Who did not receive chemoradiotherapy or antitumor treatment prior to the study enrollment; (2) who developed endometriosis, uterine fibroids, or other serious uterine lesions along with cervical cancer; (3) with other primary malignant tumors, immune system abnormalities, liver and kidney decompensation, or serious infection; and (4) with incomplete clinical data and follow-up data. Additionally, 92 patients with benign uterine disease and 86 healthy individuals were enrolled into the benign and control groups, respectively. Patients in the cervical cancer group were aged 37–69 (51.62 ± 5.87) years; had a body mass index (BMI) of 19–25 (22.75 ± 1.63) kg/m2; had a disease duration of 1–3 (2.17 ± 0.65) years; had the following pathological types: squamous cell carcinoma in 101 cases, adenocarcinoma in 25 cases, and adenosquamous carcinoma in 21 cases; had the following Federation of Gynecology and Obstetrics (FIGO)[7] stages: stage I in 42 cases, stage II in 49 cases, and stage III in 56 cases; had nerve invasion in 92 cases and no nerve invasion in 55 cases; had vascular invasion in 69 cases and no vascular invasion in 78 cases; and had lymph node metastasis in 87 cases and no lymph node metastasis in 60 cases. Patients in the benign group were aged 40–65 (51.12 ± 6.21) years; had a BMI of 19–25 (22.61 ± 1.82) kg/m2; had a disease duration of 1–3 (2.24 ± 0.59) years; and developed endometriosis in 42 cases, uterine fibroids in 36 cases, and adenomyosis in 14 cases. Patients in the control group were 35–65 (52.04 ± 5.48) years and had a BMI of 20–25 (22.84 ± 1.59) kg/m2. Among the three groups, no significant differences were observed in age or BMI (P > 0.05).
Detection of serum Spondin-2 and immune cytokine levels: Before surgery, blood samples were collected and centrifuged at 3,000 rpm for 10 min to separate the upper serum. An enzyme-linked immunosorbent assay was used to determine the serum levels of Spondin-2, interleukin (IL)-2, IL-4, tumor necrosis factor (TNF-α), and IL-5. The human factor antibody was added to approximately 100 μL of the standard samples and 100 μL of the test samples, and the reaction was performed at 37 °C for 90 min. After the reaction was completed, horseradish peroxidase enzyme was added to each well and incubated at 37 °C for 30 min, followed by the addition of substrate solution and the termination of the reaction. The optical density values were measured at 450 nm using a microplate reader, and the factor levels to be measured were compared using the standard curve.
All patients with cervical cancer underwent extensive hysterectomy and bilateral pelvic lymphadenectomy. Their clinicopathological data, including pathological type, FIGO stage, nerve invasion, vascular invasion, and lymph node metastasis, were collected. A receiver operating characteristic (ROC) curve was used to analyze the cutoff value for serum Spondin-2 Levels used in the diagnosis of cervical cancer, and the patients were divided into high Spondin-2 and low Spondin-2 groups to analyze the differences in the clinicopathological characteristics of patients with different expression levels.
Follow-up: The patients were followed up every 3 mo until July 30, 2022 or death. Progression-free survival (PFS) and overall survival (OS) were assessed. PFS was defined as the time until confirmed tumor recurrence or metastasis, whereas OS was defined as the time until confirmed death.
The data were analyzed using SPSS 22.0, and the formula mean ± SD was used to represent the measurement data in accordance with the normal distribution. A one-way analysis of variance was applied for comparison between groups, the Student–Newman–Keuls-q test was applied for pairwise comparison between groups, and an independent sample t-test was used for comparison between two groups. The enumeration data were expressed as percentages (%). The χ2 test was utilized for comparison between groups. The ROC curve was developed to determine the cutoff value of serum Spondin-2 expression for diagnosing cervical cancer. The Kaplan–Meier curve was used to analyze the PFS and OS. A P value of < 0.05 was considered statistically significant.
The serum Spondin-2 expression level was considerably higher in the cervical cancer group (P < 0.05) than in the benign and control groups (Table 1).
| Group | n | Spondin-2 (μg/L) | F | P value |
| Cervical cancer group | 147 | 25.68 ± 7.11 | 105.790 | 0.000 |
| Benign group | 92 | 17.52 ± 5.76 | ||
| Control group | 86 | 14.16 ± 4.96 |
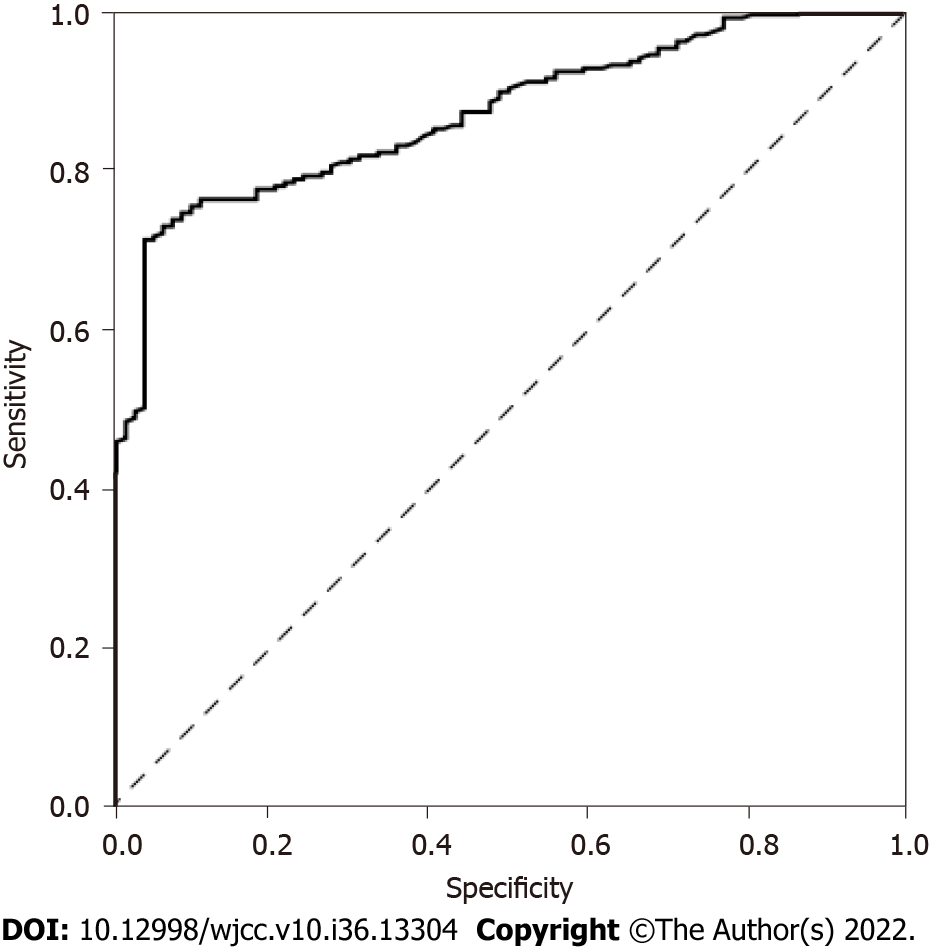
ROC curves were established, and the maximum Youden’s index was used as the cutoff value of Spondin-2 expression in patients with cervical cancer (21.20 μg/L). The sensitivity and specificity values were 71.1% and 95.3%, respectively, and the area under the curve value was 0.866 (95%CI: 0.827-0.905; P < 0.001) (Figure 1).
According to the cutoff value, patients with Spondin-2 expression levels greater than the cutoff value were assigned to the high expression group and those with lower levels were assigned to the low expression group. The proportion of patients with FIGO stage III, vascular invasion, nerve invasion, and lymph node metastasis was higher in the high Spondin-2 group than in the low Spondin-2 group (P < 0.05). No significant difference was observed in the pathogenic categories between the two groups (P > 0.05) (Table 2).
| Clinical pathology | High Spondin-2 group (n = 101) | Low Spondin-2 group (n = 46) | χ2 value | P value |
| Pathological type | ||||
| Squamous cell carcinoma | 70 | 31 | 0.595 | 0.743 |
| Adenocarcinoma | 18 | 7 | ||
| Adenosquamous carcinoma | 13 | 8 | ||
| FIGO stage | ||||
| Stage I–II | 50 | 41 | 10.501 | 0.001 |
| Stage III | 51 | 5 | ||
| Nerve invasion | ||||
| Yes | 72 | 20 | 10.438 | 0.001 |
| No | 29 | 26 | ||
| Vascular invasion | ||||
| Yes | 57 | 12 | 11.688 | 0.001 |
| No | 44 | 34 | ||
| Lymph node metastasis | ||||
| Yes | 66 | 21 | 5.075 | 0.024 |
| No | 35 | 25 |
The high Spondin-2 group had higher IL-4 and IL-5 levels than the low Spondin-2 group, whereas the low Spondin-2 group had higher IL-2 and TNF-α levels than the high Spondin-2 group (P < 0.05) (Table 3).
| Group | n | IL-2 (pg/mL) | TNF-ɑ (ng/mL) | IL-4 (pg/mL) | IL-5 (pg/mL) |
| High Spondin-2 group | 101 | 3.52 ± 0.83 | 10.62 ± 1.80 | 2.98 ± 0.53 | 4.43 ± 1.08 |
| Low Spondin-2 group | 46 | 4.12 ± 1.13 | 13.83 ± 2.56 | 2.45 ± 0.76 | 3.18 ± 0.72 |
| t value | - | 3.614 | 8.735 | 4.879 | 7.153 |
| P value | - | 0.000 | 0.000 | 0.000 | 0.000 |
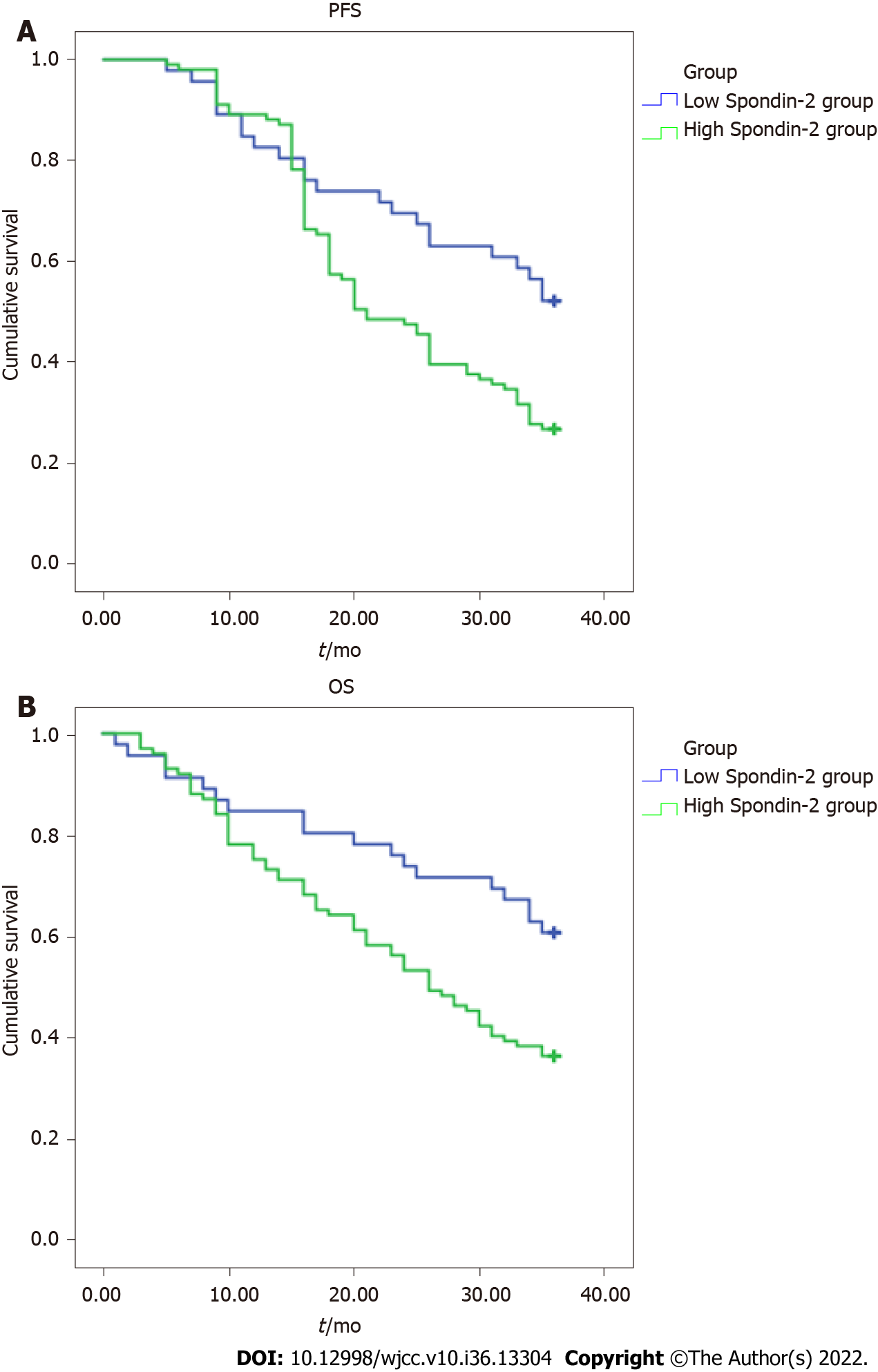
After 3 years of follow-up, PFS and OS were shorter in the high Spondin-2 group than in the low Spondin-2 group (P < 0.05) (Table 4, Figure 2A and B).
| Group | n | PFS (mo) | OS (mo) |
| High Spondin-2 group | 101 | 23.2 ± 9.3 | 26.8 ± 8.7 |
| Low Spondin-2 group | 46 | 28.9 ± 10.4 | 30.1 ± 9.7 |
| t value | - | 3.319 | 2.056 |
| P value | - | 0.001 | 0.042 |
Cervical cancer has a complicated etiology, and HPV infection is a recognized risk factor[8]. Tumor cells often have the ability to evade immune recognition[9]. Disturbed immune responses not only facilitate HPV infection but also cause persistent infection and increase the risk of epithelial malignant transformation[10,11]. In addition, cervical cancer is prone to peripheral nerve invasion and invasive metastasis, which are important factors that result in a poor prognosis[12]. Therefore, it is imperative to analyze the factors related to invasion and infiltration and the antitumor immune response to improve cervical cancer prognosis.
Spondin-2 is a secreted protein, which was originally discovered in ovarian cancer cells, and is linked to innate and acquired immunity as a pattern-recognition molecule and integrin ligand for pathogenic microorganisms[13]. Spondin-2 is reportedly a critical indicator of the initial response to innate immunity, but its expression levels differ in tumor and non-tumor cells[14]. Moreover, Spondin-2 expression levels were elevated in the sera of patients with prostate cancer[15], hepatocellular carcinoma[16], and pancreatic cancer[17], and high levels were associated with bone metastasis and lymph node metastasis, suggesting that Spondin-2 was also involved in tumor invasion and metastasis. This study found that serum Spondin-2 expression was significantly higher in the cervical cancer group than in the benign and control groups, suggesting that Spondin-2 expression is elevated in the sera of patients with cervical cancer and may play a role in the development of cervical tumors and their microenvironment. An ROC curve was used to determine the cutoff value for serum Spondin-2 diagnosis, and patients with cervical cancer were divided into high Spondin-2 and low Spondin-2 groups. The results showed that the number of patients with FIGO stage III, nerve invasion, vascular invasion, and lymph node metastasis was higher in the high Spondin-2 group than in the low Spondin-2 group, implying that serum Spondin-2 expression is related to these aspects progression of cervical cancer; thus, its high expression indicates disease progression. Tumor cells can induce neovascularization in surrounding non-malignant tissues, which not only supports tumor growth but also provides a pathway for metastasis[18]. Nerve invasion is also one of the routes of tumor metastasis and mainly refers to neurotropic invasion and peripheral spread of the tumor[19]. It involves the detachment of tumor cells into the stroma and vascular system and the formation of tumor thrombi around the lesion and other tissues and organs, forming the basis of tumor metastasis; these are high-risk indicators affecting the recurrence rate of cervical cancer and patient survival[20]. Spondin-2 influences the proliferation, invasion, and metastasis of cancer cells by regulating the Wnt signaling pathway, which may be related to the invasion and infiltration of cervical cancer cells[21]. Moreover, the susceptibility of cervical cancer to invasion and infiltration may be related to immune dysfunction[14], and Spondin-2 may influence cancer cell invasion and infiltration by participating in the body’s specific and non-specific immune responses. T lymphocytes mediate the body’s antitumor immune response, which is crucial for HPV elimination and monitoring of abnormal growing cells; when the immune function is abnormal, malignant cervical cells cannot be recognized and removed in time, which triggers the development and progression of cervical cancer. Spondin-2 acts as an integrin ligand in the extracellular matrix and can interfere with the body’s normal immune response by recruiting inflammatory cells[22]. This study revealed that IL-4 and IL-5 in high Spondin-2 group were higher than those in low Spondin-2 group, while IL-2 and TNF- α level lower than low Spondin-2 group, suggesting that Spondin-2 is involved in the antitumor immune response. CD4+ is the primary cell type that exerts specific immune effects, including T helper type 1 (Th1) and Th2 subtypes, with the former secreting IL-2 and TNF-α. In contrast, the latter secretes IL-4 and IL-5, which play an immunosuppressive role. The findings of this study indicate that Spondin-2 can inhibit the antitumor immune response and promote the immune escape of tumor cells, thus affecting the progression of the disease. After 3 years of follow-up, PFS and OS were shorter in the high Spondin-2 group than in the low Spondin-2 group, indicating that high serum Spondin-2 expression is associated with a poor prognosis of cervical cancer and further validating the involvement of Spondin-2 in the development of cervical cancer.
In conclusion, the serum level of Spondin-2 is elevated in patients with cervical cancer and is linked to tumor invasion and infiltration, antitumor immune response, and prognosis. Thus, it is a potential novel diagnostic marker and treatment target for cervical cancer. However, this study did not investigate its specific mechanism of action in depth, and further studies should be conducted.
Cervical cancer is a gynecological malignancy that is common in middle-aged and older patients, with a high mortality rate; it seriously endangers the health of patients. Spondin-2 is an important molecular marker that is involved in innate and acquired immune responses. This study focused on the relationship between serum Spondin-2 expression, tumor invasion and infiltration, and immune response in patients with cervical cancer to provide a theoretical basis for clinical practice.
The motivation of this study was to investigate the differences in serum Spondin-2 expression levels in patients with cervical cancer, patients with benign uterine lesions, and healthy subjects, as well as the relationship between serum Spondin-2 Levels, tumor invasion and infiltration, and antitumor immune response.
This study aimed to investigate the relationship between serum Spondin-2 expression, tumor invasion and infiltration, and antitumor immune response in patients with cervical cancer.
We detected Spondin-2 expression in the sera of patients with cervical cancer or benign uterine lesions and in those of healthy subjects. According to the threshold of Spondin-2 used in cervical cancer diagnosis, patients with cervical cancer were divided into high Spondin-2 and low Spondin-2 groups. Clinicopathological features, immune cytokine levels, and prognosis of patients with different levels of Spondin-2 expression were compared.
The serum Spondin-2 expression level was significantly higher in the cervical cancer group than in the benign and control groups. The proportion of patients with Federation of Gynecology and Obstetrics stage III, nerve invasion, vascular invasion, and lymph node metastasis was higher in the high Spondin-2 group than in the low Spondin-2 group. The levels of interleukin (IL)-5 and IL-4 were higher in the high Spondin-2 group than in the low Spondin-2 group, whereas the levels of IL-2 and tumor necrosis factor-α were lower in the high Spondin-2 group than in the low Spondin-2 group. After 3 years of follow-up, progression-free survival and overall survival were significantly lower in the high Spondin-2 group than in the low Spondin-2 group.
The expression of Spondin-2 in patients with cervical cancer was upregulated, and it was associated with tumor invasion and infiltration, antitumor immune response, and prognosis.
Serum Spondin-2 Levels can be used as a new diagnostic marker and therapeutic target for cervical cancer, providing a theoretical basis for clinical diagnosis and disease evaluation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arif N, Pakistan; Javed R, India S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Gnade CM, Hill EK, Botkin HE, Hefel AR, Hansen HE, Sheets KA, Mott SL, Hardy-Fairbanks AJ, Stockdale CK. Is the age of cervical cancer diagnosis changing over time? J Gynecol Obstet Hum Reprod. 2021;50:102040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Wang Y, He M, Zhang G, Cao K, Yang M, Zhang H, Liu H. The immune landscape during the tumorigenesis of cervical cancer. Cancer Med. 2021;10:2380-2395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Li Y, Lu S, Wang S, Peng X, Lang J. Identification of immune subtypes of cervical squamous cell carcinoma predicting prognosis and immunotherapy responses. J Transl Med. 2021;19:222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Zhu BP, Guo ZQ, Lin L, Liu Q. Serum BSP, PSADT, and Spondin-2 levels in prostate cancer and the diagnostic significance of their ROC curves in bone metastasis. Eur Rev Med Pharmacol Sci. 2017;21:61-67. [PubMed] |
| 5. | Yuan X, Bian T, Liu J, Ke H, Feng J, Zhang Q, Qian L, Li X, Liu Y, Zhang J. Spondin2 is a new prognostic biomarker for lung adenocarcinoma. Oncotarget. 2017;8:59324-59332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, Chon HS, Chu C, Clark R, Cohn D, Crispens MA, Damast S, Dorigo O, Eifel PJ, Fisher CM, Frederick P, Gaffney DK, Han E, Huh WK, Lurain JR, Mariani A, Mutch D, Nagel C, Nekhlyudov L, Fader AN, Remmenga SW, Reynolds RK, Tillmanns T, Ueda S, Wyse E, Yashar CM, McMillian NR, Scavone JL. Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:64-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 700] [Article Influence: 140.0] [Reference Citation Analysis (0)] |
| 7. | Liu Z, Yang X, Liu R, Bao J, An N, Jiang S, Miao S, Guo C, Qu G, Meng H. Phototherapy together with it triggered immunological response for Anti-HPV treatment of oropharyngeal cancer: Removing tumor and pathogenic virus simultaneously. Biomaterials. 2021;272:120777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Ding W, Ma Y, Ma C, Malone DC, Ma A, Tang W, Si L. The Lifetime Cost Estimation of Human Papillomavirus-related Diseases in China: A Modeling Study. J Transl Int Med. 2021;9:200-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Wang Y, Hou K, Jin Y, Bao B, Tang S, Qi J, Yang Y, Che X, Liu Y, Hu X, Zheng C. Lung adenocarcinoma-specific three-integrin signature contributes to poor outcomes by metastasis and immune escape pathways. J Transl Int Med. 2021;9:249-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Dejima H, Hu X, Chen R, Zhang J, Fujimoto J, Parra ER, Haymaker C, Hubert SM, Duose D, Solis LM, Su D, Fukuoka J, Tabata K, Pham HHN, Mcgranahan N, Zhang B, Ye J, Ying L, Little L, Gumbs C, Chow CW, Estecio MR, Godoy MCB, Antonoff MB, Sepesi B, Pass HI, Behrens C, Vaporciyan AA, Heymach JV, Scheet P, Lee JJ, Wu J, Futreal PA, Reuben A, Kadara H, Wistuba II. Immune evolution from preneoplasia to invasive lung adenocarcinomas and underlying molecular features. Nat Commun. 2021;12:2722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 11. | Dogan S, Terzioglu E, Ucar S. Innate immune response against HPV: Possible crosstalking with endocervical γδ T cells. J Reprod Immunol. 2021;148:103435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, Gopalakrishnan V, Xi Y, Zhao H, Amaria RN, Tawbi HA, Cogdill AP, Liu W, LeBleu VS, Kugeratski FG, Patel S, Davies MA, Hwu P, Lee JE, Gershenwald JE, Lucci A, Arora R, Woodman S, Keung EZ, Gaudreau PO, Reuben A, Spencer CN, Burton EM, Haydu LE, Lazar AJ, Zapassodi R, Hudgens CW, Ledesma DA, Ong S, Bailey M, Warren S, Rao D, Krijgsman O, Rozeman EA, Peeper D, Blank CU, Schumacher TN, Butterfield LH, Zelazowska MA, McBride KM, Kalluri R, Allison J, Petitprez F, Fridman WH, Sautès-Fridman C, Hacohen N, Rezvani K, Sharma P, Tetzlaff MT, Wang L, Wargo JA. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577:549-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 815] [Cited by in RCA: 1713] [Article Influence: 342.6] [Reference Citation Analysis (0)] |
| 13. | Sun R, He L, Lee H, Glinka A, Andresen C, Hübschmann D, Jeremias I, Müller-Decker K, Pabst C, Niehrs C. RSPO2 inhibits BMP signaling to promote self-renewal in acute myeloid leukemia. Cell Rep. 2021;36:109559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Lu H, Feng Y, Hu Y, Guo Y, Liu Y, Mao Q, Xue W. Spondin 2 promotes the proliferation, migration and invasion of gastric cancer cells. J Cell Mol Med. 2020;24:98-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Yang Y, Shao ZQ, Guo JX. [Expression of malignant molecules in prostate cancer tissue and its correlation with serum PSA, XAGE-1b and Spondin-2 contents]. Hainan Yixueyuan Xuebao. 2015;21:1693-1696. [DOI] [Full Text] |
| 16. | Huang C, Ou R, Chen X, Zhang Y, Li J, Liang Y, Zhu X, Liu L, Li M, Lin D, Qiu J, Liu G, Zhang L, Wu Y, Tang H, Liu Y, Liang L, Ding Y, Liao W. Tumor cell-derived SPON2 promotes M2-polarized tumor-associated macrophage infiltration and cancer progression by activating PYK2 in CRC. J Exp Clin Cancer Res. 2021;40:304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 17. | Berger MD, Ning Y, Stintzing S, Heinemann V, Cao S, Zhang W, Yang D, Miyamoto Y, Suenaga M, Schirripa M, Hanna DL, Soni S, Puccini A, Tokunaga R, Naseem M, Battaglin F, Cremolini C, Falcone A, Loupakis F, Lenz HJ. A polymorphism within the R-spondin 2 gene predicts outcome in metastatic colorectal cancer patients treated with FOLFIRI/bevacizumab: data from FIRE-3 and TRIBE trials. Eur J Cancer. 2020;131:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Sun K, Lv H, Chen B, Nie C, Zhao J, Wang S, Wang J, Xu W, Chen X. Dawning precision treatment for gastric cancer: The latest biomarkers. J Transl Int Med. 2021;9:228-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Jackson SR, Costa MFDM, Pastore CF, Zhao G, Weiner AI, Adams S, Palashikar G, Quansah K, Hankenson K, Herbert DR, Vaughan AE. R-spondin 2 mediates neutrophil egress into the alveolar space through increased lung permeability. BMC Res Notes. 2020;13:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Hwang SU, Yoon JD, Kim M, Cai L, Choi H, Oh D, Kim E, Hyun SH. R-Spondin 2 and WNT/CTNNB1 Signaling Pathways Are Required for Porcine Follicle Development and In Vitro Maturation. Animals (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Gong Y, Yuan S, Sun J, Wang Y, Liu S, Guo R, Dong W, Li R. R-Spondin 2 Induces Odontogenic Differentiation of Dental Pulp Stem/Progenitor Cells via Regulation of Wnt/β-Catenin Signaling. Front Physiol. 2020;11:918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Kuramitsu S, Masuda T, Hu Q, Tobo T, Yashiro M, Fujii A, Kitagawa A, Abe T, Otsu H, Ito S, Oki E, Mori M, Mimori K. Cancer-associated Fibroblast-derived Spondin-2 Promotes Motility of Gastric Cancer Cells. Cancer Genomics Proteomics. 2021;18:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |