Published online Dec 26, 2022. doi: 10.12998/wjcc.v10.i36.13227
Peer-review started: July 7, 2022
First decision: October 27, 2022
Revised: November 7, 2022
Accepted: December 5, 2022
Article in press: December 5, 2022
Published online: December 26, 2022
Processing time: 172 Days and 6.1 Hours
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) for the diagnosis of mediastinal and hilar lymph is poorly studied in patients with extrathoracic malignancies.
To evaluate the value of EBUS-TBNA for the diagnosis of enlarged intrathoracic lymph nodes in patients with extrathoracic malignancies.
This was a retrospective study of patients with extrathoracic malignancies who were referred to Peking University Cancer Hospital from January 2013 to December 2018 for EBUS-TBNA due to intrathoracic lymphadenopathy. The specimens were defined as positive for malignancy, negative for non-malignancy (tuberculosis, sarcoidosis, etc.), and without a definitive diagnosis. Sensitivity, negative predictive value (NPV) for malignancy, and overall accuracy were calculated. Complications were recorded.
A total of 80 patients underwent EBUS-TBNA and had a final diagnosis, among which 50 (62.5%) were diagnosed with extrathoracic malignancy with intr
EBUS-TBNA is a simple and accurate procedure for the diagnosis of intrathoracic lymphadenopathy with extrathoracic malignancy.
Core Tip: This was a retrospective study of patients referred to Peking University Cancer Hospital from January 2013 to December 2018 for endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) due to intrathoracic lymphadenopathy. The specimens were defined as positive for malignancy, negative for non-malignancy (tuberculosis, sarcoidosis, etc.), and without definite diagnosis. Sensitivity, negative predictive value for malignancy, and overall accuracy were calculated. EBUS-TBNA was found to be a simple and accurate procedure for the diagnosis of intrathoracic lymphadenopathy with extrathoracic malignancy.
- Citation: Li SJ, Wu Q. Endobronchial ultrasound-guided transbronchial needle aspiration in intrathoracic lymphadenopathy with extrathoracic malignancy. World J Clin Cases 2022; 10(36): 13227-13238
- URL: https://www.wjgnet.com/2307-8960/full/v10/i36/13227.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i36.13227
Intrathoracic lymphadenopathy is a common incidental finding by computed tomography (CT) or positron emission tomography (PET)-CT in cases with synchronous or metachronous extrathoracic malignancies[1-3]. In such conditions, the causes of mediastinal or hilar nodal enlargement may be distal metastasis of the extrathoracic lesion, metastasis from a primary lung cancer synchronous with the extrathoracic malignancy, or even benign lesions including tuberculosis, granulomatous inflammation, and reactive changes[3-5]. In all of these situations, pathologic confirmation of the enlarged lymph node is crucial for the proper staging and management of patients[1].
Mediastinoscopy is considered the “gold standard” in nodal evaluation for intrathoracic lymphadenopathy, but it is invasive and requires general anesthesia[6]. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is currently the preferred modality to sample both mediastinal and hilar lymph nodes in primary lung cancer because it is not only minimally invasive but also can be performed under moderate conscious sedation or even under intratracheal surface anesthesia only[7,8]. Tournoy et al[9] showed that EBUS-TBNA is a diagnostic test for lung lesions after failed diagnostic bronchoscopy. A number of studies have shown that EBUS-TBNA is of value for the staging of lung cancer, as reviewed by Sehgal et al[10]. EBUS-TBNA is also of value for the diagnosis of mediastinal lymphoma[11]. In clinical practice, EBUS-TBNA could be considered the first-line examination for suspicious mediastinal lymph nodes in patients with extrathoracic cancer, preventing surgery in 50% of them[12]; in contrast, the use of EBUS-TBNA in intrathoracic lymphadenopathy in patients with extrathoracic solid organ malignancy is a relatively less investigated topic[13,14].
Therefore, the aim of this study was to evaluate the value of EBUS-TBNA for the diagnosis of enlarged intrathoracic lymph nodes in patients with extrathoracic malignancies.
This study was designed as a single-center retrospective case series study. Data from patients who were referred to Peking University Cancer Hospital (Beijing, China) from January 2013 to December 2018 for EBUS-TBNA due to intrathoracic lymphadenopathy were retrieved from the hospital database. The study was conducted according to good clinical practice and the Declaration of Helsinki. The protocol was approved by the ethics committee of Peking University Cancer Hospital (No. 2018YJZ72), which waived the need for individual consent.
The inclusion criteria for patients were synchronous or metachronous extrathoracic solid organ malignancy, and available radiological data including from CT or PET-CT scan. The exclusion criteria were synchronous or metachronous lymphoma or leukemia, or follow-up of < 12 mo when no definite diagnosis could be obtained by EBUS-TBNA with/without other interventional procedures including mediastinoscopy or thoracoscopy.
Before EBUS-TBNA, the target lymph nodes for sampling were selected according to enlarged mediastinal or hilar lymph node with a short axis > 10 mm in thorax CT or maximum standardized uptake (SUVmax) value > 2.5 in PET-CT. The lymph node map was determined according to the classification proposed by the International Association for the Study of Lung Cancer[15].
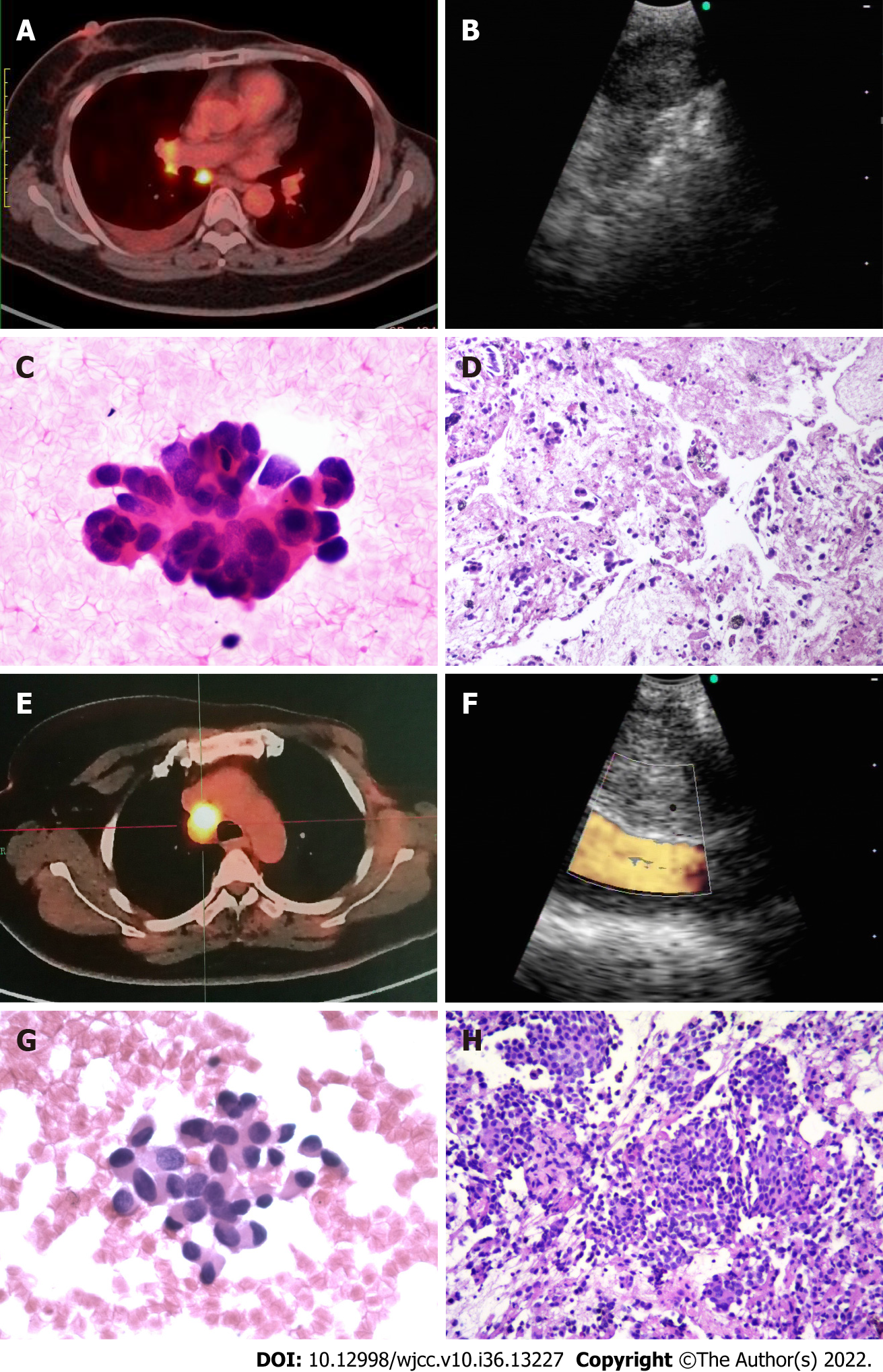
EBUS-TBNA was performed in an outpatient setting using a flexible bronchoscope (BF-UC260F-OL8; Olympus, Tokyo, Japan) by 1 of 2 experienced endoscopists (QW or SJL). First, local anesthesia was applied via aerosol inhalation and intratracheal spray of 2% lidocaine. Then, the EBUS scope was introduced, and all reachable lymph node stations were examined. Sampling was performed from mediastinal and hilar lymph nodes that had been previously identified as suspicious by imaging and were able to be accessed by EBUS-TBNA. For each target lesion, real-time punctures were made using a standard 22-gauge needle (ECHO-HD-22-EBUS-O; Cook Medical, Bloomington, IN, United States). Attempts were made to acquire both cytological and histological specimens during sampling, if possible (Figure 1). Major complications (e.g., serious hemorrhage > 100 mL, pneumothorax, and post-procedure infection) that occurred during and/or after surgery were recorded.
The cytological samples were prepared as air-dried smears on glass slides and in liquid fixative for thinprep cytological test. They histological specimens were fixed in formalin solution, and immunohistochemistry was performed when necessary (Figure 1).
The specimens obtained from EBUS-TBNA were defined as positive for malignancy, negative for non-malignancy (tuberculosis, sarcoidosis, etc.), and without definite diagnosis. In cases of no definite diagnosis, mediastinoscopy, video-assisted thoracic surgery (commonly known as VATS), or repeated EBUS-TBNA was recommended; patients who refused were subject to at least 12 mo of radiological and clinical follow-up every 3 mo. Follow-up was censored on December 31, 2018.
SPSS 20.0 software (IBM Corp., Armonk, NY, United States) was used for the statistical analyses. Continuous variables are presented as either means ± SD (normal distribution, Kolmogorov-Smirnov test) or median (minimum-maximum) (non-normal distribution). Categorical variables are presented as numbers and percentages and were analyzed using either the χ2 test or the Fisher’s exact test, as appropriate. Sensitivity for malignancy, negative predictive value (NPV) for non-malignancy, and overall accuracy were calculated. Predictors of malignant lymphadenopathy were modeled using logistic regression (enter method); variables with P < 0.05 in univariate analyses were included in multivariate analysis. P < 0.05 was considered statistically significant.
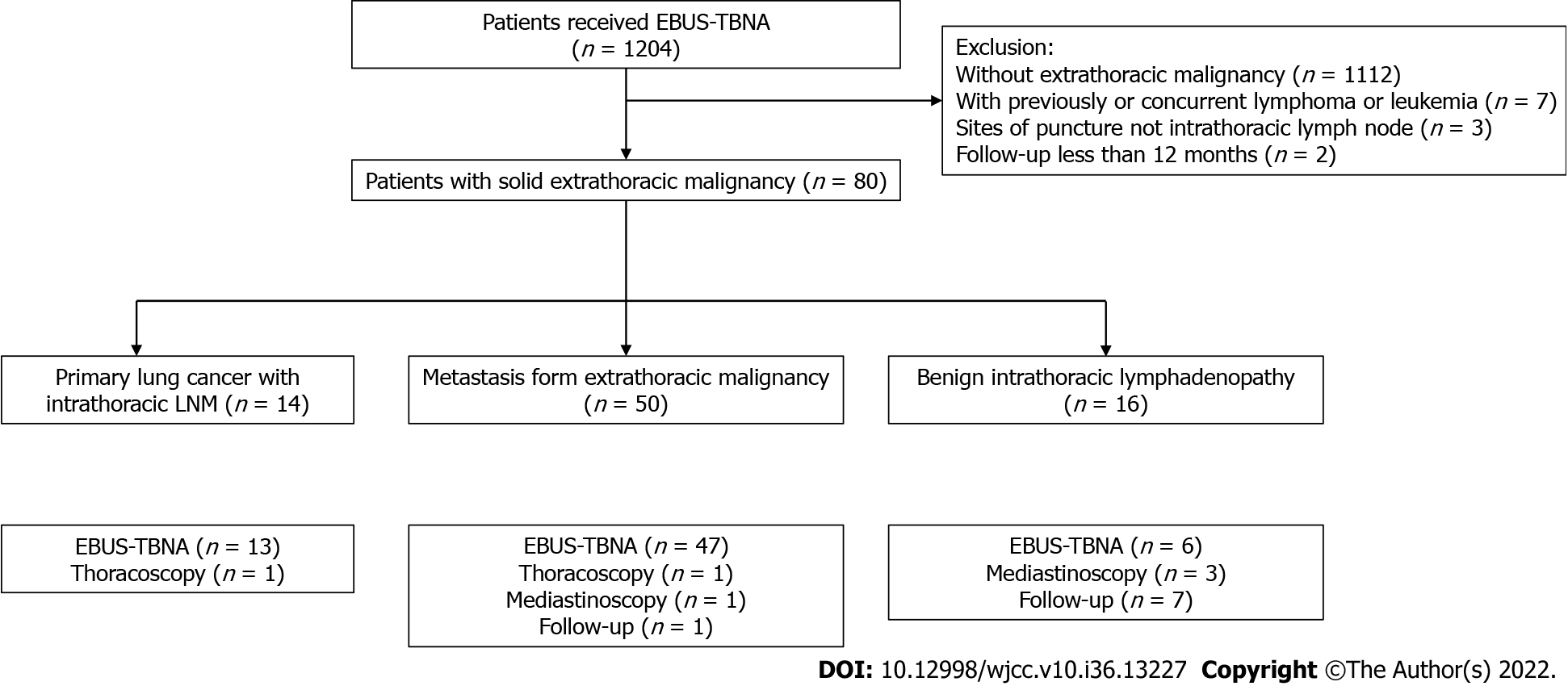
A total of 1204 patients who underwent EBUS-TBNA were reviewed. After exclusion of 1124 cases [without known extrathoracic malignancy (n = 1112), with previously or concurrent lymphoma or leukemia (n = 7), with sites of puncture not at the intrathoracic lymph node (n = 3), and with follow-up of less than 12 mo (n = 2)], 80 fulfilled the eligibility criteria (Figure 2). Among the cases included in the study (Table 1), the most common extrathoracic malignancies (65%) were breast, colorectal, and gastric cancers.
| Characteristics | n (%) or mean ± SD |
| Age (yr) | 58.5 ± 8.8 |
| Male sex | 36 (45.0) |
| Site of the primary extrathoracic malignancy | |
| Breast | 18 (22.5) |
| Colon | 12 (15.0) |
| Rectal | 11 (13.8) |
| Gastric | 11 (13.8) |
| Renal cell | 7 (8.8) |
| Thyroid | 6 (7.5) |
| Head and neck1 | 5 (6.3) |
| Endometrial | 3 (3.8) |
| Hepatic cell | 2 (2.5) |
| Other2 | 5 (6.3) |
| Site of intrathoracic lymphadenopathy | |
| Mediastinal only | 43 (53.8) |
| Mediastinal and hilar | 21 (26.2) |
| Hilar only | 16 (20.0) |
| Status of the extrathoracic malignancy | |
| Metachronous | 66 (82.5) |
| Synchronous | 14 (17.5) |
| CT findings | |
| Intrathoracic nodal enlargement with pulmonary lesion | 39 (48.8) |
| Short axis of the target lymph node in mm | 18.1 ± 6.7 |
| PET-CT findings | |
| Patients with PET-CT examination | 49 (61.3) |
| SUVmax of the target lymph nodes | 9.2 ± 5.0 |
The 80 included patients had a total of 123 enlarged lymph nodes sampled by EBUS-TBNA (median of one lymph node every patient; range: 1-4). Cytological specimens were successfully acquired from all 80 patients, and histological specimens were available for 74 (92.5%). The paratracheal region was the most common site (42.2%) for puncture (Table 2). The median number of puncture times was two for each lymph node (range: 1-6).
| Extrathoracic malignancy | n (%) | Location | EBUS | Surgery | Follow-up | |||||||
| Paratracheal | Subcarinal | Hilar and lobar | ETM LNM | PLC LNM | Benign | ETM LNM | PLC LNM | Benign | FO | ETM LNM | ||
| Breast cancer | 18 (22.5) | 12 | 8 | 8 | 11 | 3 | 2 | - | - | 1 | 1 | - |
| Colon cancer | 12 (15.0) | 8 | 4 | 4 | 7 | 3 | - | - | - | - | 1 | 1 |
| Rectal cancer | 11 (13.8) | 4 | 2 | 8 | 7 | 3 | - | 1 | - | - | - | - |
| Gastric cancer | 11 (13.8) | 7 | 5 | 6 | 6 | 2 | - | - | 1 | 1 | - | |
| Renal cell cancer | 7 (8.8) | 6 | 4 | 2 | 6 | - | - | 1 | - | - | - | - |
| Thyroid cancer | 6 (7.5) | 6 | 2 | 2 | 1 | 2 | 3 | - | - | - | - | - |
| Head and neck cancer1 | 5 (6.3) | 3 | 3 | 2 | 3 | - | - | - | 1 | 1 | - | - |
| Endometrial cancer | 3 (3.8) | 1 | 2 | 2 | 2 | - | 1 | - | - | - | - | - |
| Hepatic cell cancer | 2 (2.5) | 1 | 1 | 0 | 2 | - | - | - | - | - | - | - |
| Other2 | 5 (6.3) | 4 | 2 | 4 | 2 | - | 2 | - | - | - | 1 | - |
| Total | 80 (100.0) | 52 | 33 | 38 | 47 | 13 | 9 | 2 | 1 | 3 | 5 | |
EBUS-TBNA diagnosed intrathoracic nodal metastasis from extrathoracic malignancy in 47 (58.8%) patients, and intrathoracic lymphadenopathy due to primary lung cancer in 13 (16.3%) patients (including 8 Lung adenocarcinomas, 3 Lung squamous carcinomas, and 2 small cell lung cancers). Regarding the 9 patients with benign diagnosis, sarcoidosis was diagnosed in 3, caseous granulomatosis with suspected tuberculosis in 3, and non-caseating granulomatous inflammation in 3. For the last 3, the inflammation was clinically inconsistent with sarcoidosis because of negative fungal and mycobacterial cultures. All 3 refused further interventional procedures, and after a median follow-up of 15 (14-19) mo, clinical and radiological results suggested benign behavior (all enlarged lymph nodes remained stable).
EBUS-TBNA found normal lymph node tissue or non-specific tissue for pathologic diagnosis in 11 (13.8%) patients. Six of them received surgical intervention (four mediastinoscopy procedures and two VATS procedures) to obtain pathologic results. Reactive change was found in 2 patients, tuberculosis was found in 1, squamous carcinoma of lung with hilar lymph node metastasis in 1, rectal cancer lung metastasis with nodal involvement in 1, and renal cell cancer with mediastinal nodal involvement in 1. The remaining 5 patients who refused surgical intervention received periodical clinical and radiologic follow-up. One patient had progressive lymphadenopathy that was clinically considered metastasis from extrathoracic malignancy (colon cancer) and accepted the recommendation of systemic chemotherapy by multidisciplinary team. The remaining 4 patients showed a favorable outcome (1 was stable and 3 showed regressive lymphadenopathy) during a median follow-up of 16 (13-18) mo.
Ultimately, 50 (62.5%) patients were diagnosed with extrathoracic malignancy with intrathoracic lymph nodes metastasis and 14 (17.5%) with primary lung cancer with nodal involvement; the remaining 16 (20.0%) patients exhibited benign behavior including tuberculosis, sarcoidosis and reactive lymphadenitis, or who had benign follow-up. The diagnostic sensitivity, NPV, and accuracy of EBUS-TBNA for intrathoracic lymphadenopathy in patients with extrathoracic malignancy were 93.8% (n = 60/64), 80.0% (n = 16/20), and 95.0% (n = 76/80), respectively.
Univariate analyses revealed that longer short axis of the lymph node and synchronous lung lesion were associated with the presence of metastatic lymphadenopathy (P = 0.003 and P = 0.001, respectively). In the logistic regression multivariate model, longer short axis of the lymph node (OR: 1.200, 95%CI: 1.024-1.407) and synchronous lung lesion (OR: 19.449, 95%CI: 1.875-201.753) were independently associated with malignant intrathoracic lymphadenopathy (P = 0.024 and P = 0.013, respectively; Table 3).
| Covariates | Univariate analysis | Multivariate analysis | ||
| OR (95%CI) | P | OR (95%CI) | P | |
| Sex | ||||
| Female | 1 | |||
| Male | 1.720 (0.727-4.071) | 0.217 | ||
| Age (yr) | ||||
| ≤ 60 | 1 | |||
| > 60 | 1.647 (0.696-3.900) | 0.257 | ||
| Size of sampled lymph node | 1.113 (1.036-1.196) | 0.003 | 1.200 (1.024-1.407) | 0.024 |
| Site of lymphadenopathy | ||||
| Mediastinal and hilar | 1 | |||
| Mediastinal only | 1.244 (0.528-2.932) | 0.617 | ||
| Hilar only | 6.788 (0.819-56.257) | 0.076 | ||
| Status of synchronous lung lesion | ||||
| Without | 1 | 1 | ||
| With | 8.082 (2.292-28.501) | 0.001 | 19.449 (1.875-201.753) | 0.013 |
| Status of synchronous ETM | ||||
| Synchronous | 1 | |||
| Metachronous | 1.057 (0.629-1.776) | 0.834 | ||
| SUVmax of sampled lymph node | 0.987 (0.887-1.098) | 0.806 | ||
Univariate analyses demonstrated that no characteristics of the lymph nodes themselves and EBUS-TBNA were associated with the yield of malignant intrathoracic lymphadenopathy (Table 4).
| Covariates | Accurate number, | Univariate analysis | |
| n (%) | OR (95%CI) | P value | |
| Sex | |||
| Female | 46 (92.0) | 1 | |
| Male | 41 (93.2) | 1.783 (0.310-10.246) | 0.571 |
| Age (yr) | |||
| ≤ 60 | 47 (92.2) | 1 | |
| > 60 | 40 (93.0) | 1.702 (0.296-9.785) | 0.551 |
| Location of sampled lymph node | |||
| Hilar | 27 (93.1) | 1 | |
| Paratracheal | 36 (92.3) | 2.000 (0.312-12.815) | 0.465 |
| Subcarinal | 24 (92.3) | 2.667 (0.260-27.381) | 0.409 |
| Determination of target lymph node | |||
| PET-CT and CT | 52 (90.0) | 1 | |
| CT only | 35 (97.2) | 3.365 (0.377-30.052) | 0.277 |
| Size of short axis in sampled lymph node in mm | 18.9 ± 7.0 | 1.093 (0.975-1.248) | 0.191 |
| SUVmax of sampled lymph node | 8.8 ± 5.1 | 0.877 (0.753-1.015) | 0.077 |
| Number of passes per lymph node, times, median (range) | 2 (1-5) | 2.097 (0.691-6.253) | 0.193 |
| Histological specimen acquired | |||
| No | 7 (87.5) | 1 | |
| Yes | 80 (93.0) | 2.286 (0.233-22.387) | 0.478 |
| Operator of EBUS-TBNA | |||
| Dr. LSJ | 70 (92.1) | 1 | |
| Dr. WQ | 17 (94.4) | 1.124 (0.133-11.085) | 0.863 |
No major complications occurred during the EBUS-TBNA procedures. Twelve (15.0%) patients experienced transient hypoxemia and all recovered after immediate increase of oxygen flow.
EBUS-TBNA for the diagnosis of mediastinal and hilar lymph is poorly studied in patients with extrathoracic malignancies. This study’s evaluation of the value of EBUS-TBNA for the diagnosis of enlarged intrathoracic lymph nodes in patients with extrathoracic malignancies suggested that the procedure is simple and accurate for diagnosis of intrathoracic lymphadenopathy with extrathoracic malignancy. Moreover, there were no complications.
With the rapid development of cancer therapies, overall survival of patients has improved significantly[16]. Regular follow-up and surveillance are essential and, unfortunately, the detection of intrathoracic lymphadenopathy is not uncommon, due in part to improvements in imaging technologies. Up to 30% of extrathoracic malignancies may lead to intrathoracic lymph node metastasis[17]. The most common solid malignancies responsible include breast, colorectal, head and neck, melanoma, kidney, and stomach cancers[14,18,19]. This study showed that breast, colorectal, gastric, and renal cell cancers accounted for 73.4% (59/80) of the extrathoracic malignancies in our patients; of note, however, differences in other cancer types might simply be due to the differences in cancer types treated at our hospital.
Primary lung cancer with nodal involvement accounts for the majority of mediastinal and hilar lymphadenopathy cases[20], but Mehta et al[13] showed a high frequency of benign diagnoses. EBUS-TBNA is considered safe and feasible for tissue sampling with access to both mediastinal and hilar lymph nodes, which is recommended by both the American College of Chest Physicians and European Society of Thoracic Surgeons in primary lung cancer[7,8]. In a meta-analysis that included nine studies and 1066 patients, EBUS-TBNA had pooled sensitivity of 90%, accuracy of 96%, and NPV of 93% for mediastinal staging[21]. EBUS-TBNA also showed an acceptable diagnostic ability in determining intrathoracic lymphadenopathy with extrathoracic malignancy. In a meta-analysis of six studies (553 patients) by Yang et al[17], EBUS-TBNA provided pooled sensitivity of 85%, accuracy of 85%, and negative likelihood ratio of 16%. The present study revealed sensitivity, accuracy, and NPV of 93.8%, 95.0%, and 80.0%, respectively, by EBUS-TBNA for intrathoracic lymphadenopathy in patients with extrathoracic malignancy, in accordance with a previous study[17]. As intrathoracic metastasis always indicates an advanced stage of the primary extrathoracic malignancy, early and accurate identification of the nature of the lymph node is important for staging, treatment strategy, and prognosis. Considering the promising diagnostic value of EBUS-TNBA, the procedure could be recommended as the first diagnostic procedure for mediastinal and hilar lymphadenopathies seen in extrathoracic malignancies.
Regarding granulomatous lymphadenitis, the diagnostic yield of EBUS-TBNA seems efficacious. A meta-analysis of 15 studies with 553 patients found that the pooled diagnostic accuracy for sarcoidosis was 79% by EBUS-TBNA[22]. Concerning tuberculous lymphadenitis diagnosis, in a meta-analysis of 14 studies with 684 patients, EBUS-TBNA showed a pooled diagnostic yield of 80%[23]. In regard to EBUS-TBNA for non-caseating granulomatous, more attention should be paid. Sanz-Santos et al[14] reported a case of non-caseating granulomatous detected by EBUS-TBNA that was diagnosed with lymphoma after 6 mo of follow-up. Kitamura et al[24] reported that 6% of patients with thoracic malignancy may have sarcoid-like reactions in non-metastatic lymph nodes. The present study found 3 patients with tuberculosis and 3 with sarcoidosis, but we could not obtain specimens for the 3 granulomatous cases and their benign behavior was merely determined through follow-up. These results suggest that in the case of the possibility of undiscovered malignancy, all granulomatous lymphadenitis cases diagnosed by EBUS-TBNA without obvious infection or sign of sarcoidosis should be followed with more attention or surgical interventions should be advised. Evison et al[25] suggested that for suspicious mediastinal and/or hilar lymph nodes but negative EBUS-TBNA, follow-up rather than resampling could be an appropriate approach.
The radiologic diagnosis of nodal involvement is mostly based on morphologic changes such as increase in size, coexistence of pulmonary lesions on CT scans, and lymph nodes > 2 cm in short axis[26]. In this study, both univariate and multivariate regression suggested that the diameter of the lymph node and synchronous lung lesion can be malignancy indicators. As intrathoracic lymph node metastasis is relatively common in primary lung cancer[4], patients with a history of extrathoracic malignancy, exhibiting intrathoracic lymphadenopathy combined with lung lesion, should be distinguished between distal metastasis and extrathoracic lesion and between intrathoracic metastasis and lung cancer. In such conditions, EBUS-TBNA may provide help in pathologic evidence acquisition.
PET-CT is widely used in cancer staging and distant metastasis detection. Generally, a SUVmax value > 2.5 could be clinically correlated with the risk of malignancy, and a SUVmax value > 6.3 is considered malignant with sensitivity and specificity of 70.6% and 83.3%, respectively[27]. In the present study, no significant relationship was found between the SUVmax value and the presence of malignancy. This may be due to the possibility that increased SUVmax values can also be seen in benign pathologies involving inflammation such as sarcoidosis and infectious disease[5]. Moreover, not all patients in our study underwent PET-CT, which may have also caused data bias.
As with other interventional procedures, the high diagnostic yield of EBUS-TBNA is associated not only with lesion-related factors such as size, location, and metabolic activity but also with procedure-related factors such as the operator’s experience, number of passes, and number of lymph nodes sampled[28]. In this study, none of the above factors showed a significant relationship with accurate diagnosis in malignancy. That may be caused by mixture of multiple lesion categories.
This study had a couple of limitations. First, it was a retrospective study, which intrinsically has a certain selection bias. Second, several cases without definite diagnosis by EBUS-TBNA did not have histological confirmation. In the future, a well-designed prospective study should overcome the limitations above.
In conclusion, EBUS-TBNA is a simple and accurate procedure for the diagnosis of intrathoracic lymphadenopathy with extrathoracic malignancy. Its application resulted in no major complications.
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is an established technique for the diagnosis of mediastinal and hilar lymphadenectasis in primary lung cancer, but is poorly studied in patients with extrathoracic malignancies.
Regular follow-up and surveillance are essential in cancer patients, and the detection of intrathoracic lymphadenopathy in those with extrathoracic malignancies is not uncommon. EBUS-TBNA is recommended for tissue sampling both in mediastinal and hilar lymph nodes in lung cancer. Data on the usefulness of this technique in patients with extrathoracic malignancies remain limited.
In this study, we describe our experience with the use of EBUS-TBNA in patients with extrathoracic malignancies due to intrathoracic lymphadenopathy.
The results of the sample acquired by EBUS-TBNA were defined as positive for malignancy, negative for non-malignancy (tuberculosis, sarcoidosis, etc.), and without definite diagnosis. Sensitivity, negative predictive value (NPV) for malignancy, and overall accuracy were ca
The diagnostic sensitivity, NPV, and accuracy of EBUS-TBNA for intrathoracic lymphadenopathy in patients with extrathoracic malignancy were 93.8% (n = 60/64), 80.0% (n = 16/20), and 95.0% (n = 76/80), respectively. Longer short axis of the lymph node (P = 0.024) and synchronous lung lesion (P = 0.013) were independently associated with malignant intrathoracic lymphadenopathy. No major complication was observed.
EBUS-TBNA is a simple and accurate procedure for the diagnosis of intrathoracic lymphadenopathy with extrathoracic malignancy. Its application resulted in no major complications.
This retrospective study demonstrates that EBUS-TBNA is effective and safe for diagnosis of intrathoracic lymphadenopathy in patients with extrathoracic malignancy. Additional prospective studies are warranted to establish standards for higher diagnostic yield.
We thank Zhong-Hu He for his generous help with the data analyses.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Esch M, Germany; Moshref L, Saudi Arabia S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Munden RF, Carter BW, Chiles C, MacMahon H, Black WC, Ko JP, McAdams HP, Rossi SE, Leung AN, Boiselle PM, Kent MS, Brown K, Dyer DS, Hartman TE, Goodman EM, Naidich DP, Kazerooni EA, Berland LL, Pandharipande PV. Managing Incidental Findings on Thoracic CT: Mediastinal and Cardiovascular Findings. A White Paper of the ACR Incidental Findings Committee. J Am Coll Radiol. 2018;15:1087-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 2. | Frank L, Quint LE. Chest CT incidentalomas: thyroid lesions, enlarged mediastinal lymph nodes, and lung nodules. Cancer Imaging. 2012;12:41-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Nin CS, de Souza VV, do Amaral RH, Schuhmacher Neto R, Alves GR, Marchiori E, Irion KL, Balbinot F, Meirelles GS, Santana P, Gomes AC, Hochhegger B. Thoracic lymphadenopathy in benign diseases: A state of the art review. Respir Med. 2016;112:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Shroff GS, Viswanathan C, Carter BW, Benveniste MF, Truong MT, Sabloff BS. Staging Lung Cancer: Metastasis. Radiol Clin North Am. 2018;56:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Larici AR, Glaudemans AW, Del Ciello A, Slart RH, Calandriello L, Gheysens O. Radiological and nuclear medicine imaging of sarcoidosis. Q J Nucl Med Mol Imaging. 2018;62:14-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | McNally PA, Arthur ME. Mediastinoscopy. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. [PubMed] |
| 7. | Silvestri GA, Gonzalez AV, Jantz MA, Margolis ML, Gould MK, Tanoue LT, Harris LJ, Detterbeck FC. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e211S-e250S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 1015] [Article Influence: 84.6] [Reference Citation Analysis (0)] |
| 8. | De Leyn P, Dooms C, Kuzdzal J, Lardinois D, Passlick B, Rami-Porta R, Turna A, Van Schil P, Venuta F, Waller D, Weder W, Zielinski M. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2014;45:787-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 561] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 9. | Tournoy KG, Rintoul RC, van Meerbeeck JP, Carroll NR, Praet M, Buttery RC, van Kralingen KW, Rabe KF, Annema JT. EBUS-TBNA for the diagnosis of central parenchymal lung lesions not visible at routine bronchoscopy. Lung Cancer. 2009;63:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Sehgal IS, Agarwal R, Dhooria S, Prasad KT, Aggarwal AN. Role of EBUS TBNA in Staging of Lung Cancer: A Clinician's Perspective. J Cytol. 2019;36:61-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Dhooria S, Mehta RM, Madan K, Vishwanath G, Sehgal IS, Chhajed PN, Prakash G, Gupta N, Bal A, Agarwal R. A Multicenter Study on the Utility of EBUS-TBNA and EUS-B-FNA in the Diagnosis of Mediastinal Lymphoma. J Bronchology Interv Pulmonol. 2019;26:199-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Fournier C, Hermant C, Gounant V, Escarguel B, Thibout Y, Lachkar S, Raspaud C, Vergnon JM. Diagnostic of mediastinal lymphadenopathy in extrathoracic cancer: A place for EBUS-TBNA in real life practice? Respir Med Res. 2019;75:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Mehta RM, Biraris P, Patil S, Singla A, Kallur K, Gasparini S. Utility of EBUS-TBNA in PET-positive mediastinal lymph nodes in subjects with extra-thoracic malignancy. PLoS One. 2019;14:e0213437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Sanz-Santos J, Cirauqui B, Sanchez E, Andreo F, Serra P, Monso E, Castellà E, Llatjós M, Mesa M, Ruiz-Manzano J, Rosell R. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of intrathoracic lymph node metastases from extrathoracic malignancies. Clin Exp Metastasis. 2013;30:521-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P; Members of IASLC Staging Committee. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:568-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 812] [Article Influence: 50.8] [Reference Citation Analysis (1)] |
| 16. | Chen W, Sun K, Zheng R, Zeng H, Zhang S, Xia C, Yang Z, Li H, Zou X, He J. Cancer incidence and mortality in China, 2014. Chin J Cancer Res. 2018;30:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 703] [Article Influence: 100.4] [Reference Citation Analysis (2)] |
| 17. | Yang B, Li F, Shi W, Liu H, Sun S, Zhang G, Jiao S. Endobronchial ultrasound-guided transbronchial needle biopsy for the diagnosis of intrathoracic lymph node metastases from extrathoracic malignancies: a meta-analysis and systematic review. Respirology. 2014;19:834-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Navani N, Nankivell M, Woolhouse I, Harrison RN, Munavvar M, Oltmanns U, Falzon M, Kocjan G, Rintoul RC, Janes SM. Endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of intrathoracic lymphadenopathy in patients with extrathoracic malignancy: a multicenter study. J Thorac Oncol. 2011;6:1505-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Tertemiz KC, Alpaydin AO, Karacam V. The role of endobronchial ultrasonography for mediastinal lymphadenopathy in cases with extrathoracic malignancy. Surg Endosc. 2017;31:2829-2836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e78S-e92S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 332] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 21. | Dong X, Qiu X, Liu Q, Jia J. Endobronchial ultrasound-guided transbronchial needle aspiration in the mediastinal staging of non-small cell lung cancer: a meta-analysis. Ann Thorac Surg. 2013;96:1502-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Agarwal R, Srinivasan A, Aggarwal AN, Gupta D. Efficacy and safety of convex probe EBUS-TBNA in sarcoidosis: a systematic review and meta-analysis. Respir Med. 2012;106:883-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 168] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 23. | Li W, Zhang T, Chen Y, Liu C, Peng W. Diagnostic Value of Convex Probe Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration in Mediastinal Tuberculous Lymphadenitis: A Systematic Review and Meta-Analysis. Med Sci Monit. 2015;21:2064-2072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Kitamura A, Takiguchi Y, Kurosu K, Takigawa N, Saegusa F, Hiroshima K, Nakajima T, Tanabe N, Nakatani Y, Yoshino I, Tatsumi K. Feasibility of cytological diagnosis of sarcoidosis with endobronchial US-guided transbronchial aspiration. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29:82-89. [PubMed] |
| 25. | Evison M, Crosbie PA, Morris J, Martin J, Barber PV, Booton R. A study of patients with isolated mediastinal and hilar lymphadenopathy undergoing EBUS-TBNA. BMJ Open Respir Res. 2014;1:e000040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Brufau BP, Cerqueda CS, Villalba LB, Izquierdo RS, González BM, Molina CN. Metastatic renal cell carcinoma: radiologic findings and assessment of response to targeted antiangiogenic therapy by using multidetector CT. Radiographics. 2013;33:1691-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 27. | Kandemir Z, Sentürk A, Ozdemir E, Yildirim N, Hasanoğlu HC, Keskin M, Türkölmez S. The evaluation of hypermetabolic mediastinal-hilar lymph nodes determined by PET/CT in pulmonary and extrapulmonary malignancies: correlation with EBUS-TBNA. Turk J Med Sci. 2015;45:1234-1242. [PubMed] |
| 28. | Muthu V, Sehgal IS, Dhooria S, Prasad KT, Gupta N, Aggarwal AN, Agarwal R. Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: Techniques and Challenges. J Cytol. 2019;36:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |