Published online Dec 26, 2022. doi: 10.12998/wjcc.v10.i36.13208
Peer-review started: September 16, 2022
First decision: October 19, 2022
Revised: October 30, 2022
Accepted: November 30, 2022
Article in press: November 30, 2022
Published online: December 26, 2022
Processing time: 101 Days and 7.1 Hours
Hypersplenism associated with cirrhotic portal hypertension is a common condition often resulting from hepatitis B-related cirrhosis. However, the levels of immunoglobulin (Ig) and complement in patients with hypersplenism associated with cirrhotic portal hypertension remain unclear. This study was undertaken to determine the levels of Ig and complement in these patients, the relationship between these levels and Child-Pugh class and their clinical significance.
To investigate the antibody (Ig) and complement levels in patients with hypersplenism associated with cirrhotic portal hypertension and their clinical significance.
Clinical data of 119 patients with hypersplenism associated with cirrhotic portal hypertension were statistically analyzed and compared with those of 128 control patients.
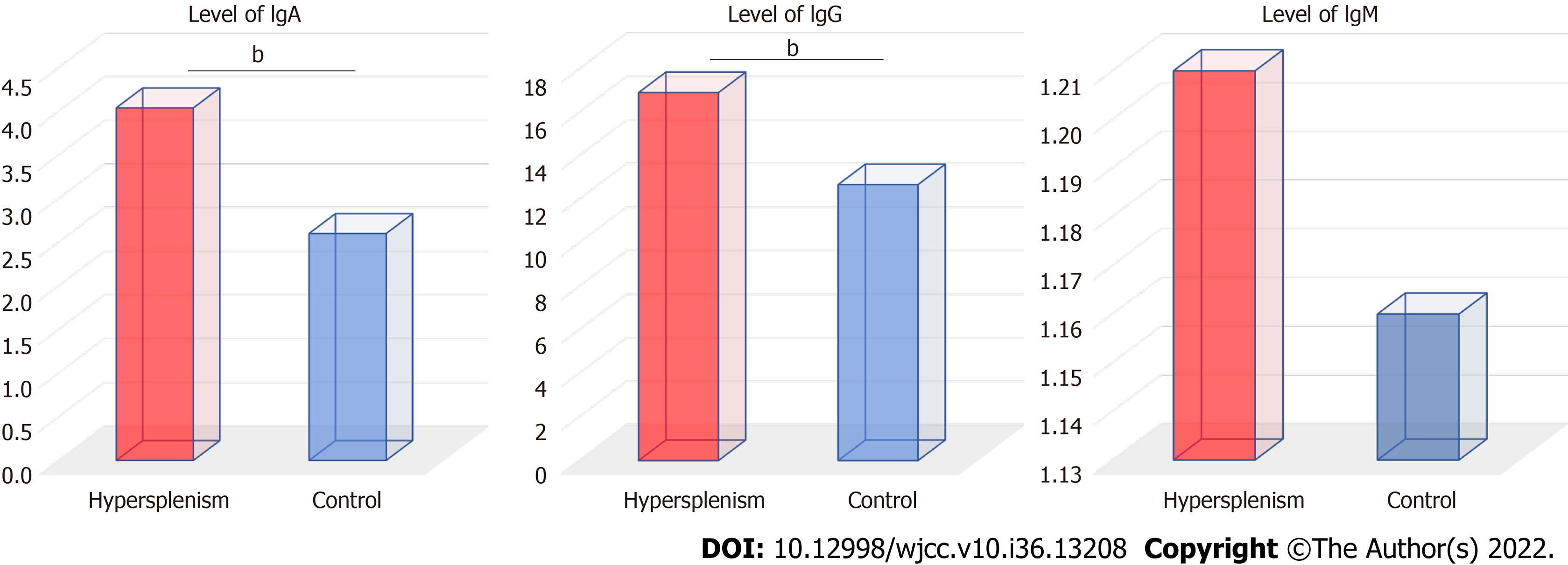
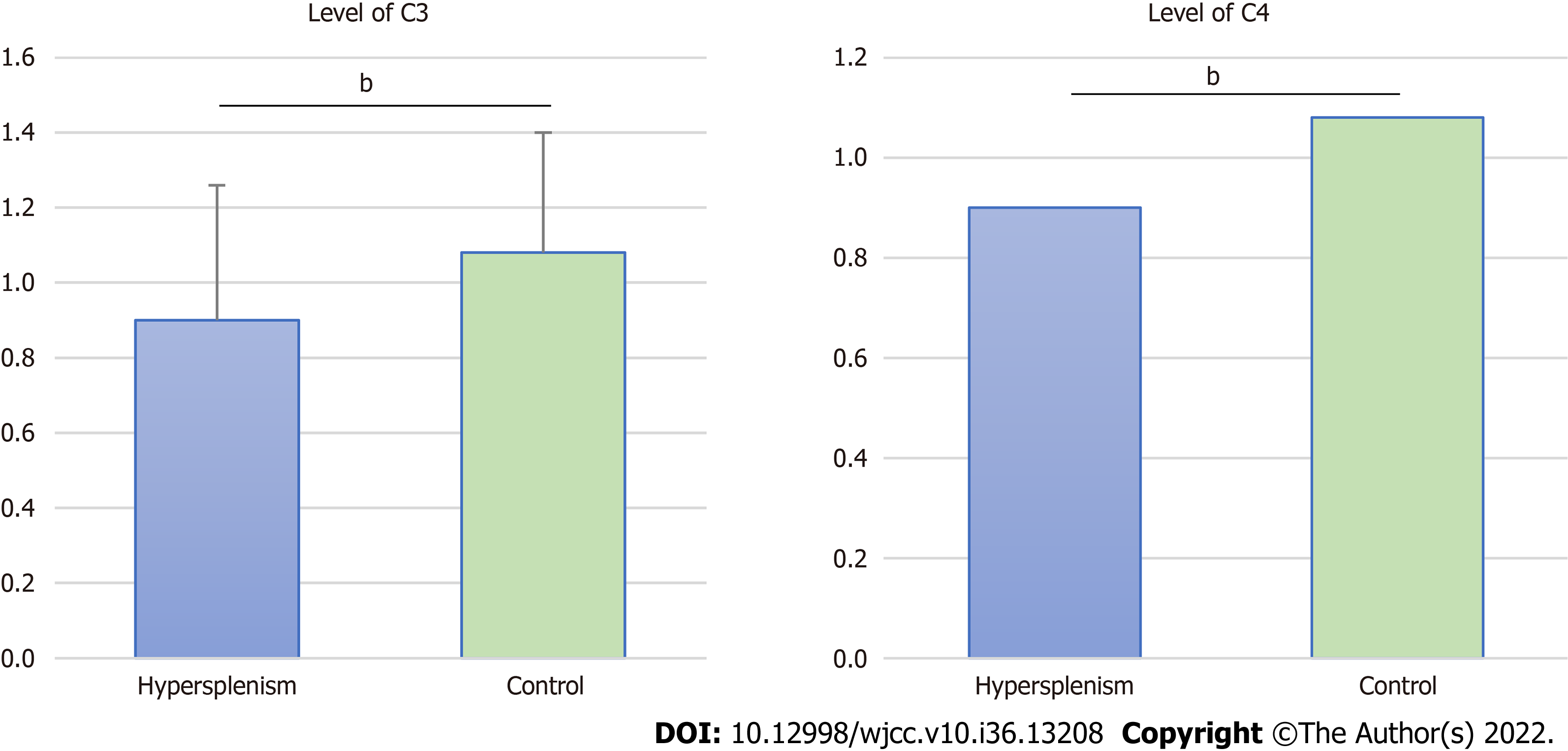
IgA and IgG levels in patients with hypersplenism were significantly higher than controls (P < 0.001). There was no significant difference in IgM between the two groups (P = 0.109). C3 and C4 levels in patients with hypersplenism were significantly lower than controls (P < 0.001). As liver function decreased, IgA and IgG levels increased (P < 0.001), and C3 and C4 levels decreased (P < 0.001).
Patients with hypersplenism associated with cirrhotic portal hypertension have significantly higher antibody (IgA and IgG) levels and significantly lower complement (C3 and C4) levels, which are both related to liver damage. Clinically, the administration of anti-hepatitis virus agents and protection of liver function should be strengthened.
Core Tip: Patients with cirrhotic portal hypertension and hypersplenism are clinically common. The spleen is an important immune organ, but studies on antibody and complement levels in patients are scarce. This study found that IgA and IgG levels increased and complement levels decreased in our patient population compared to the healthy controls. These findings indicate liver damage, supporting the need for anti-viral treatment in these patients.
- Citation: Zhang K, Zeng M, Li YJ, Wu HF, Wu JC, Zhang ZS, Zheng JF, Lv YF. Antibody and complement levels in patients with hypersplenism associated with cirrhotic portal hypertension and therapeutic principles. World J Clin Cases 2022; 10(36): 13208-13215
- URL: https://www.wjgnet.com/2307-8960/full/v10/i36/13208.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i36.13208
Hypersplenism associated with cirrhotic portal hypertension is a common condition often resulting from hepatitis B-related cirrhosis[1]. Hepatitis B is a chronic infectious disease caused by hepatitis B virus (HBV) infection[2]. Under normal circumstances, the immune system protects the body by defending against external invading pathogens, maintaining physiological balance and eliminating diseased cells through cellular and/or humoral immunity. An abnormal immune response, whether hyperactive (i.e. allergy) or hypoactive (i.e. immunodeficiency), can cause tissue damage and immunopathological reactions[3].
HBV infection is a global public health issue; in particular, China has a high prevalence of HBV[4]. The pathogenesis of HBV infection is not yet fully understood. Numerous studies have shown that the immunopathological response and the interaction between the virus and host cells are the main causes of liver cell damage[5]. The progression and outcome of HBV infection is therefore related to the host’s immune response. Immunosuppression or immune system disorder can cause HBV replication, leading to chronic infection, and then develop cirrhosis. Liver cirrhosis caused by any reason may lead to hypersplenism related to portal hypertension[6] and possibly liver cancer.
Antibodies are important effector molecules that mediate humoral immunity by binding to specific antigens. They are immunoglobulins (Ig) produced by plasma cells, which are differentiated from B cells and memory B cells in the immune system under antigen stimulation[7]. They are distributed in the serum, tissue fluid, exocrine fluid and on the surface of some cell membranes. They demonstrate antibody-dependent cell-mediated cytotoxicity and play a role in neutralization, opsonization and complement activation[8,9].
By combining different heavy and light chains, Igs form complete antibody molecules that can be classified into five types: IgG, IgM, IgA, IgD and IgE. IgG is the only antibody that can cross the placental barrier and is the main component of serum Igs[10]. It is the main antibody produced during the immune response and the “main force” to fight against infection; in fact, most antibody activity in the serum is related to IgG. It activates complement through the classical pathway and binds to Fc receptors on the surface of macrophages and natural killer cells to regulate antibody-dependent cell-mediated cytotoxicity[11]. IgM accounts for 5%-10% of the total serum Ig pool. It is the first antibody to be produced during ontogeny[12] and to appear in the primary humoral immune response, serving as the “vanguard” for specific defense against infection. IgA is an exocrine Ig that participates in local mucosal immunity and plays an important role as the ”border guard” for local defense against infection. IgE is mainly present in the allergic response[13], and IgD is present only in trace amounts[14,15].
Complement proteins play vital roles in the immune response, affecting both innate and adaptive immunity, regulating the immune response at different stages and influencing the immunological function of antibodies. In particular, the complement protein C3 plays a critical role. The level of serum C3 is proportional to the total amount of complement, and the serum C3 and C4 levels provide a good estimate for the total serum complement level[16,17]. However, the levels of Ig and complement in patients with hypersplenism associated with cirrhotic portal hypertension remain unclear. This study was undertaken to determine the levels of Ig and complement in these patients, the relationship between these levels and Child-Pugh class and their clinical significance.
A total of 119 patients with hypersplenism were compared with a control group of 128 patients. All methods were performed in accordance with the relevant guidelines and regulations/Declaration of Helsinki. Informed consent was obtained from all patients.
The hypersplenism group was composed of hypersplenism caused by cirrhosis and portal hypertension. Patients with hypersplenism caused by non-cirrhotic portal hypertension, such as lymphoma, pulmonary tuberculosis, connective tissue and inflammatory diseases, were excluded. The 119 patients included 93 males and 26 females, with a male-to-female ratio of 3.6:1. Their ages ranged from 41 years to 82 years, with a mean of 51 years. Among them, 95 patients (80.0%) had hepatitis B cirrhosis, 13 (10.9%) had hepatitis C cirrhosis, 3 (2.5%) had biliary cirrhosis, and 8 (6.6%) had other types of cirrhosis. Liver cirrhosis and splenomegaly (as assessed by B-Mode ultrasound and computed tomography), mono- or multilineage peripheral cytopenias (as assessed by laboratory tests) and moderate or severe varices in the lower esophagus and gastric fundus (as assessed by computed tomography and endoscopy), were found in all patients.
Furthermore, all patients underwent surgical treatment. Specifically, 45 patients (37.8%) underwent hepatic lobectomy for concomitant liver cancer, 33 (27.7%) underwent devascularization of the lower esophagus and gastric fundus + splenectomy for massive gastrointestinal bleeding (≥ 1000 mL), 12 (10.1%) underwent splenectomy for splenomegaly in which the spleen extends beyond the midline of the abdomen or below the line joining the two anterior superior iliac spines-and reduced quality of life, 15 (12.6%) underwent splenectomy + portal-azygos disconnection for moderate or severe hypersplenism, 11 (9.3%) underwent splenectomy alone, and 3 (2.5%) underwent portacaval shunt alone. Liver tissue was collected during the operation and sent for pathological examination, which revealed cirrhosis.
The control group was composed of surgical patients without hypersplenism associated with cirrhotic portal hypertension. These patients had no history of hepatitis virus infection or cirrhosis caused by other reasons. The liver function was normal, and the spleen volume was not enlarged. The 128 control patients included 65 males and 63 females, with a male-to-female ratio of 1:1. Their ages ranged from 20 years to 93 years, with a mean of 49 years. Specifically, 49 patients with cholecystolithiasis and 9 with gallbladder polyps underwent laparoscopic cholecystectomy, 38 with choledocholithiasis underwent choledocholithotomy, 19 with inguinal hernia and femoral hernia underwent laparoscopic hernia repair, and 13 with nodular goiter underwent subtotal thyroidectomy.
Ig, C3 and C4 were detected by turbidimetric inhibition immunoassay. Briefly, 2 mL of peripheral venous blood was drawn from the patient, placed in a dry test tube and sent to the hospital laboratory for routine detection.
Statistical analysis was performed using SPSS software v25.0 (IBM Corp., Armonk, NY, United States). Measurement data were expressed as (average of x ± standard deviation) and [mean (P25, P75)]. The t/z test and Wilcoxon rank sum test for two independent samples were used for comparison between groups. P < 0.05 or P < 0.001 were considered statistically significant.
There were significantly more males than females in the hypersplenism group compared to controls (P < 0.05). There was no significant difference in age between the two groups (P > 0.05).
Compared with the control group, IgA and IgG levels were significantly higher in the hypersplenism group (Z = -6.61 and -7.16, respectively; P < 0.001). There was no significant difference in IgM levels between the two groups (Z = -1.60, P = 0.109) (Figure 1).
Compared with the control group, C3 and C4 levels were significantly lower in the hypersplenism group (t/z = 4.28 and -6.65, respectively; P < 0.001) (Figure 2).
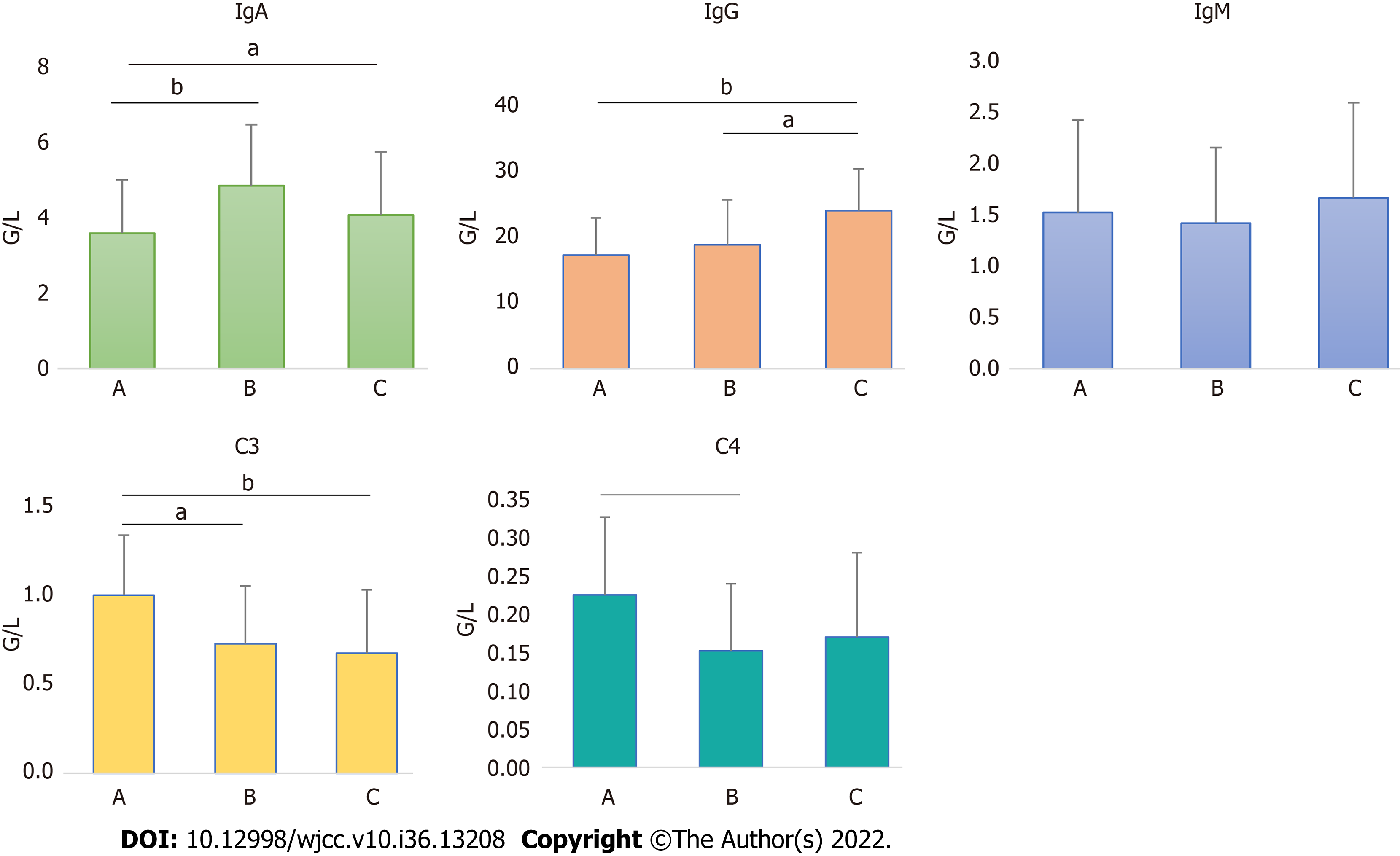
For comparison of IgA, there were significant differences between class A and B (Z = 3.773, P < 0.001) and class A and C (Z = 2.373, P = 0.018) but not between class B and C (Z = 0.190, P = 0.850). For comparison of IgG, there were significant differences between class A and C (t = 3.732, P < 0.001) and class B and C (t = 2.225, P = 0.032) but not between class A and B (t = 1.252, P = 0.213). There were no statistically significant differences between the IgM groups. For comparison of C3, there were significant differences between class A and B (t = 3.149, P = 0.002), and class A and C (t = 3.857, P < 0.001) but not between class B and C (t = 0.486, P = 0.630). For comparison of C4, there were significant differences between class A and B (Z = 3.364, P < 0.001) but not between class A and C (Z = 1.851, P = 0.064) nor class B and C (Z = 0.298, P = 0.765) (Figure 3).
Based on B-Mode ultrasound and computed tomography findings, endoscopy revealed moderate-to-severe varices in the lower esophagus and gastric fundus. Liver tissue was collected during the operation and sent for pathological examination. These findings supported the diagnosis of cirrhotic portal hypertension. Dameshek[18] proposed four criteria for a diagnosis of hypersplenism: (1) Splenomegaly; (2) One or several types of cytopenia; (3) Bone marrow is normal or in hyperplastic state; and (4) Pathological changes of blood cells disappeared after splenectomy. The clinical manifestations of portal hypertension include splenomegaly, and for patients with hypersplenism, peripheral cytopenia should be present and blood counts should become normal after splenectomy[4]. The diagnosis of hypersplenism in cirrhotic portal hypertension is consistent with all patients[19].
Although there was a significant difference in sex composition between the two groups, there was no significant difference in age. Hence, there should be no age-related effect on the Ig and complement measurements between the hypersplenism patients and controls. IgA and IgG levels were significantly higher than controls, indicating that patients with hypersplenism associated with cirrhotic portal hypertension had elevated serum levels of the two dominant Igs. This phenomenon has gained the attention of clinicians[11] and has been repeatedly confirmed[20,21]. Elevated serum Ig levels are of great significance in clinical diagnosis[20] and are suggestive of liver damage.
In this study, hypersplenism patients with Child-Pugh class C had significantly higher IgA and IgG levels than those with class A or B, indicating that the liver function was inversely correlated with serum levels of the two main Igs. There was no significant difference in IgM among the Child-Pugh classes, which may be related to its low total amount in all groups. There are two reasons for the increased Ig levels in the class C patients. Namely, a large number of antibodies are produced to eliminate the virus, and cirrhosis caused by HBV results in liver cell dysfunction and a reduced ability to remove antibodies[22]. Further research is required to determine whether and to what extent enhanced splenic macrophage function is correlated with increased Ig levels in patients with hypersplenism associated with cirrhotic portal hypertension[23].
The significantly lower serum levels of complements C3 and C4 in patients with hypersplenism associated with cirrhotic portal hypertension are also related to liver function impairment. One possible explanation is the reduced ability of damaged liver cells to synthesize complements. Another possibility is the development of a portal systemic collateral circulation, allowing a large amount of endotoxin to enter the bloodstream, which simultaneously activates the classical and alternative pathways, resulting in a large amount of complements to be consumed[24] and a substantial reduction in C3 and C4 levels[25].
This study shows that increased liver function impairment corresponds to lower levels of C3 and C4. As a type of globulin with antibody activity in human serum or fluid, Igs are antimicrobial and antiviral and enhance phagocytosis. Moreover, they can kill or dissolve pathogenic microorganisms with the assistance of complements, which is an important defense function in anti-infection immunity. Therefore, understanding serum Ig levels and their relationship to liver function in patients with hypersplenism associated with cirrhotic portal hypertension is of great clinical significance for assessing the disease progression and strengthening liver-protective treatment.
Patients with hypersplenism associated with cirrhotic portal hypertension have significantly higher antibody (IgA and IgG) levels and significantly lower complement (C3 and C4) levels. The increase of antibodies and the decrease of complement are related to liver function damage. Clinically, the administration of anti-hepatitis virus agents and protection of liver function should be strengthened[26-28].
The antibody and complement levels in patients with cirrhosis and hypersplenism due to portal hypertension are not clear, which affects the diagnosis and treatment to some extent.
There are no studies determining the levels of immunoglobulins (Ig) and complements in patients with hypersplenism due to cirrhosis and portal hypertension, which affects the diagnosis and treatment.
To investigate the antibody (Ig) and complement levels in patients with hypersplenism due to liver cirrhosis and portal hypertension and how to treat them.
The levels of IgA, IgG, IgM, C3 and C4 were determined and compared in 119 patients with confirmed hypersplenism and 128 control patients.
The levels of IgA and IgG in the hypersplenism group were significantly higher than those in the control group (P < 0.001). The levels of C3 and C4 in the hypersplenism group were significantly lower than those in the control group (P < 0.001). The worse the liver function was, the higher the IgA and IgG levels were (P < 0.001) and the lower the C3 and C4 levels were (P < 0.001).
Antibodies in patients with liver cirrhosis and portal hypertension and hypersplenism were significantly increased, while complements (C3 and C4) were significantly decreased. Both the increase of antibody and the decrease of complement are related to the damage of liver function.
It is important to know the antibody (Ig) and complement levels of patients with hypersplenism due to cirrhosis and portal hypertension. Anti-hepatitis virus and liver function protection should be strengthened in treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Immunology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Abi-Mosleh L, India; Sripongpun P, Thailand S-Editor: Xing YX L-Editor: Filipodia P-Editor: Xing YX
| 1. | Lv Y, Wu H, Lau WY, Zheng J, Wu J, Zeng M. Impact of total splenectomy on peripheral lymphocytes and their subsets in patients with hypersplenism associated with cirrhotic portal hypertension. Sci Rep. 2021;11:21246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Kennedy M, Alexopoulos SP. Hepatitis B virus infection and liver transplantation. Curr Opin Organ Transplant. 2010;15:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Rouse BT. Virus-induced immunopathology. Adv Virus Res. 1996;47:353-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Lv Y, Yee Lau W, Wu H, Han X, Gong X, Liu N, Yue J, Li Q, Li Y, Deng J. Causes of peripheral cytopenia in hepatitic cirrhosis and portal hypertensive splenomegaly. Exp Biol Med (Maywood). 2017;242:744-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Duriez M, Mandouri Y, Lekbaby B, Wang H, Schnuriger A, Redelsperger F, Guerrera CI, Lefevre M, Fauveau V, Ahodantin J, Quetier I, Chhuon C, Gourari S, Boissonnas A, Gill U, Kennedy P, Debzi N, Sitterlin D, Maini MK, Kremsdorf D, Soussan P. Alternative splicing of hepatitis B virus: A novel virus/host interaction altering liver immunity. J Hepatol. 2017;67:687-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Baig S, Alamgir M. The extrahepatic manifestations of hepatitis B virus. J Coll Physicians Surg Pak. 2008;18:451-457. [PubMed] |
| 7. | Zabel F, Mohanan D, Bessa J, Link A, Fettelschoss A, Saudan P, Kündig TM, Bachmann MF. Viral particles drive rapid differentiation of memory B cells into secondary plasma cells producing increased levels of antibodies. J Immunol. 2014;192:5499-5508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Vaerman JP. [Effector mechanisms of IgA]. Ann Biol Clin (Paris). 1984;42:61-70. [PubMed] |
| 9. | Sawa T, Kinoshita M, Inoue K, Ohara J, Moriyama K. Immunoglobulin for Treating Bacterial Infections: One More Mechanism of Action. Antibodies (Basel). 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Hädge D. [Evolution of the immunoglobulins]. Allerg Immunol (Leipz). 1985;31:231-243. [PubMed] |
| 11. | Feizi T. Immunoglobulins in chronic liver disease[J]. Sci Rep. 1968;9:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 132] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Zhang Q, Gao Y, Peng Y, Fu M, Liu YQ, Zhou QJ, Yu J, Zheng XQ. Epidemiological survey of human cytomegalovirus antibody levels in children from Southeastern China. Virol J. 2014;11:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Antunes J, Kochuyt AM, Ceuppens JL. Perioperative allergic reactions: experience in a Flemish referral centre. Allergol Immunopathol (Madr). 2014;42:348-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Liu A H, Jena P K, Wysocki L J. Tracing the development of single memory-lineage B cells in a highly defined immune response. J Exp Med. 1996;183:2053-2063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Richard K S Loh, Sandra Vale, Andrew McLean-Tooke. Quantitative serum immunoglobulin tests. Aust Fam Physician. 2013;42:195-198. [PubMed] |
| 16. | Yang X, Sun J, Gao Y, Tan A, Zhang H, Hu Y, Feng J, Qin X, Tao S, Chen Z, Kim ST, Peng T, Liao M, Lin X, Zhang Z, Tang M, Li L, Mo L, Liang Z, Shi D, Huang Z, Huang X, Liu M, Liu Q, Zhang S, Trent JM, Zheng SL, Xu J, Mo Z. Genome-wide association study for serum complement C3 and C4 levels in healthy Chinese subjects. PLoS Genet. 2012;8:e1002916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Ritchie RF, Palomaki GE, Neveux LM, Navolotskaia O, Ledue TB, Craig WY. Reference distributions for complement proteins C3 and C4: a practical, simple and clinically relevant approach in a large cohort. J Clin Lab Anal. 2004;18:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Dameshek W. Hypersplenism. Bull N Y Acad Med. 1955;31:113-136. [PubMed] |
| 19. | Lv Y, Gong X, Xie X, Wang B, Yang Y, Li Y. Clinical study on the relationship between hematocytopenia and splenomegaly caused by cirrhotic portal hypertension. Cell Biochem Biophys. 2014;70:355-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Lin S, Sun Q, Mao W, Chen Y. Serum Immunoglobulin A (IgA) Level Is a Potential Biomarker Indicating Cirrhosis during Chronic Hepatitis B Infection. Gastroenterol Res Pract. 2016;2016:2495073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Watt K, Uhanova J, Gong Y, Kaita K, Doucette K, Pettigrew N, Minuk GY. Serum immunoglobulins predict the extent of hepatic fibrosis in patients with chronic hepatitis C virus infection. J Viral Hepat. 2004;11:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Ortarık Z, Toyran A, Sen S, Mart Kömürcü SZ, Güvener E. [Evaluation of serum IgG, IgA and IgM levels as indicators of hepatic fibrosis in patients with chronic hepatitis C infection]. Mikrobiyol Bul. 2011;45:296-305. [PubMed] |
| 23. | Gonzàlez-Quintela A, Alende MR, Gamallo R, Gonzàlez-Gil P, López-Ben S, Tomé S, Otero E, Torre JA. Serum immunoglobulins (IgG, IgA, IgM) in chronic hepatitis C. A comparison with non-cirrhotic alcoholic liver disease. Hepatogastroenterology. 2003;50:2121-2126. [PubMed] |
| 24. | Zhu C, Song H, Xu F, Yi W, Liu F, Liu X. Hepatitis B virus inhibits the expression of complement C3 and C4, in vitro and in vivo. Oncol Lett. 2018;15:7459-7463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Chang WY, Chuang WL. Complements as new diagnostic tools of hepatocellular carcinoma in cirrhotic patients. Cancer. 1988;62:227-232. [PubMed] [DOI] [Full Text] |
| 26. | Lei Z, Xia Y, Si A, Wang K, Li J, Yan Z, Yang T, Wu D, Wan X, Zhou W, Liu J, Wang H, Cong W, Wu M, Pawlik TM, Lau WY, Shen F. Antiviral therapy improves survival in patients with HBV infection and intrahepatic cholangiocarcinoma undergoing liver resection. J Hepatol. 2018;68:655-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Xu XF, Xing H, Han J, Li ZL, Lau WY, Zhou YH, Gu WM, Wang H, Chen TH, Zeng YY, Li C, Wu MC, Shen F, Yang T. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma: A Multicenter Study From China. JAMA Surg. 2019;154:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 394] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 28. | Huang G, Li PP, Lau WY, Pan ZY, Zhao LH, Wang ZG, Wang MC, Zhou WP. Antiviral Therapy Reduces Hepatocellular Carcinoma Recurrence in Patients With Low HBV-DNA Levels: A Randomized Controlled Trial. Ann Surg. 2018;268:943-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |