Published online Dec 6, 2022. doi: 10.12998/wjcc.v10.i34.12768
Peer-review started: September 20, 2022
First decision: October 13, 2022
Revised: October 21, 2022
Accepted: November 11, 2022
Article in press: November 11, 2022
Published online: December 6, 2022
Processing time: 73 Days and 0.6 Hours
Colonic duplication refers to a spherical or tubular cavity which shows similar properties with the native colon and is attached to the mesenteric side of the alimentary tract. It is the rarest in alimentary tract duplications. Based upon anatomic feature, colonic duplications can be classified as spherical (cystic) or tubular, with the latter being less common (approximately 20%). Symptoms of colonic duplication are dependent on the duplication site and extent, and patient age, etc. Usually, patients with colonic duplication manifest typical intestinal obstruction, potentially accompanied by recurrent dark or bright red bloody stool, varying degrees of anemia-related symptoms, and body wasting.
A young male patient was admitted to our hospital due to recurrent abdominal pain. No definite diagnosis was achieved by computed tomography (CT) or electronic colonoscopy, and the bowel preparation efficacy was suboptimal. Hirschsprung disease was suspected, and thus laparoscopic exploration was performed. An approximately 60-cm-long inverted duplicated colon with severe edema and dilation was identified. It originated from the mesenteric side of the transverse colon and ended in the terminal part of the descending colon with a blind end. The parallel native colon had a thickened colonic wall, became stiff, and was poor in peristalsis. The patient then underwent subtotal colectomy and was discharged 7 d after the surgery. From 3 mo post-surgery to date, the patient had regular bowel movement once daily and a steady increase in body weight.
Tubular colonic duplication is a rare type of alimentary tract duplication that can be detected by ultrasonography, CT, or magnetic resonance imaging based on the actual clinical situation. Surgical resection of aberrant colon (including the duplicated colonic segment and other potentially involved colonic segments) is the only approach to cure this medical condition.
Core Tip: The incidence of tubular duplication malformations of the colon is very low. Patients usually present with intestinal obstruction. Accurate imaging is not yet fixed, and the diagnosis is usually clear only during surgery, which is the only effective treatment.
- Citation: Zhang ZM, Kong S, Gao XX, Jia XH, Zheng CN. Colonic tubular duplication combined with congenital megacolon: A case report. World J Clin Cases 2022; 10(34): 12768-12774
- URL: https://www.wjgnet.com/2307-8960/full/v10/i34/12768.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i34.12768
There have been some studies reporting alimentary tract duplications. According to previous reports, alimentary tract duplications majorly happen in the small intestine, while most of the cases correspond anatomically to the cystic lesions independent of the normal alimentary tract. This study reports a case of tubular colonic duplication, which is extremely rare in alimentary tract duplications.
An 18-year-old male patient visited our hospital with recurrent intestinal obstruction and 2-wk exacerbation as the chief complaint.
The patient previously underwent conservative treatment for recurrent intestinal obstruction in a local hospital and was discharged after symptoms resolved. However, he responded poorly to the recent several conservative treatments.
The patient previously had no other disease, food or drug allergy, surgery, or trauma.
There was no relevant family history or other inherited disorders.
Physical examination revealed distended abdomen, tenderness in the left upper abdomen, no rebound tenderness or muscle tension, flatness to percussion, no shifting dullness, and weak bowel sound.
All laboratory tests were in the normal range.
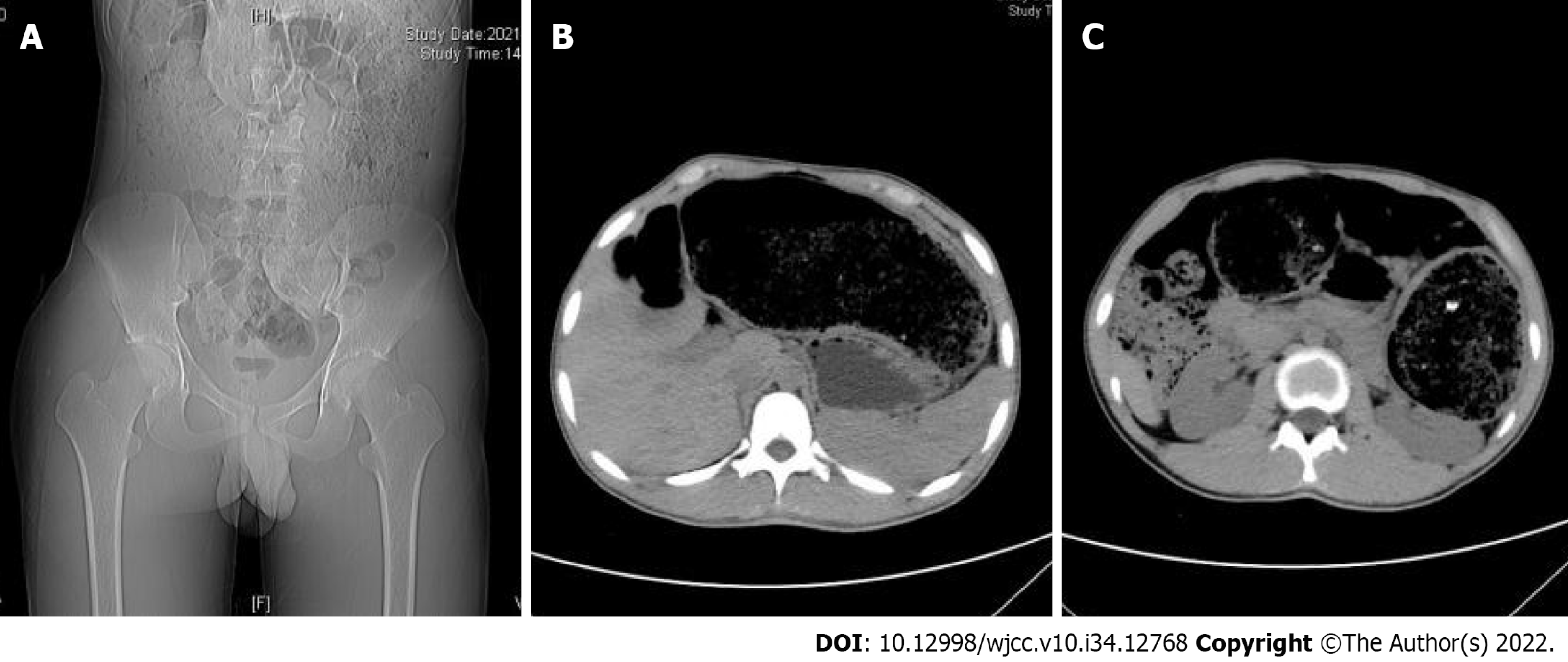
On abdominal plain computed tomography (CT), distinct distention of the colonic lumen was identified. It was significant in the transverse and descending colons, compressing the adjacent structures. Megacolon was suggested (Figure 1).
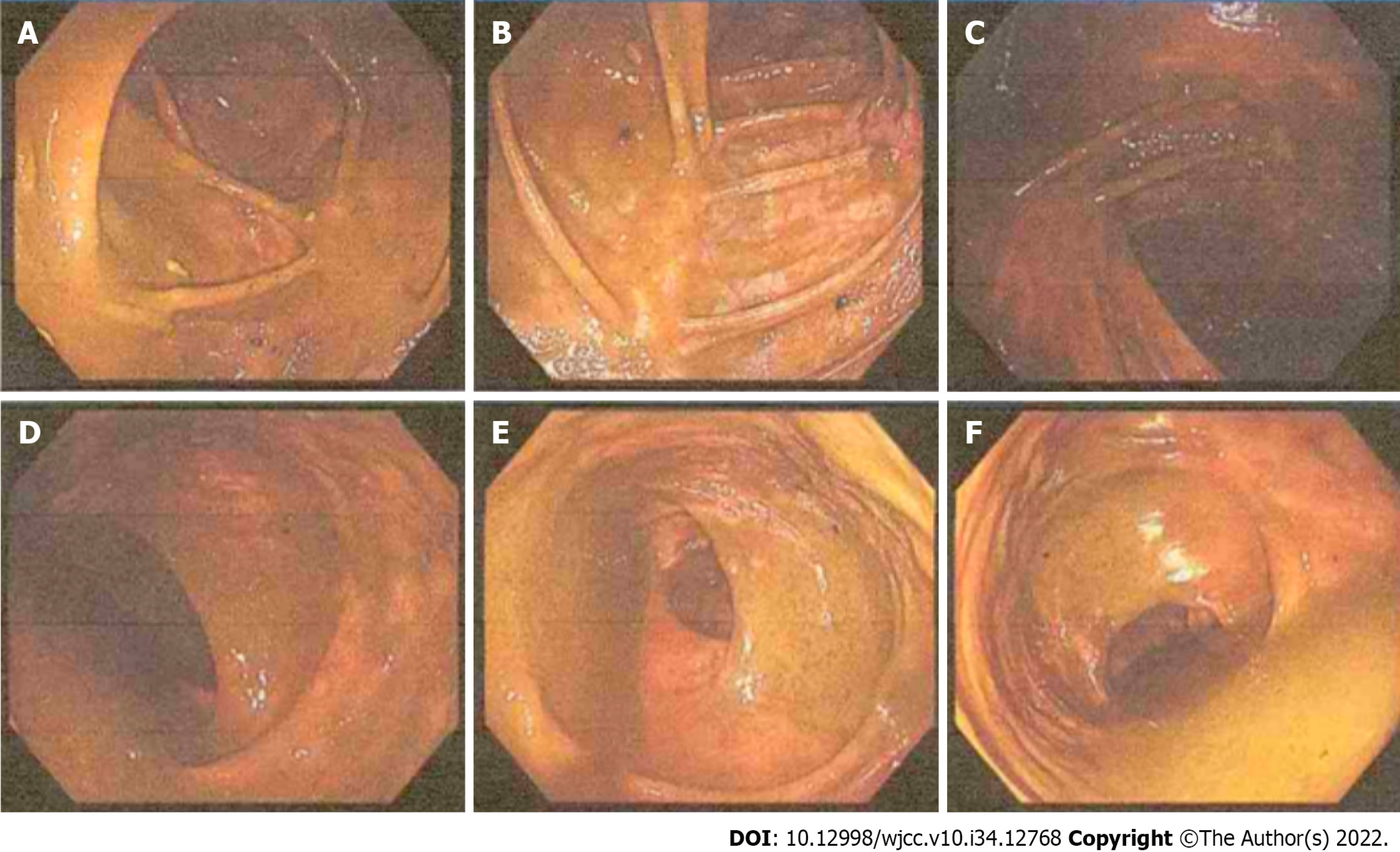
Colonoscopy was applied with an insertion depth of 70 cm reaching the ileocecum. A large amount of residual fecal material was observed in the lumen with a smooth mucosal surface, and the visible distention predominantly happened in the ascending colon, hepatic flexure, and transverse colon. No structural abnormalities were found. Laparoscopic exploration was then conducted (Figure 2).
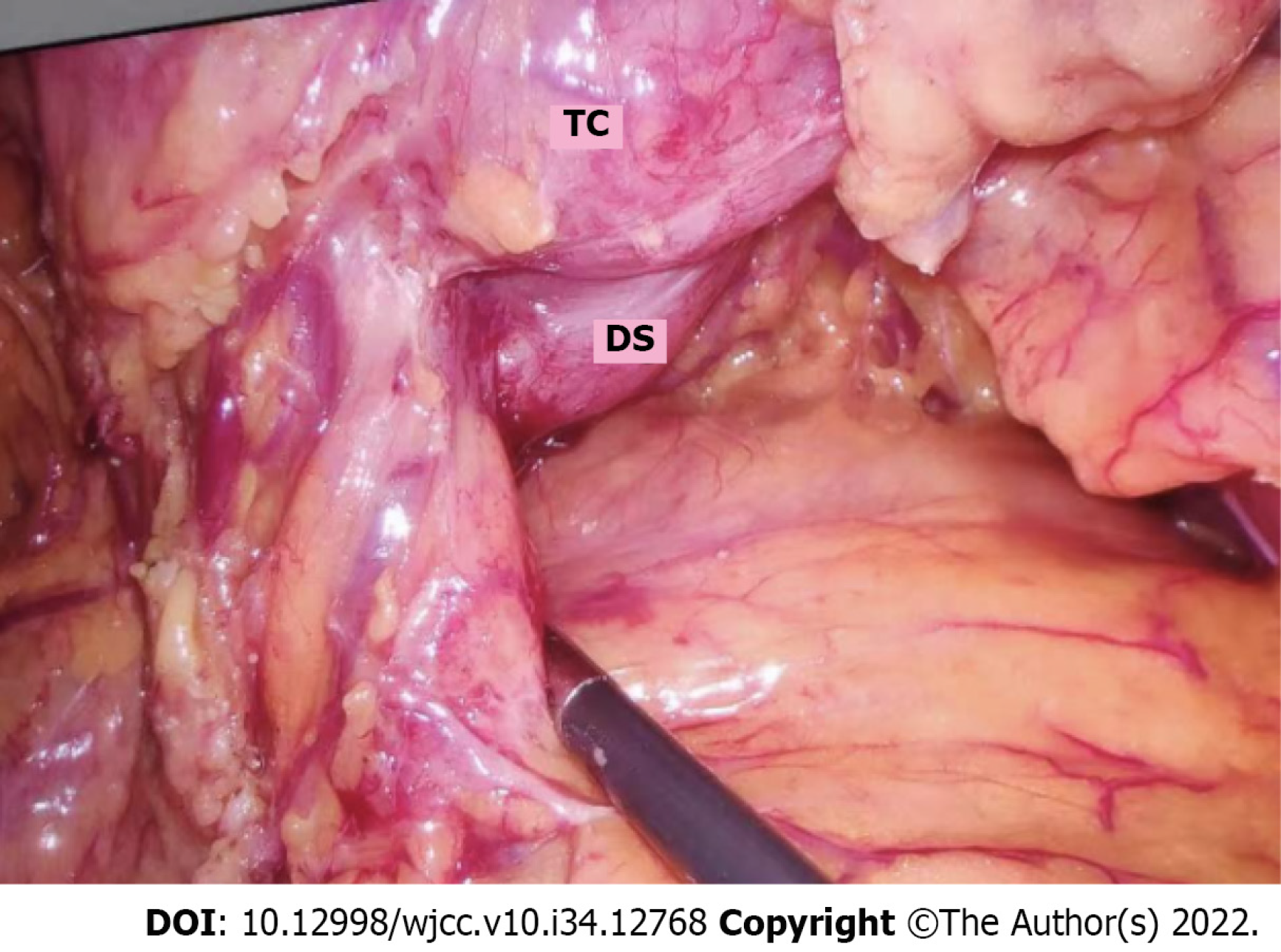
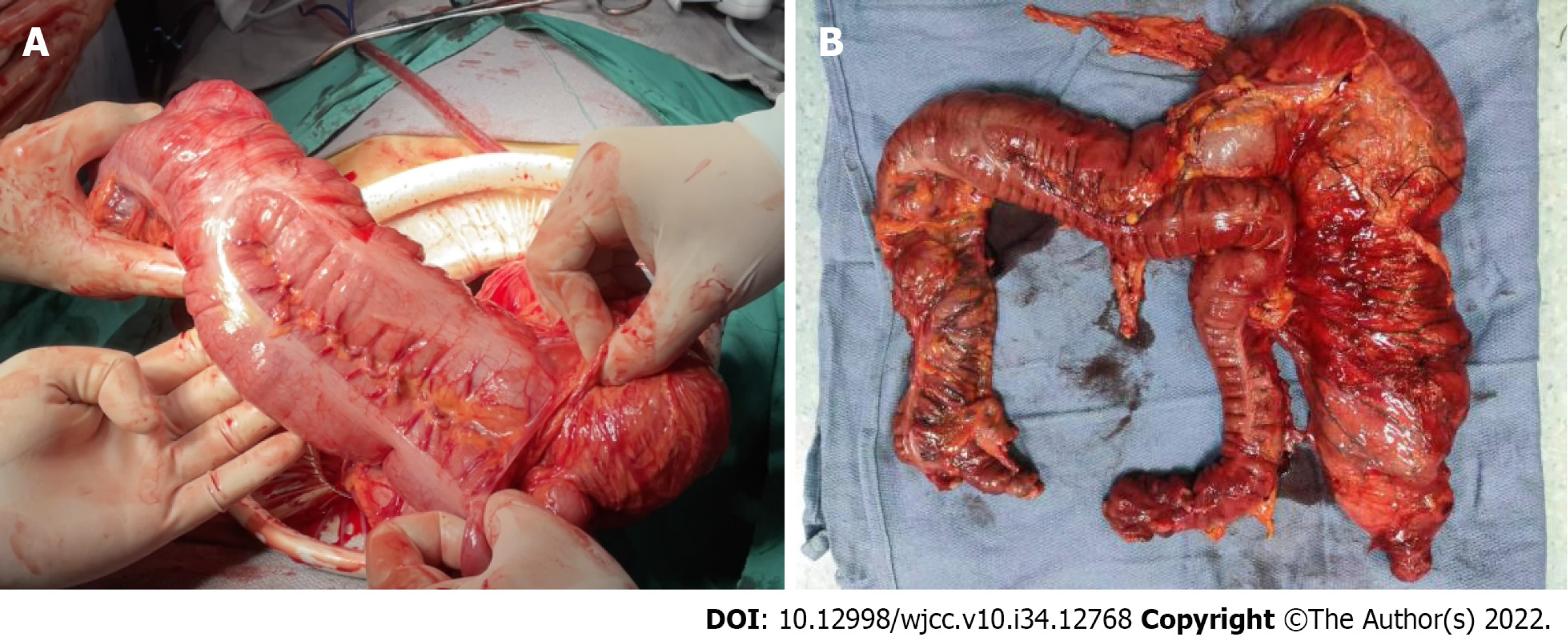
Tubular colonic duplication was confirmed intraoperatively. A dilated bowel segment originating from the transverse colon at the flexure was identified. The surgery was converted to open surgery. A bowel segment arising from the transverse colon at the flexure and ended in the terminal part of the descending colon with a blind end was observed. It was parallel to the normal mesenteric side of the transverse colon, and shared blood supply with the native colon. The duplicated bowel segment was about 60 cm in length and in a "Y" shape. It was hard to separate the native and duplicated bowel segment due to their intimate anatomical relationship. Besides, the two parts shared a common set of blood-supplying vessels, although only the branches issuing from the arch vessels from the most distal part of the native colon supplied blood to the duplicated bowel segment. Moreover, the native colon presented with a thickened colonic wall, stiffness, and poor peristalsis, which might be associated with the chronic inflammation because of colonic duplication. Considering the characteristics of colonic duplication, the direction of peristalsis in the colon, and the patient’s chief complaint, we suspected that the peristalsis in the native colon was also affected (loss of ganglion cells indicated by postoperative pathology). In this context, there might be a high risk of developing complications after resection of the duplicated bowel segment. Therefore, subtotal colectomy was decided. After discussion with the patient family about the situation, subtotal colectomy was performed to fulfill sigmoidal colon and terminal ileum anastomosis (Figures 3 and 4).
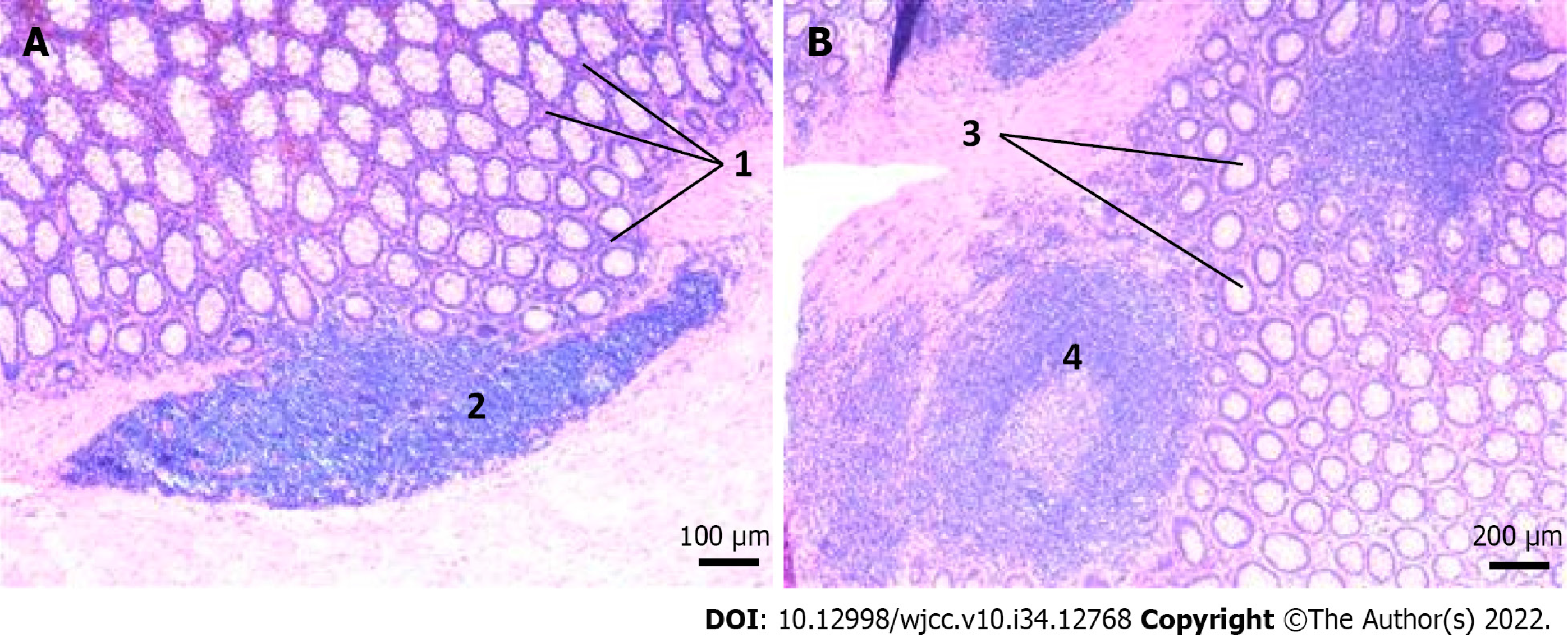
Postoperative pathology demonstrated: (1) Chronic mucositis (colonic) accompanied by pigmentophage proliferation, consistent with melanosis coli; (2) absence of ganglion cells in the tangent of dilated or stenosed segment; and (3) acute and chronic appendicitis (Figure 5).
A final diagnosis of tubular colonic duplication complicated by congenital megacolon was made.
Subtotal colon resection was performed.
During postoperative hospitalization (7 d), no signs of discomfort, surgery-related complications, or other adverse events were observed. Flatus was fulfilled on postoperative day 4, and the patient was fed a liquid diet on the same day. Symptoms such as abdominal distension and pain were monitored. One month after surgery, the patient had bowel movement four times daily, presenting with shapeless yellow loose stools. By the second month after surgery, the bowel movement frequency was reduced to twice daily, and the stools presented as shaped, yellow, and soft. From the third month after surgery to date, the patient had normal bowel movement once daily with the stools presenting as yellow and soft. Satisfactory results were found at the recent follow-up, including body gain from preoperative 60 kg to 70 kg and BMI increase from 17.54 kg/m2 to 20.47 kg/m2. Overall, the patient was doing well during the 10-mo follow-up.
Tubular colonic duplication is the rarest malformation of the alimentary tract[1]. The etiology remains elusive, and potential causes include genetic factors, smoking, antidepressants, etc[2-5]. There is a notion that the endothelium of the nourishing vessels in the end of the intestinal diverticulum, which has been present during fetal life, gets damaged due to maternal intrauterine ischemia, resulting in unexpected vascular alteration. This alteration can gradually separate the diverticulum from the intestinal wall and then transform it into blind end tubular bowel segment[6]. Usually, the duplicated tubular colon shares blood vessels with the native colon and also has full-developed wall structures[7,8]. Tubular duplications commonly present with a proximal blind end and communicate with the native colon with the distal opening. In patients with such characteristic, enteroscopy is highly efficient to find the disorder after the appearance of clinical symptoms or abdominal discomfort. In contrast, the case reported here presented with a distal blind end and a connection to the native colon with the proximal end. This explained why there was no abnormality on enteroscopy. The anterograde (peristaltic) direction of the proximal opening makes it difficult to observe the intestinal abnormalities on enteroscopy, by which inverted observation is not available.
The diagnosis of tubular colonic duplication is difficult, and the majority of patients are admitted for acute abdomen[9]. Ultrasonography (USG), computed tomography (CT), and magnetic resonance imaging (MRI) are commonly used diagnostic tools with their own advantages and disadvantages. USG is non-radioactive and easy to operate, but it generates poor-quality images and has a limited capability to define the boundaries between the duplication and surrounding healthy tissues. Relatively, CT and MRI images are clearer. While for patients unable to take care of themselves, such as infants, analgesia and sedation are required sometimes before MRI. Besides, MRI also has some limitations, such as heavy noise, long duration, and high cost, which should also be considered for diagnosis. Okur et al[10] recommended CT for diagnosis, but it failed to make a definite diagnosis in the patient reported here. The patient here was initially diagnosed with Hirschsprung disease preoperatively and then underwent laparoscopic exploration to obtain a definite diagnosis as tubular colonic duplication.
Inoue et al[11] previously reported two cases of colonic duplication complicated by colonic mali
The patient reported here was admitted for recurrent intestinal obstruction and diagnosed with tubular colonic duplication, which is the rarest among alimentary tract duplications. Preoperative examinations failed to make a valuable diagnosis. Postoperative pathology suggested a diagnosis of melanosis coli, which shows a possible relationship with colonic malignancy and has potential pathological changes that may lead to carcinogenesis. Surgical resection is the only treatment to cure colonic duplication, while the procedure is dependent on the specific anatomic features of the lesion. In this patient, single surgical resection of the duplicated colonic segment had a high risk of developing complications, due to the intimate anatomical relationship with and the alterations in the native colon (including thickening and stiffness of the colonic wall, and poor peristalsis). Therefore, subtotal colectomy was selected[16].
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Haddadi S, Algeria; Pietroletti R, Italy S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YL
| 1. | Parker BC, Guthrie J, France NE, Atwell JD. Gastric duplications in infancy. J Pediatr Surg. 1972;7:294-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43:1731-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1527] [Cited by in RCA: 1621] [Article Influence: 77.2] [Reference Citation Analysis (0)] |
| 3. | Perry MF, Mulcahy H, DeFranco EA. Influence of periconception smoking behavior on birth defect risk. Am J Obstet Gynecol. 2019;220:588.e1-588.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Khanna K, Sharma S, Pabalan N, Singh N, Gupta DK. A review of genetic factors contributing to the etiopathogenesis of anorectal malformations. Pediatr Surg Int. 2018;34:9-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Malm H. Prenatal exposure to selective serotonin reuptake inhibitors and infant outcome. Ther Drug Monit. 2012;34:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Ildstad ST, Tollerud DJ, Weiss RG, Ryan DP, McGowan MA, Martin LW. Duplications of the alimentary tract. Clinical characteristics, preferred treatment, and associated malformations. Ann Surg. 1988;208:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 158] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Grande Moreillo C, Margarit Mallol J, Fuentes Carretero S. Intestinal duplication isolated from the digestive tract: an entity to be considered. Cir Pediatr. 2022;35:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Erginel B, Soysal FG, Ozbey H, Keskin E, Celik A, Karadag A, Salman T. Enteric Duplication Cysts in Children: A Single-Institution Series with Forty Patients in Twenty-Six Years. World J Surg. 2017;41:620-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Hugele F, Dumont C, Boulot P, Couture A, Prodhomme O. Does prenatal MRI enhance fetal diagnosis of intra-abdominal cysts? Prenat Diagn. 2015;35:669-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Okur MH, Arslan MS, Arslan S, Aydogdu B, Türkçü G, Goya C, Uygun I, Cigdem MK, Önen A, Otcu S. Gastrointestinal tract duplications in children. Eur Rev Med Pharmacol Sci. 2014;18:1507-1512. [PubMed] |
| 11. | Inoue Y, Nakamura H. Adenocarcinoma arising in colonic duplication cysts with calcification: CT findings of two cases. Abdom Imaging. 1998;23:135-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Zhang Y, Zhan TT, Dong ZY, Sun HH, Wang JW, Chen Y, Xu SC. Melanosis coli: A factor not associated with histological progression of colorectal polyps. J Dig Dis. 2022;23:302-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Kassim SA, Abbas M, Tang W, Wu S, Meng Q, Zhang C, Naeem S, Li X, Chen R. Retrospective study on melanosis coli as risk factor of colorectal neoplasm: a 3-year colonoscopic finding in Zhuhai Hospital, China. Int J Colorectal Dis. 2020;35:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Wang ZC, Gao J, Zi SM, Yang M, Du P, Cui L. Aberrant expression of sonic hedgehog pathway in colon cancer and melanosis coli. J Dig Dis. 2013;14:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Oniscu A, James RM, Morris RG, Bader S, Malcomson RD, Harrison DJ. Expression of Sonic hedgehog pathway genes is altered in colonic neoplasia. J Pathol. 2004;203:909-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Merrot T, Anastasescu R, Pankevych T, Tercier S, Garcia S, Alessandrini P, Guys JM. Duodenal duplications. Clinical characteristics, embryological hypotheses, histological findings, treatment. Eur J Pediatr Surg. 2006;16:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |