Published online Dec 6, 2022. doi: 10.12998/wjcc.v10.i34.12761
Peer-review started: September 11, 2022
First decision: October 13, 2022
Revised: October 20, 2022
Accepted: November 4, 2022
Article in press: November 4, 2022
Published online: December 6, 2022
Processing time: 81 Days and 20.4 Hours
Acephalic spermatozoa syndrome (ASS) is an extremely rare form of severe teratozoospermia, where in most of the sperm either appear to lack heads or have disconnected or poorly connected heads and tails.
We reported the case of a male patient with secondary infertility whose sperm showed typical ASS upon morphological analysis. Whole-exome sequencing was performed on the patient’s peripheral blood, which revealed two heterozygous variants of the PMFBP1 gene: PMFBP1c.414+1G>T (p.?) and PMFBP1c.393del (p.C132Afs*3).
It is speculated that the compound homozygous mutation of PMFBP1 may be the cause of ASS. We conducted a literature review in order to provide the basis for genetic counseling and clinical diagnosis of patients with ASS.
Core Tip: Acephalic spermatozoa syndrome (ASS) is an extremely rare form of teratozoospermia. Patients with ASS are often unable to conceive naturally, and intracytoplasmic sperm injection is the main method used for such patients to produce biological offspring. Recent studies have shown that ASS is associated with inherited genetic mutations.
- Citation: Deng TQ, Xie YL, Pu JB, Xuan J, Li XM. Compound heterozygous mutations in PMFBP1 cause acephalic spermatozoa syndrome: A case report. World J Clin Cases 2022; 10(34): 12761-12767
- URL: https://www.wjgnet.com/2307-8960/full/v10/i34/12761.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i34.12761
According to World Health Organization (WHO) estimates, 15% of the couples of childbearing ages face fertility issues, with 50% attributed to male factors[1]. Sperm quality remains an important indicator of male fertility, with the main manifestations of poor quality being oligospermia, asthenozoospermia, teratozoospermia, or azoospermia. Acephalic spermatozoa syndrome (ASS) (OMIM: 617187) is an extremely rare form of teratozoospermia[2]. Patients with ASS are often unable to conceive naturally, and intracytoplasmic sperm injection (ICSI) is the main method used for such patients to produce biological offspring[3]. In recent years, the development of next-generation sequencing has provided a technical basis for genetic research on ASS. To date, the followings have been identified as causative genes related to azoospermia: SUN5, BRDT, PMFBP1, TSGA10, HOOK1, DNAH6, and CEP112[4-10]. Here we report a case of ASS caused by a heterozygous mutation in the polyamine modulated factor 1 binding protein 1 (PMFBP1) gene.
A 31-year-old male patient attended the outpatient clinic of our hospital’s reproductive medicine center with a complaint of “three years without pregnancy despite not using contraception”.
Three years without pregnancy.
In 2014, the patient’s wife had undergone an induced abortion, and the patient had undergone a high ligation of his left varicocele in 2018; however, sperm quality did not appear to have improved upon re-examination after the surgery.
The patient has no history of hypertension or diabetes. Her parents were non-consanguineous and had no family history of hereditary diseases.
In terms of appearance, the patient’s height was 165 cm, weight was 65 kg, and body mass index was 23.88 Kg/m2. Specialist examination showed that his pubic hair was distributed like an inverted triangle, his penis measured approximately 6 cm long when flaccid, the urethral opening showed no visible abnormalities, bilateral testicular volume was approximately 12 mL with a tough texture, the bilateral vas deferens and epididymis showed no observable abnormalities upon palpation, and no varicocele was found.
Through two routine semen analyses and rapid morphology staining (Diff-Quik method), the patient was diagnosed with secondary infertility and ASS. Given the patient’s informed consent, 5 mL of his peripheral blood was collected. Our study was approved by the Ethics Committee of the Shenzhen Maternal and Child Health Care Hospital and was conducted with the patient’s informed consent.
After three to five days of abstinence, the patient masturbated to enable sperm extraction, which was liquified in a water bath at 37℃. Analyses were conducted twice according to the WHO laboratory manual (Fifth Edition)[11]. Eosin staining was conducted to detect sperm viability and sperm morphology was determined after staining the sperm smear using a rapid staining solution (Diff-Quik method), and 200 sperms were counted. Morphological defects were divided into four categories: normal, abnormal head-neck configuration, detached heads, and headless, and the percentage of each category was calculated.
Peripheral blood was collected and used for karyotype analysis through cell culture and Y chromosome microdeletion testing through PCR-capillary electrophoresis.
For exome sequencing, we fragmented 1-3 μg of genomic DNA, extracted from each sample, to an average size of 180 bp with a Bioruptorsonicator (Diagenode). Paired-end sequencing libraries then were prepared using a DNA sampleprep reagent set 1 (NEBNext). Library preparation included end repair, adapter ligation and PCRenrichment, and was carried out as recommendedby Illumina protocols.
The amplified DNA was captured use GenCap Deafness capture kit (MyGenostics GenCap Enrichment technologies). The DNA probes were designed to tile along the exon regions and the known non-exon pathogenic region of human genes. The capture experiment was conducted according to manufacturer’s protocol. The PCR product was purified using SPRI beads (Beckman Coulter) according to manu
After sequencing, the rawdata were saved as a FASTQ format, then followed the bioinformatics analysis,the data would be transformed to VCF format, variants were further annotated by ANNOVA Rand associated with multiple databases, such as,1000 genome, ESP6500, dbSNP , EXAC, Inhouse (MyGenostics), HGMD, and predicted by SIFT, PolyPhen-2, MutationTaster, GERP++.
five steps using to select the potential pathogenic mutations in downstream analysis: (1) Mutation reads should be more than 5, mutation ration should be no less than 30%; (2) Removing the mutation, the frequency of which showed more than 5% in 1000 g, ESP6500 and Inhouse database; (3) If the mutations existed in InNormal database (MyGenostics), then dropped; (4) Removing the synonymous; and (5) After (1),(2),(3), if the mutations were synonymous and they were reported in HGMD, left them. When finished above jobs, the mutations which were left should be the pathogenic mutations.
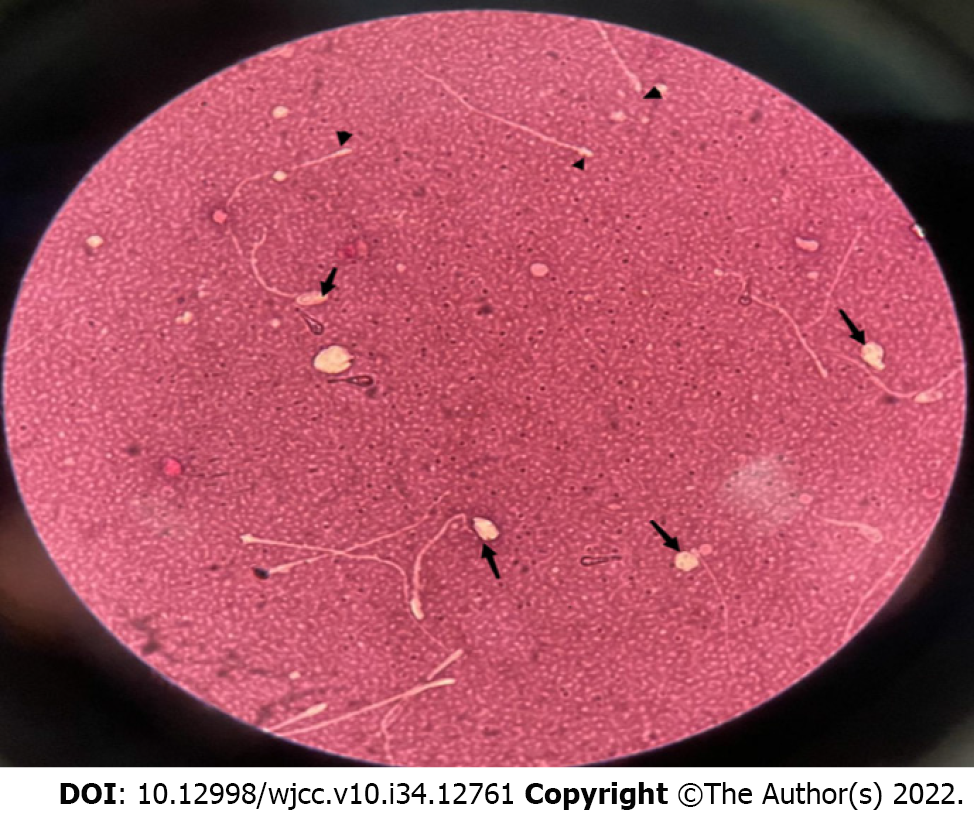
When performed under conditions of low sperm activity, neither method revealed morphologically normal sperm. High percentages of sperm with abnormal head-neck connections, detached heads, and headless sperm were observed, indicating complete teratozoospermia with oligoasthenozoospermia (Table 1). Eosin staining showed that there were no normal sperms in the semen; instead, some were intact (with head and tail at an angle), most had headless, and very few had sperm heads without tails (Figure 1).
| Parameter | December 29, 2021 | March 25, 2022 |
| Semen volume (mL) | 2.0 | 2.8 |
| Semen concentration (106/mL) | 10.65 | 11.28 |
| Rapidly progressivey (%) | 3.61 | 2.27 |
| Sperm motility (%) | 25.30 | 6.82 |
| Sperm survival (%) | 50 | 45 |
| Proportion of morphologically normal sperm (%) | 0 | 0 |
Peripheral blood karyotype analysis (G banding): 46, XY; Y chromosome microdeletion: no deletion observed.
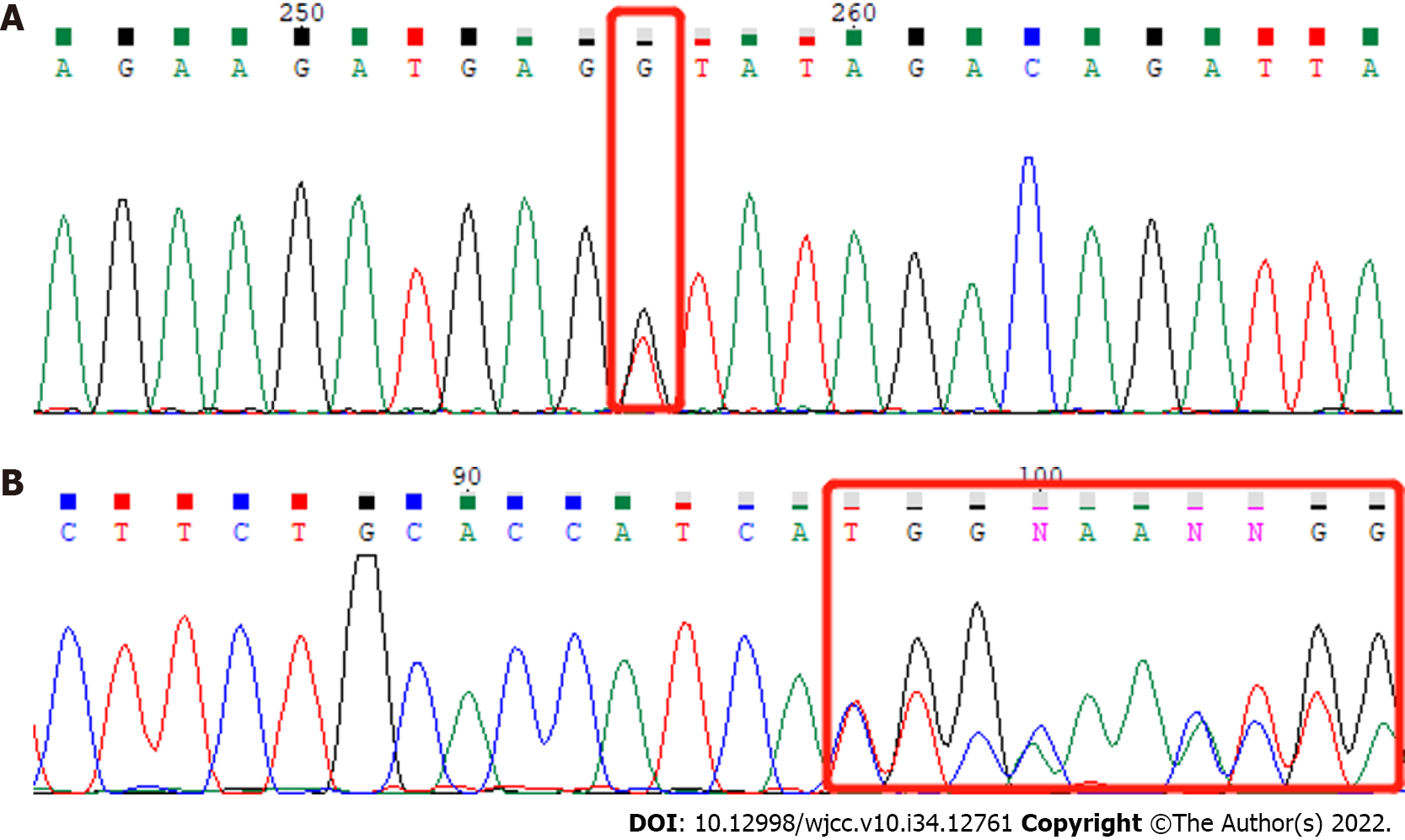
We analyzed exons and adjacent splicing regions of genes associated with male infertility, with a focus on known pathogenic genes related to severe oligozoospermia and the patient’s clinical background. We found two heterozygous variants of the PMFBP1 gene (Figure 2): PMFBP1c.414+1G>T (p.?) and PMFBP1c.393del (p.C132Afs*3). The heterozygous splicing variant c.414+1G>T (p.?) of gene PMFBP1 was in intron 4 (the PMFBP1 gene contains 20 introns) and was likely to interfere with the mRNA splicing signal. This was a classical splicing variant, which may lead to loss of amino acids but might not destroy the reading frame. The heterozygous frameshift variant c.393del (p.C132Afs*3) of the PMFBP1 gene caused the replacement of cysteine by alanine at position 132, followed by a frameshift and a premature stop codon, resulting in the early termination of protein coding. The detected frameshift mutation may lead to > 10% amino acid loss, resulting in the occurrence of nonsense-mediated mRNA decay, which may be a non-functional mutation. According to the American College of Medical Genetics and Genomics Guidelines, these variants are probable pathogenic variants (Table 2)[12].
| Gene | Chromosomal location (hg19) | Mutation name | Group frequency in East Asian population | Zygote type | Mutation rating | Related diseases |
| PMFBP1 | chr16:72188109 | NM_031293.3: c.414+1G>T (p.) | 0.00021 | Heterozygous | Probable pathogen | Spermatogenesis Disease Type 31 (618112, AR) |
| PMFBP1 | chr16:72188131 | NM_031293.3: c.393del (p.C132Afs*3) | Not recorded | Heterozygous | Probable pathogen |
Combined with the patient’s Whole-exome sequencing and semen results, the final diagnosis was acephalic spermatozoa syndrome.
The patient was recommended assisted reproductive technology, the sperm with head-tail junction were selected for ICSI. Due to the low proportion of the sperm with head-tail junction in the semen sample and the fragility of the head-tail junction, we used the upstream method to process the semen samples.Base on the Racowesky method, if D3 blastomeres ≥ 6 were considered as high-quality embryos (I-II). According to the condition of embryos and endometrium of patients,one or two fresh high-quality embryo was transferred, and luteal support was performed after transfer.
On the third day, two embryos were obtained, and one blastocyst was obtained on the fifth day. His wife did not conceive during the two transplants, now there are no available embryos.
ASS can initially be diagnosed by observing sperm morphology under light microscopy; 30 to 100% of the sperms in patients’ semen appear to be headless, have disconnected or loosely connected heads and tails, or have “very small heads” preceding the flagella, which appear as opaque dots[13]. There are individual differences in semen quality parameters of patients, where most have oligoasthenozoospermia, but some may also have normal sperm counts. The PMFBP1 gene is located on chromosome 16 (q22.2) and contains 27 exons. The coding region of the gene consists of 3024 bases and encodes 1007 amino acids[14]. The PMFBP1 gene is highly expressed in both human and mouse testes[15]. In animal testes, the PMFBP1 protein is localized in the implantation fossa and basal body of the sperm and located between the SUN5 and SPATA6 proteins, forming a “sandwich” structure[16]. PMFBP1 may act as a scaffolding protein to stabilize the sperm’s head and tail. Spermatogenic failure 31 (OMIM 618112) can be caused by homozygous or compound heterozygous variants of the PMFBP1 gene and is often inherited in an autosomal recessive pattern[14]. Its main clinical manifestations are oligospermia, with a large proportion of immotile sperm and a high proportion (90%) of acephalic sperm. Many sperms have abnormal head-tail connections or are tailless, with a very small proportion of sperm appearing morphologically normal. Approximately 34.61% of ASS cases are related to mutations in the PMFBP1 gene.
Normal sperm necks consist of an implantation fossa, basal body, proximal centriole, and its surrounding segmented columns. ASS can be divided into three subtypes, which are I, II, and III, according to ultrastructural observations of the breakage site near the midpiece[17]. Mutations in the SUN5, PMFBP1, and HOOK1 genes are related to type II ASS occurrence[8]. Studies have reported no significant differences in the sperm quality of patients with ASS and with PMFBP1, SUN5, BRDT, and TSGA10 mutations; however, other studies show that patients with ASS caused by PMFBP1 mutations not only have rounded or amorphous heads in ejaculated sperm but also have lower sperm concentration, suggesting that PMFBP1 mutations cause a more severe phenotype of acephalic sperm.
The efficacy of drug treatment in ASS is not clear, and patients are often unable to conceive naturally. ICSI is the primary method used for patients to produce biological offspring, wherein sperms with abnormal head-tail connections and sperms that are headless or tailless can be injected into the egg cytoplasm[18]. If the patient’s ejaculate does not contain any sperm heads, testicular puncture can be performed to extract testicular sperm for ICSI. Some ASS patients have had success in producing offspring; however, some patients were unable to conceive high-quality embryos even with ICSI adjuvant therapy, possibly due to genetic mutation. For Type I ASS patients, ICSI outcomes were poor due to a lack of distal centrioles[16]; Type II ASS patients typically achieved better ICSI outcomes than those of other subtypes, with successful cases of pregnancies and live births[14]. Mutations in the TSGA10 and BRDT genes are associated with type III ASS, which often lead to embryonic developmental arrest due to paternal centriole defects, resulting in clinical pregnancy failure[19].
The pathogenic onset of ASS is attributed to mutations in the PMFBP1 gene. A novel PMFBP1 genetic mutation provides accurate genetic diagnosis for patients with ASS and serves as theoretical support and practical guidance for the application of assisted reproductive therapy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Andrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hegazy AA, Egypt; Zhang C, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1241] [Cited by in RCA: 1380] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 2. | Perotti ME, Giarola A, Gioria M. Ultrastructural study of the decapitated sperm defect in an infertile man. J Reprod Fertil. 1981;63:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Elkhatib RA, Paci M, Longepied G, Saias-Magnan J, Courbière B, Guichaoua MR, Lévy N, Metzler-Guillemain C, Mitchell MJ. Homozygous deletion of SUN5 in three men with decapitated spermatozoa. Hum Mol Genet. 2017;26:3167-3171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Zhu F, Wang F, Yang X, Zhang J, Wu H, Zhang Z, He X, Zhou P, Wei Z, Gecz J, Cao Y. Biallelic SUN5 Mutations Cause Autosomal-Recessive Acephalic Spermatozoa Syndrome. Am J Hum Genet. 2016;99:1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Pivot-Pajot C, Caron C, Govin J, Vion A, Rousseaux S, Khochbin S. Acetylation-dependent chromatin reorganization by BRDT, a testis-specific bromodomain-containing protein. Mol Cell Biol. 2003;23:5354-5365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 219] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 6. | Sha YW, Wang X, Xu X, Ding L, Liu WS, Li P, Su ZY, Chen J, Mei LB, Zheng LK, Wang HL, Kong SB, You M, Wu JF. Biallelic mutations in PMFBP1 cause acephalic spermatozoa. Clin Genet. 2019;95:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Ye Y, Wei X, Sha Y, Li N, Yan X, Cheng L, Qiao D, Zhou W, Wu R, Liu Q, Li Y. Loss-of-function mutation in TSGA10 causes acephalic spermatozoa phenotype in human. Mol Genet Genomic Med. 2020;8:e1284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Chen H, Zhu Y, Zhu Z, Zhi E, Lu K, Wang X, Liu F, Li Z, Xia W. Detection of heterozygous mutation in hook microtubule-tethering protein 1 in three patients with decapitated and decaudated spermatozoa syndrome. J Med Genet. 2018;55:150-157. [PubMed] [DOI] [Full Text] |
| 9. | Cannarella R, Condorelli RA, Duca Y, La Vignera S, Calogero AE. New insights into the genetics of spermatogenic failure: a review of the literature. Hum Genet. 2019;138:125-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Kumar A, Rajendran V, Sethumadhavan R, Purohit R. CEP proteins: the knights of centrosome dynasty. Protoplasma. 2013;250:965-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | World Health Organization. WHO laboratory manual for the examination and processing of human semen. Ed. 5. Geneva, Switzerland: WHO Press; 2010. |
| 12. | Brandt T, Sack LM, Arjona D, Tan D, Mei H, Cui H, Gao H, Bean LJH, Ankala A, Del Gaudio D, Knight Johnson A, Vincent LM, Reavey C, Lai A, Richard G, Meck JM. Adapting ACMG/AMP sequence variant classification guidelines for single-gene copy number variants. Genet Med. 2020;22:336-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 13. | Perotti ME, Gioria M. Fine structure and morphogenesis of "headless" human spermatozoa associated with infertility. Cell Biol Int Rep. 1981;5:113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Ohuchi J, Arai T, Kon Y, Asano A, Yamauchi H, Watanabe T. Characterization of a novel gene, sperm-tail-associated protein (Stap), in mouse post-meiotic testicular germ cells. Mol Reprod Dev. 2001;59:350-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Zhu F, Liu C, Wang F, Yang X, Zhang J, Wu H, Zhang Z, He X, Zhou P, Wei Z, Shang Y, Wang L, Zhang R, Ouyang YC, Sun QY, Cao Y, Li W. Mutations in PMFBP1 Cause Acephalic Spermatozoa Syndrome. Am J Hum Genet. 2018;103:188-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 16. | Wu B, Gao H, Liu C, Li W. The coupling apparatus of the sperm head and tail†. Biol Reprod. 2020;102:988-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Nie H, Tang Y, Qin W. Beyond Acephalic Spermatozoa: The Complexity of Intracytoplasmic Sperm Injection Outcomes. Biomed Res Int. 2020;2020:6279795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Fang J, Zhang J, Zhu F, Yang X, Cui Y, Liu J. Patients with acephalic spermatozoa syndrome linked to SUN5 mutations have a favorable pregnancy outcome from ICSI. Hum Reprod. 2018;33:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Li L, Sha Y, Wang X, Li P, Wang J, Kee K, Wang B. Whole-exome sequencing identified a homozygous BRDT mutation in a patient with acephalic spermatozoa. Oncotarget. 2017;8:19914-19922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |