Published online Dec 6, 2022. doi: 10.12998/wjcc.v10.i34.12742
Peer-review started: September 6, 2022
First decision: October 12, 2022
Revised: October 20, 2022
Accepted: October 27, 2022
Article in press: October 27, 2022
Published online: December 6, 2022
Processing time: 86 Days and 19.8 Hours
Oral liposarcoma is an extremely rare lesion that is often clinically misdiagnosed as a benign tumor due to its asymptomatic and indolent clinical course. Here, we report a case of massive low-grade myxoid liposarcoma (MLS) of the floor of the mouth.
A 71-year-old man presented with a huge mass in the left floor of the mouth. A biopsy was performed, and a diagnosis of a myxoid tumor suspicious for low-grade MLS or myxoma was made. Gadolinium-enhanced T1-weighted magnetic resonance imaging showed an intensely enhanced tumor lesion that occupies the left sublingual space and extends to the submandibular space. Submandibular dissection, tumor resection, and reconstruction with a radial forearm flap were performed. The surgical specimen exhibited histologically low-grade MLS. Fused in sarcoma (FUS, also known as TLS) and DNA damage-inducible transcript 3 (DDIT3, also known as CHOP) break-apart was not detected in the fluorescence in situ hybridization analysis. The tumor was completely encapsulated and did not require additional treatment. Furthermore, no recurrence was reported 40 mo after surgery.
We experienced an extremely rare, massive, low-grade MLS emerging from the floor of the mouth. Oftentimes, an MLS of the floor of the mouth lacks significant clinical findings and is often misdiagnosed. Although no FUS-DDIT3 fusion gene was detected, a low-grade MLS was ultimately diagnosed based on the histological findings.
Core Tip: Liposarcoma emerging from the floor of the mouth is extremely rare and often lacks significant clinical findings. This type of liposarcoma is usually misdiagnosed as ranula or benign tumor. In the present case, a definitive diagnosis could not be made preoperatively; however, myxoid liposarcoma was suggested, and complete resection could be achieved with appropriate imaging. A preoperative biopsy can help prevent incomplete resection. Clinical and radiological surveillance is necessary due to the possibility of local recurrence.
- Citation: Kugimoto T, Yamagata Y, Ohsako T, Hirai H, Nishii N, Kayamori K, Ikeda T, Harada H. Massive low-grade myxoid liposarcoma of the floor of the mouth: A case report and review of literature. World J Clin Cases 2022; 10(34): 12742-12749
- URL: https://www.wjgnet.com/2307-8960/full/v10/i34/12742.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i34.12742
Liposarcomas are among the most common malignant mesenchymal neoplasms of the adipose tissue, which were first described by Virchow in 1857[1]. They account for approximately 20% of all soft tissue sarcomas and mostly occur in the lower extremities and retroperitoneum[2]; up to 9% of them are detected in the head and neck region[3]. Although more common in other areas, it is rarely seen in the floor of the mouth[4]. Liposarcomas can become quite large, but those observed in the head and neck region rarely exceed 10 cm[2]. Liposarcoma is categorized into four subtypes: atypical lipomatous tumor/well-differentiated liposarcoma (ALT/WDL), myxoid liposarcoma (MLS), pleomorphic liposarcoma (PLS), and dedifferentiated liposarcoma (DDLS)[5]. Here we report a rare case of a massive low-grade MLS of the floor of the mouth in a 71-year-old male patient.
A 71-year-old male presented to his physician stating that a mass had started to develop in the left floor of his mouth a year before and had slowly continued to enlarge.
The patient was referred to our division 3 mo later.
The patient had hypertension and alcoholic liver disease, but no other chronic disease.
The patient heavily consumed alcohol, approximately 1000 mL of beer daily for 50 years. He denied any family history of malignant tumors.
Physical examination upon presentation showed an 8.0 cm × 7.0 cm elastic, hard tumor extending from the submental to the submandibular region. The left-side floor of the mouth was elevated by the painless, smooth, nontender tumor.
The laboratory tests conducted were as follows: Blood routine test, red blood cell: 5.32 × 1012/L and hematocrit: 47.5%; biochemical test, γ-glutamyl transpeptidase: 82 U/L.
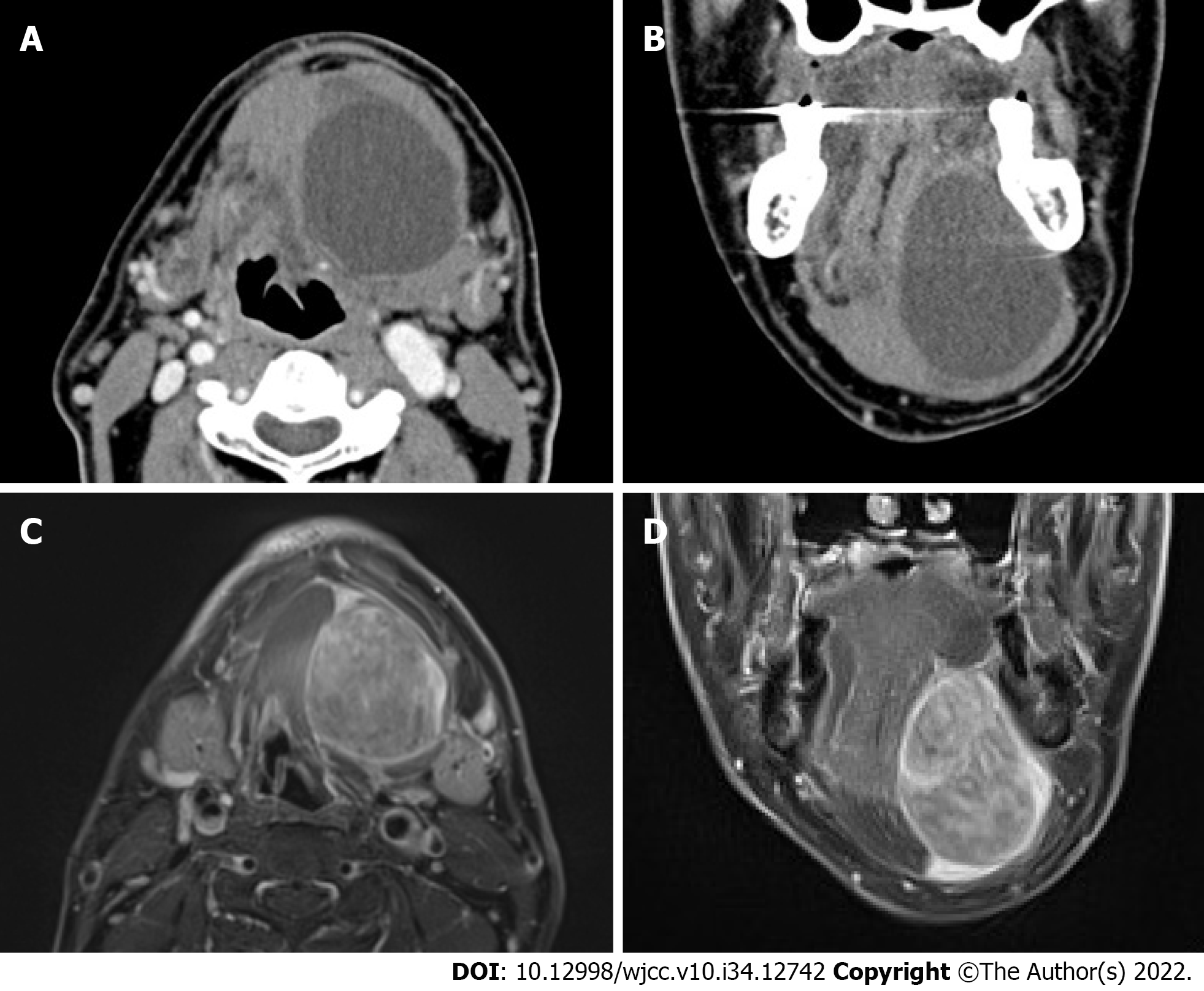
Contrast-enhanced computed tomography (CT) showed an unenhanced cystic lesion separating from the surrounding muscle tissue (Figure 1A and B). Gadolinium-enhanced T1-weighted magnetic resonance imaging (MRI) revealed an intensely enhanced tumor lesion with a well-circumscribed solid mass. The tumor had compressed the genioglossus, digastric, and mylohyoid muscles and occupied the sublingual space and extended to the submandibular space (Figure 1C and D). A malignant salivary gland tumor emerging from the sublingual gland was suspected. 2-Deoxy-2-[18F]fluoro-D-glucose positron emission tomography/CT (FDG-PET/CT) showed a slight uptake in the tumor but no abnormal uptake in other organs. The maximum standardized uptake value (SUVmax) was 3.0.
Incisional biopsy of the floor of the mouth revealed a myxoid tumor, and a low-grade MLS or myxoma was suspected. Preoperative assessment was performed at the Oral Oncology Conference, which was attended by oral surgeons, radiation therapists, pathologists, and oncologist, who jointly decided on a treatment policy.
We ultimately diagnosed this patient with MLS.
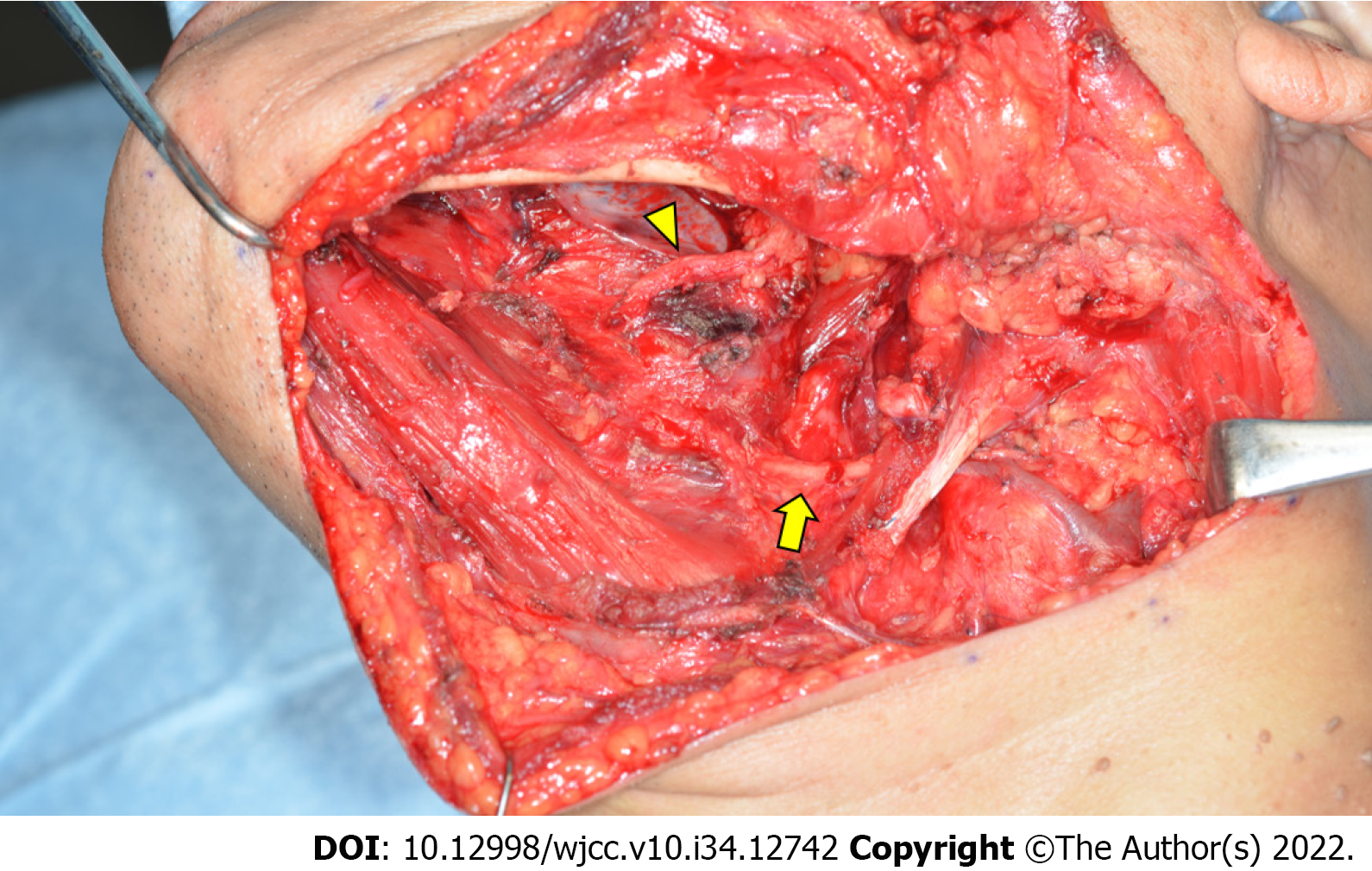
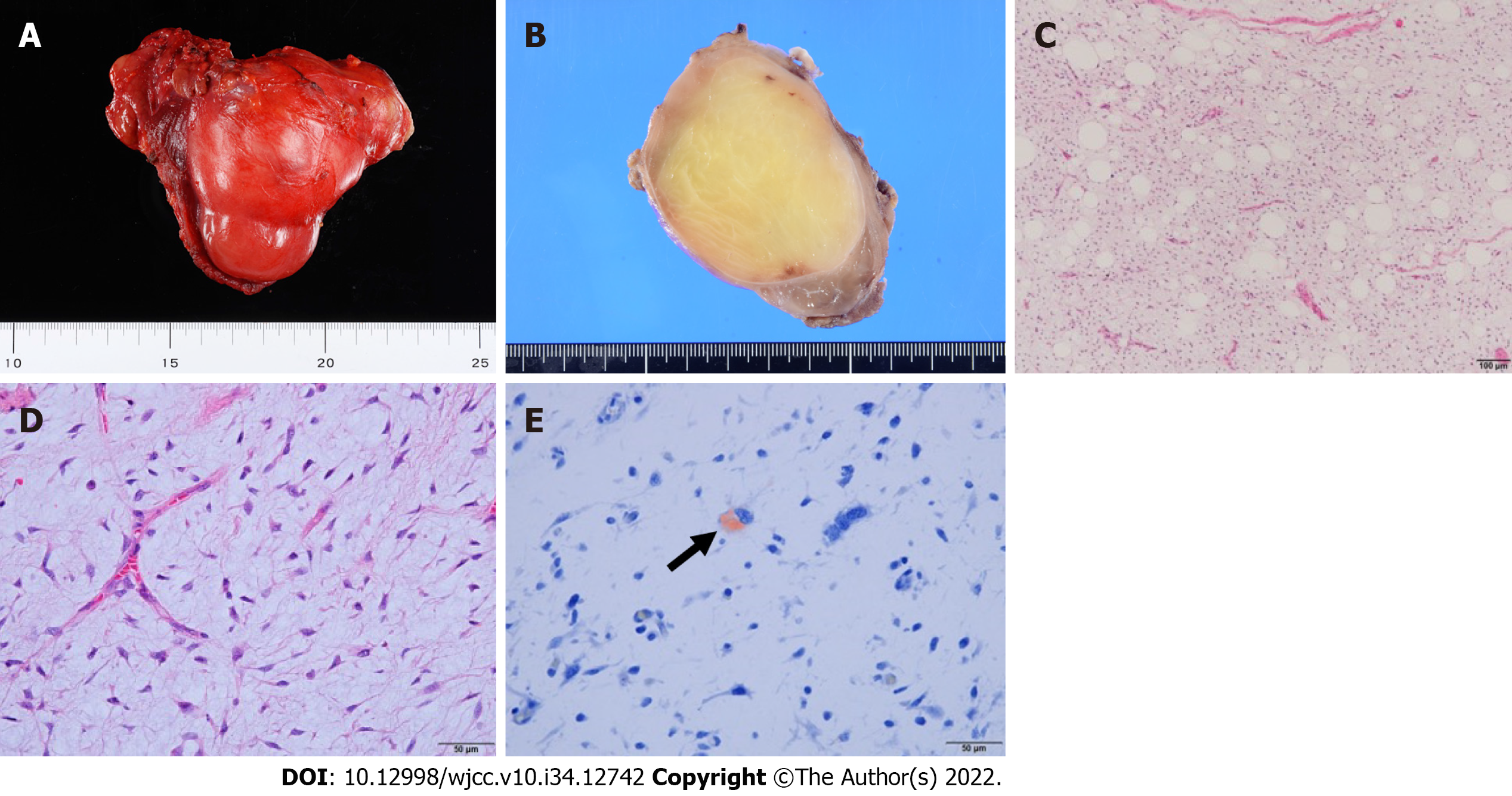
Submandibular dissection, tumor resection, and reconstruction with a forearm flap were performed. The tumor was resected based on the transoral and transcervical approaches. The floor of the mouth was reconstructed using a vascularized forearm flap following tumor resection. The tumor had a capsule, and the muscle tissue was excluded; no infiltration into the compressed mylohyoid, genioglossus, geniohyoid, and hyoglossus muscles was seen. These muscles, except for mylohyoid, were preserved. The lingual and hypoglossal nerves were not affected by the tumor and were therefore preserved. The periosteum of the mandible in contact with the tumor was resected as a margin (Figure 2). The resected tumor had a size of 8.5 cm × 6.5 cm × 5.5 cm (Figure 3A).
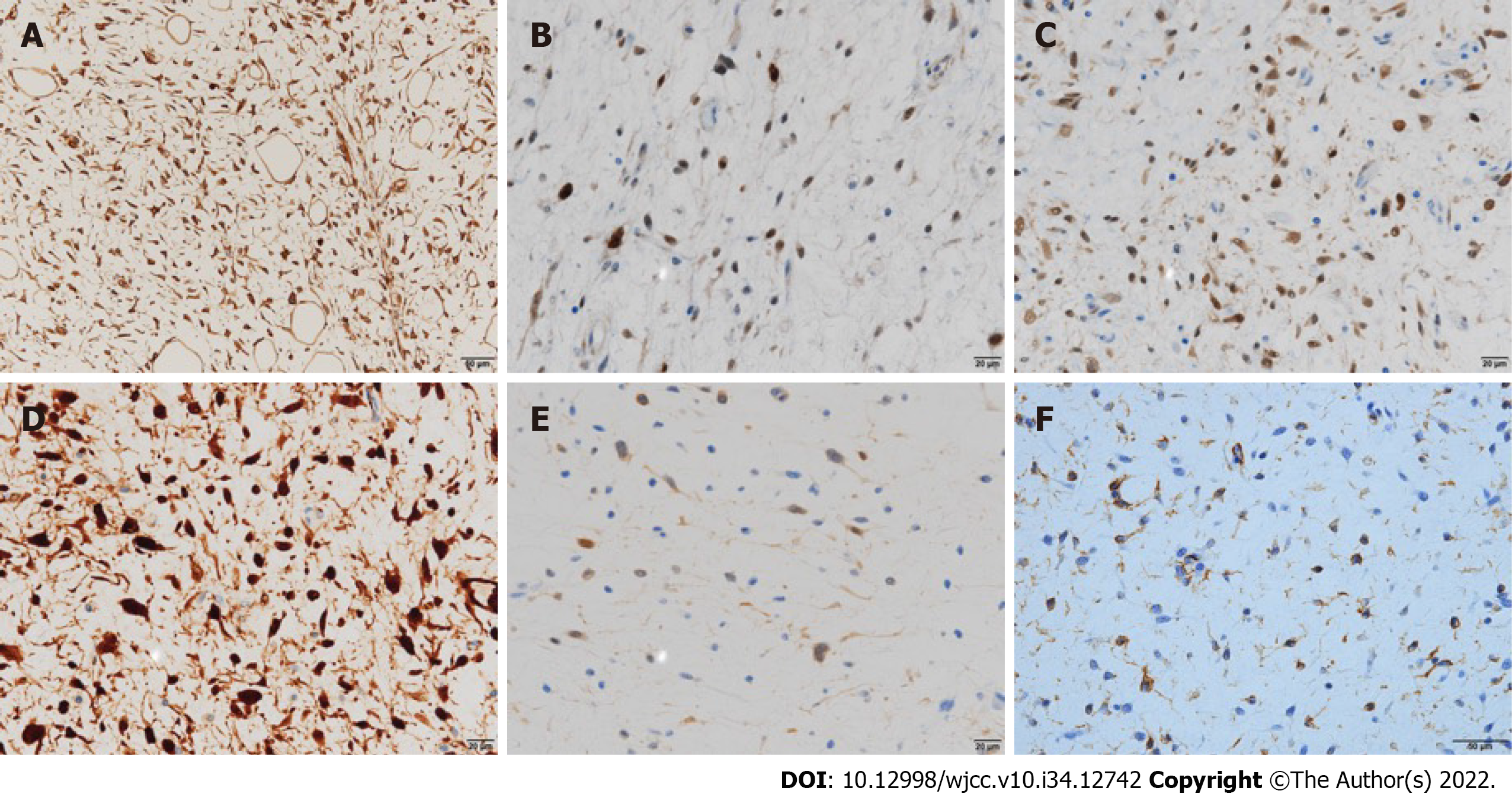
The macroscopic and histological features of the tumor are presented in Figure 3B-E, respectively. The tumor cells lacked nuclear pleomorphism, and the mitotic activity was low. The immunohistochemical staining results are presented in Figure 4. Fused in sarcoma (FUS, also known as TLS) and DNA damage-inducible transcript 3 (DDIT3, also known as CHOP) break-apart was not detected in the fluorescence in situ hybridization (FISH) analysis. However, based on the histological findings, including the presence of Oil Red O (ORO)-positive lipoblasts, a low-grade MLS was ultimately diagnosed. No cervical node metastasis was histopathologically observed.
At the 48-mo follow-up, the patient remained free of the disease, with no obvious signs of recurrence on clinical examination.
Oral liposarcoma is extremely rare[2,6,7]. Ohta et al[4] reviewed 120 cases of oral liposarcoma and found that the most common subtypes are ALT/WDL (60.8%), MLS (17.5%), DDLS (6.7%), and PLS (3.3%). The most common location was the tongue (40.7%), followed by the cheek/buccal mucosa (31.4%), gingiva (7.6%), palate (5.9%), floor of the mouth (5.1%), lip (4.2%), and other areas (5.1%). Since 1960, eight cases of liposarcoma of the floor of the mouth have been reported in the English-language literature (Table 1)[7-13]. Clinically, liposarcoma presents as a soft, painless mass with a slow growth and relatively well-defined boundaries; it is also covered with a pseudocapsule. Liposarcoma is commonly known to often lack significant clinical findings. In fact, in a past report of liposarcoma of the floor of the mouth, five patients were clinically diagnosed with ranula or benign tumor and underwent excisional biopsy (Table 1). In the present case, the tumor developed in the deep part of the floor of the mouth. It grew asymptomatically, forming a massive mass occupying the sublingual space and extending to the submandibular space, and malignant salivary gland tumor was suspected. This was a rare case in which the clinical diagnosis of a malignant tumor was made.
| Case No. | Ref. | Year | Age | Sex | Size (cm) | Clinical diagnosis | Biopsy | Histology | Additional therapy | Follow-up (mo) | Result |
| 1 | Enterline et al[8] | 1960 | 65 | Male | Egg size | NA | Incisional | MLS | R | 24 | DOD |
| 2 | Baden et al[9] | 1977 | 55 | Female | 0.7–6.5 | Ranula | Excisional | MLS | None | 36 | DID |
| 3 | Nakahara et al[7] | 1994 | 48 | Female | 2.2 | Benign tumor | Excisional | WDL | S + C | 12 | NED |
| 4 | Govender and Pillay[10] | 1998 | 34 | Male | 13 × 7.0 × 4.0 | Ranula | Excisional | MLS | R | 6 | NED |
| 5 | Nascimento et al[11] | 2002 | 35 | Female | 4.0 | NA | NA | ALT/WDL | NA | 25 | NA |
| 6 | 49 | Female | 8.0 | NA | NA | ALT/WDL | NA | 66 | NA | ||
| 7 | Nili et al[12] | 2016 | 45 | Female | 6.0 | Benign tumor | Excisional | ALT/WDL | None | 24 | NED |
| 8 | Nimura et al[13] | 2018 | 69 | Male | 6.0 × 4.5 × 4.5 | Tumor or cyst | Excisional | DDLS | R (60 Gy) | 68 | NED |
| 9 | Present case | 2022 | 71 | Male | 8.5 × 6.5 × 5.5 | Malignant salivary gland tumor | Incisional | MLS | None | 48 | NED |
MRI and CT scan are commonly employed to diagnose liposarcoma. Among them, MRI is the most useful as it can reveal tumor features that suggest malignancy. On MRI, liposarcoma is clearly demarcated; has thick, fibrous septa; and appears nodular and lobulated. The presence of bleeding, edema, and/or necrotic areas in addition to these findings is suggestive of malignancy. A low-grade liposarcoma tends to show a slight enhancement compared with a high grade liposarcoma, which usually enhances contrast very well. On FDG-PET/CT, MLS commonly presents as a mildly FDG-avid mass with overlapping metabolic characteristic. Due to the low FDG activity of a primary MLS lesion, the usefulness of FDG-PET/CT in tumor follow-up is questionable[14]. In the present case, MRI showed an uneven enhancement inside the tumor, and FDG-PET/CT showed a slight uptake in the tumor; both the MRI and PET/CT findings were consistent with the characteristics of MLS.
Although each liposarcoma subtype has its own pathological features, the key to diagnosis is the presence of lipoblasts. The myxoid variant comprises proliferating lipoblasts at varying stages of differentiation, signet-ring cells, a plexiform capillary pattern, and a myxoid matrix[15]. The smallest cell morphology is considered a low-grade tumor and is usually enriched with spindle-shaped cells. Large cell morphology is considered to be of high grade and usually shows abundant round cells. Many soft tissue neoplasms, whether benign or malignant, can demonstrate myxoid changes, including extraskeletal myxoid chondrosarcoma, myxofibrosarcoma/myxoid-type malignant fibrous histiocytoma, aggressive angiomyxoma, WDL with myxomatous change, and myxoma, and making a definitive histological diagnosis is sometimes difficult. In the present case, myxoma was part of the differential diagnosis of MLS, and it was difficult to make a definitive diagnosis in the small biopsy specimen.
MLS is characterized by a reciprocal translocation that generates a fusion transcription factor oncogene. In more than 95% of cases, a translocation, t (12,16) (q13; p11), which indicates fusing FUS with DDIT3, is present[16]. In the present case, we conducted FISH analysis to determine whether the FUS-DDIT3 fusion gene was present, but the break-apart between FUS and DDIT3 was not detected. Based on the histological findings, including the presence of ORO-positive lipoblasts, low-grade MLS was ultimately diagnosed. The fusion gene would have been detectable with polymerase chain reaction analysis, but we did not have a chance to conduct such an analysis. However, specific gene mutation is not found in all cases of each tumor type, and if histological diagnosis is reliable, it may not be necessary to change the diagnosis depending on the presence or absence of the gene mutation[17-19]. We ultimately made the diagnosis of MLS. Genetictesting for soft tissue tumors should be conducted in a well-controlled facility in close proximity to histological and clinical findings.
Liposarcoma is known to have a small island-shaped tumor tissue around the main tumor tissue, and inadequate excision is considered as the main reason for local recurrence, estimated at approximately 30%[20,21]. The optimal treatment for head and neck liposarcoma is surgery with negative margins; however, in the head and neck region, radical surgical approaches can cause functional and aesthetic disorders and may not provide sufficient resection margins. As MLS is radiosensitive, preoperative radiotherapy is considered effective to achieve a negative margin[22]. However, in the present case, a definitive diagnosis of MLS was not made preoperatively, and preoperative irradiation was not performed due to unclear tumor boundary and possibility of complete resection. Postoperative irradiation is considered for large tumors, but the efficacy of radiation therapy has been obscure due to the absence of systemic studies comparing the outcomes of treatment with or without radiotherapy[23]. Although metastasis is unlikely, local recurrence is common, with the MLS recurrence rates ranging from 33% to 53%[8,24]. Lymph node metastases are quite rare[25]; thus, neck dissection is not necessary unless there is specific evidence of lymph node metastasis.
The 5-year disease-specific survival rate is 100% for ALT/WDL, 100% for MLS, 60% for round cell liposarcoma, and 45% for PLS in the head and neck region[26]. Tumor sizes > 5.0 cm[27] and > 3.6 cm[28] have been reported to be poor prognostic factors for liposarcoma in the oral region, and careful follow-up is thus needed, as done for the patient in the present case.
In conclusion, we treated an extremely rare, massive, low-grade MLS of the floor of the mouth. Oftentimes, an MLS of the floor of the mouth lacks significant clinical findings and is misdiagnosed. A preoperative histological diagnosis via incisional biopsy allows the largest possible primary resection. In the present case, complete resection was performed, and no lymph node metastasis was observed; however, a tumor size > 5 cm is a risk factor for poor prognosis; thus, careful follow-up is essential. Although no FUS-DDIT3 fusion gene was detected, low-grade MLS was ultimately diagnosed based on the histological findings.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jain S, India; Liu J, China S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Virchow R. A case of malignant occurring in part in the form of fat neuroma tumors. Virchows Arch Pathol Anat. 1857;11:281-288. |
| 2. | Piperi E, Tosios KI, Nikitakis NG, Kyriakopoulos VF, Tzerbos F, Koutlas IG, Sklavounou A. Well-differentiated liposarcoma/atypical lipomatous tumor of the oral cavity: report of three cases and review of the literature. Head Neck Pathol. 2012;6:354-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Kim YB, Leem DH, Baek JA, Ko SO. Atypical lipomatous tumor/well-differentiated liposarcoma of the gingiva: a case report and review of literature. J Oral Maxillofac Surg. 2014;72:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Ohta K, Yoshimura H, Matsuda S, Imamura Y, Sano K. Oral liposarcoma in elderly: Case report and literature analysis. Medicine (Baltimore). 2020;99:e18985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Dei Tos AP, Marino-Enriquez A, Pedeutour F, Rossi S. Dedifferentiated liposarcoma. In: Fletcher C, Bridge J, Hogendoorn P and Mertens F, editors. WHO Classification of Tumours of Soft Tissue and Bone. 4th edition. Lyon: IARC Press; 2013: 37-38. |
| 6. | Cheng J, Wang Y, Cheng A, Wang L, Tian Z, Yu H, Wang X, Wu Y, Shen G. Primary liposarcoma in oral and maxillofacial region. J Craniofac Surg. 2011;22:1765-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Nakahara H, Shirasuna K, Terada K. Liposarcoma of the floor of the mouth: a case report. J Oral Maxillofac Surg. 1994;52:1322-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Enterline HT, Culberson JD, Rochlin DB, Brady LW. Liposarcoma. A clinical and pathological study of 53 cases. Cancer. 1960;13:932-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Baden E, Newman R. Liposarcoma of the oropharyngeal region. Review of the literature and report of two cases. Oral Surg Oral Med Oral Pathol. 1977;44:889-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Govender D, Pillay P. Primary myxoid liposarcoma with rhabdomyoblastic differentiation. Arch Pathol Lab Med. 1998;122:740-742. [PubMed] |
| 11. | Nascimento AF, McMenamin ME, Fletcher CD. Liposarcomas/atypical lipomatous tumors of the oral cavity: a clinicopathologic study of 23 cases. Ann Diagn Pathol. 2002;6:83-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Nili F, Baghai F, Aghai A, Etebarian A. Well-differentiated liposarcoma of the floor of the mouth: Report of a rare case and review of the literature. J Oral Maxillofac Pathol. 2016;20:312-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Nimura F, Nakasone T, Matsumoto H, Maruyama T, Matayoshi A, Maruyama N, Yoshimi N, Arasaki A, Nishihara K. Dedifferentiated liposarcoma of the oral floor: A case study and literature review of 50 cases of head and neck neoplasm. Oncol Lett. 2018;15:7681-7688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Lunn BW, Littrell LA, Wenger DE, Broski SM. 18F-FDG PET/CT and MRI features of myxoid liposarcomas and intramuscular myxomas. Skeletal Radiol. 2018;47:1641-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Fusetti M, Silvagni L, Eibenstein A, Chiti-Batelli S, Hueck S, Fusetti M. Myxoid liposarcoma of the oral cavity: case report and review of the literature. Acta Otolaryngol. 2001;121:759-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Antonescu CR, Tschernyavsky SJ, Decuseara R, Leung DH, Woodruff JM, Brennan MF, Bridge JA, Neff JR, Goldblum JR, Ladanyi M. Prognostic impact of P53 status, TLS-CHOP fusion transcript structure, and histological grade in myxoid liposarcoma: a molecular and clinicopathologic study of 82 cases. Clin Cancer Res. 2001;7:3977-3987. [PubMed] |
| 17. | Coindre JM, Pelmus M, Hostein I, Lussan C, Bui BN, Guillou L. Should molecular testing be required for diagnosing synovial sarcoma? Cancer. 2003;98:2700-2707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Neuville A, Ranchère-Vince D, Dei Tos AP, Montesco MC, Hostein I, Toffolatti L, Chibon F, Pissaloux D, Alberti L, Decouvelaere AV, Albert S, Rossi CR, Blay JY, Coindre JM. Impact of molecular analysis on the final sarcoma diagnosis: a study on 763 cases collected during a European epidemiological study. Am J Surg Pathol. 2013;37:1259-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Thway K, Rockcliffe S, Gonzalez D, Swansbury J, Min T, Thompson L, Fisher C. Utility of sarcoma-specific fusion gene analysis in paraffin-embedded material for routine diagnosis at a specialist centre. J Clin Pathol. 2010;63:508-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Dei Tos AP. Liposarcoma: new entities and evolving concepts. Ann Diagn Pathol. 2000;4:252-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 222] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 21. | Gagari E, Kabani S, Gallagher GT. Intraoral liposarcoma: case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Gonzalez RJ, Gronchi A, Pollock RE. Soft tissue sarcomas. In: Brunicardi FC, Andersen DK, Billiar TR, Dunn DL, Hunter JG, Kao LS, editors. Schwartz’s Principles of Surgery. New York: McGraw-Hill Education. 2019: 1577. |
| 23. | Quilichini R, Hadida A, Daumas B, Dugenet P. [A case of diffuse syphilitic osteoperiostitis]. Mars Med. 1970;107:499-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Enzinger FM, Winslow DJ. Liposarcoma. A study of 103 cases. Virchows Arch Pathol Anat Physiol Klin Med. 1962;335:367-388. [PubMed] |
| 25. | Gritli S, Khamassi K, Lachkhem A, Touati S, Chorfa A, Ben Makhlouf T, El May A, Gammoudi A. Head and neck liposarcomas: a 32 years experience. Auris Nasus Larynx. 2010;37:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Davis EC, Ballo MT, Luna MA, Patel SR, Roberts DB, Nong X, Sturgis EM. Liposarcoma of the head and neck: The University of Texas M. D. Anderson Cancer Center experience. Head Neck. 2009;31:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Fanburg-Smith JC, Furlong MA, Childers EL. Liposarcoma of the oral and salivary gland region: a clinicopathologic study of 18 cases with emphasis on specific sites, morphologic subtypes, and clinical outcome. Mod Pathol. 2002;15:1020-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Cheng J, Yu H, Wang L, Wang X, Shen G. Primary oral and maxillofacial liposarcoma: a clinicopathological and immunohistochemical study of eleven cases. Arch Med Sci. 2012;8:316-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |