Published online Dec 6, 2022. doi: 10.12998/wjcc.v10.i34.12703
Peer-review started: August 9, 2022
First decision: October 12, 2022
Revised: October 13, 2022
Accepted: November 4, 2022
Article in press: November 4, 2022
Published online: December 6, 2022
Processing time: 115 Days and 7.8 Hours
Immunoglobulin light chain (AL) amyloidosis is a rare disease characterized by deposition of ALs essentially in any organ or tissue, with cardiac involvement being very frequent (61%). Early diagnosis is of high importance because early initiation of treatment in AL amyloidosis may improve outcomes. Despite the administration of immunotherapeutic agents, in particular bortezomib and daratumumab, which have improved the outcomes of AL amyloidosis, anti-plasma cell therapy remains suboptimal for some patients.
We report the case of a 55-year-old man presenting with heart failure who was diagnosed with cardiac AL amyloidosis by an endomyocardial biopsy. He experienced a short-term hematological remission with no organ response after being administered a bortezomib-daratumumab containing regimen. The treatment was switched to pomolidomide due to pulmonary involvement and progressive pleural effusion, in which flow cytometry analysis showed abnormal plasma cells. After two cycles of this regimen, the pleural effusion was controlled effectively with no recurrence.
This case emphasizes the crucial role of endomyocardial biopsy in early diagnosis of cardiac amyloidosis and suggests that pomolidomide may be an effective treatment for patients with AL amyloidosis that is relapsed/refractory to both bortezomib and daratumumab.
Core Tip: We report the case of a 55-year-old man presenting with heart failure who was diagnosed with cardiac immunoglobulin light chain (AL) amyloidosis by an endomyocardial biopsy, in whom pomolidomide corrected severe pleural effusion after resistance to both bortezomib and daratumumab. This case emphasizes the crucial role of endomyocardial biopsy in early diagnosis of cardiac amyloidosis and suggests that pomolidomide may be an effective treatment for patients with AL amyloidosis that is relapsed/refractory to both bortezomib and daratumumab.
- Citation: Li X, Pan XH, Fang Q, Liang Y. Pomolidomide for relapsed/refractory light chain amyloidosis after resistance to both bortezomib and daratumumab: A case report. World J Clin Cases 2022; 10(34): 12703-12710
- URL: https://www.wjgnet.com/2307-8960/full/v10/i34/12703.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i34.12703
Immunoglobulin light chain (AL) amyloidosis is a rare monoclonal B-cell or plasma cell disorder characterized by the aggregation of misfolded monoclonal light chain kappa or lambda, which form insoluble amyloid fibrils with a beta-sheet structure[1] that are deposited and accumulated in nearly any organ or tissue[2]. Cardiac involvement is very frequent in AL amyloidosis (61% of patients) and can lead to heart failure because of infiltrative heart disease, which is the main prognostic factor and carries a poor prognosis[3]. Early diagnosis is therefore of high importance because early initiation of treatment in AL amyloidosis may improve outcomes[4]. Many diagnostic tools are included in the workup for detection of amyloidosis. However, a biopsy of a clinically affected organ is the most sensitive method[5].
Traditional conventional chemotherapy regimens are associated with a significantly lower response rate because clonal plasma cells in most AL amyloidosis patients are less chemosensitive than those in patients with multiple myeloma[5]. Despite the application of novel drugs that have been developed, in particular bortezomib and daratumumab, which have improved AL amyloidosis outcomes, anti-plasma cell therapy remains suboptimal for some patients[6].
The present study describes an AL amyloidosis patient diagnosed by endomyocardial biopsy who was treated with pomolidomide after being resistant to both bortezomib and daratumumab.
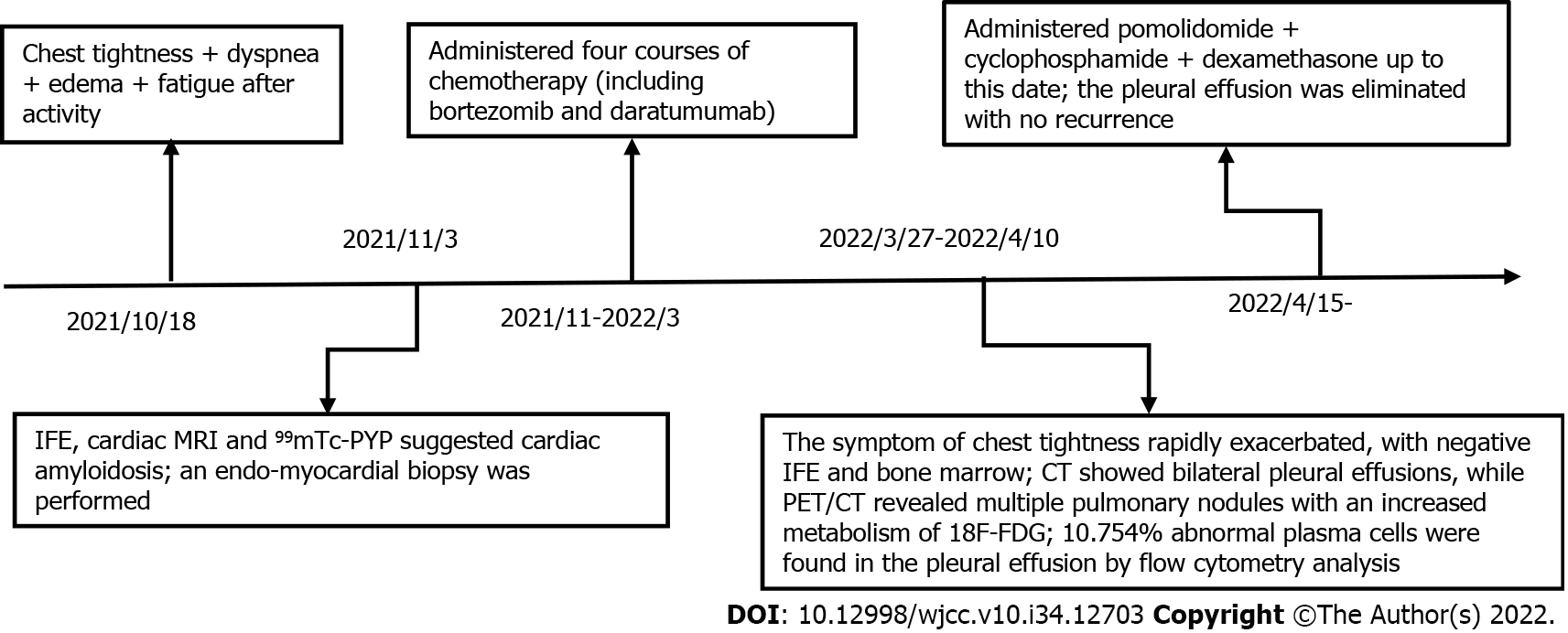
A 55-year-old man was admitted to The Second Affiliated Hospital of Zhejiang University School of Medicine (Hangzhou, China) in October 2021 with rapidly worsening heart failure.
The patient had been experiencing heart failure for 3 mo, but had begun to experience chest tightness, dyspnea, edema, and fatigue after activity.
The patient had undergone a pulmonary resection 20 years prior to address carcinoma in situ; his current disease state was considered stable.
The patient’s personal and family histories were unremarkable.
The patient’s blood pressure was 87/49 mmHg, pulse rate was 101 beats per minute with a regular rhythm, and O2 saturation was 97% at room air. Physical examination revealed that he had clear lungs and normal heart sounds with no murmurs or gallops on auscultation. His lower extremities showed mild bilateral pitting edema. No enlargement of lymph nodes, liver, or spleen was found.
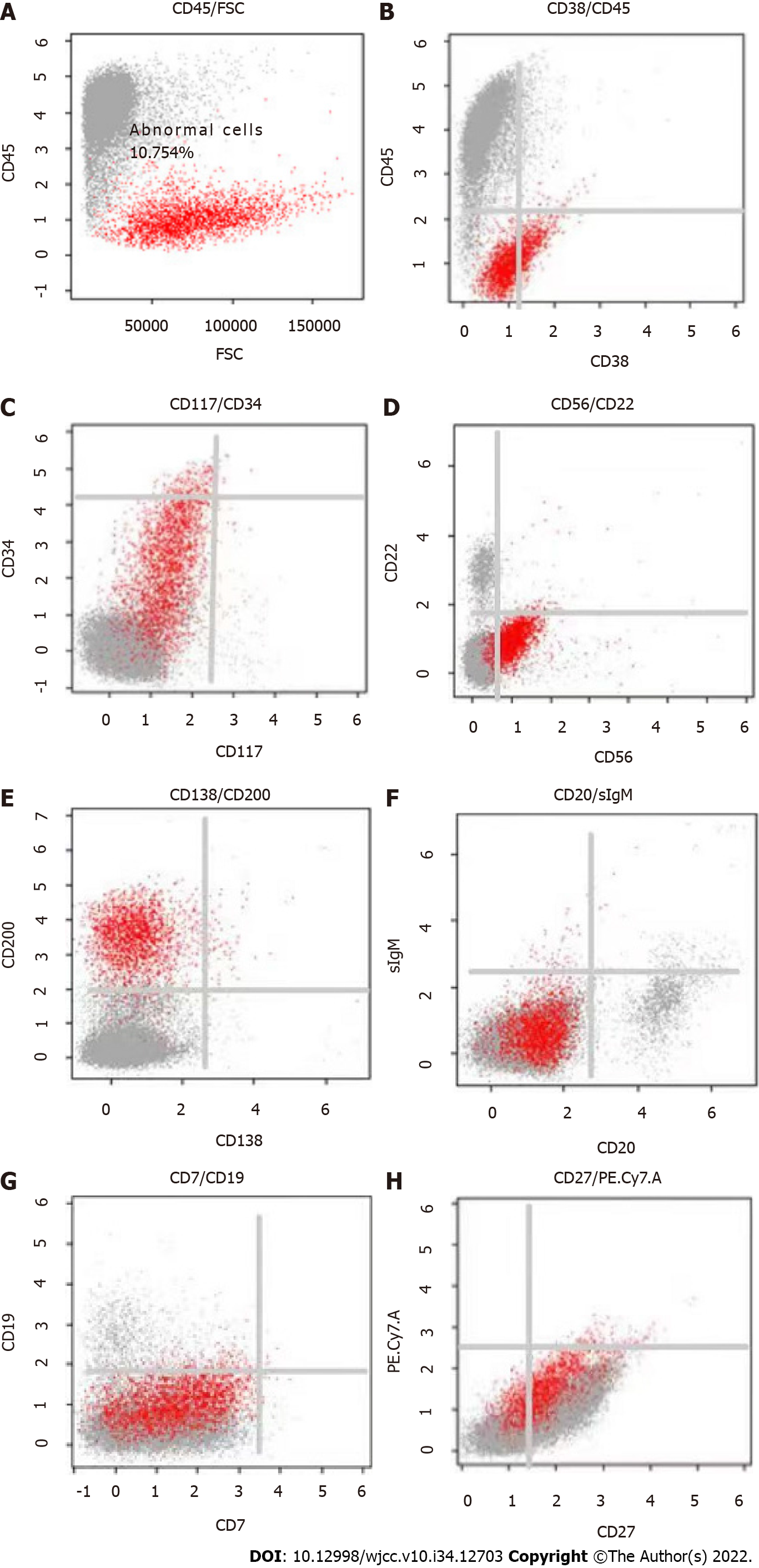
Laboratory tests showed normal findings in a complete blood count and comprehensive metabolic panel. The levels of serum lactate dehydrogenase and β2-microglobulin were within normal limits. However, the levels of brain natriuretic peptide (385.7 pg/mL; normal: < 100) and troponin T (0.058 ng/mL; normal: < 0.014) were over the normal upper limits. Creatinine was within the normal range (at 51.9 μmol/L), while urine analysis was negative for protein. Quantitation of 24-h urinary lambda light chain showed a level of 365.2 mg. Quantitative serum immunoglobulin analyses demonstrated normal levels of IgG (at 10.5 g/L), IgA (at 2.01 g/L), and IgM (at 0.28 g/L). Serum immunofixation was used to evaluate an underlying gammopathy and showed lambda light chain proteinemia. The level of serum-free lambda light chain (FLC) was normal (at 15.56 mg/L), with the difference between the involved and uninvolved serum FLC levels being 6.75 mg/L. A bone marrow aspirate smear showed 5% infiltration of plasma cells, while flow cytometry analysis showed an abnormal population of plasma cells that accounted for 5.8% of normal cells, most of which were positive for surface CD38, CD56, CD138, and cytoplasmic λ light-chain. The fluorescence in situ hybridization test was negative, which included a 1q21 amplification, 13q14 deletion, p53 deletion, and translocation of t (4; 14), t (11; 14), t (14; 16).
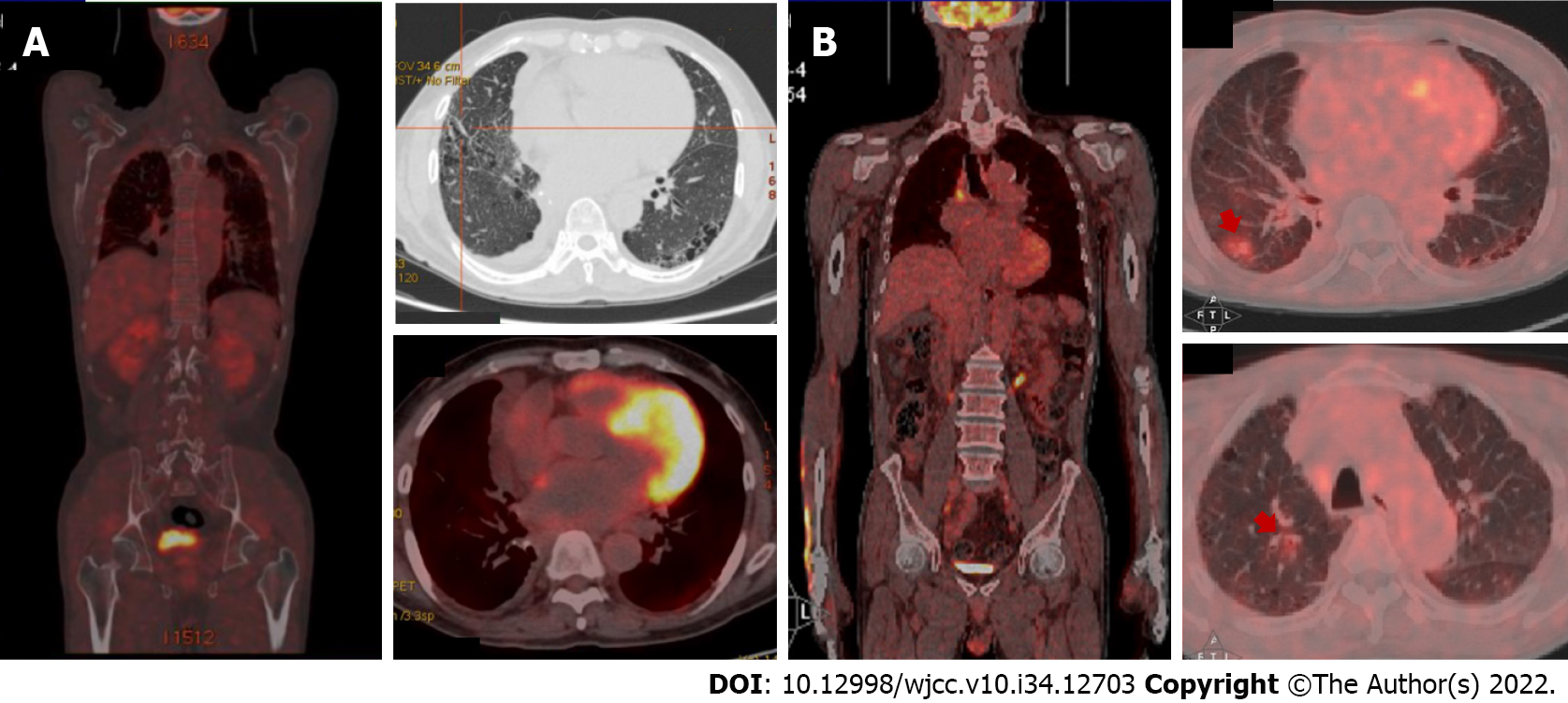
Positron emission tomography/computerized tomography (PET/CT) was performed and showed no abnormal metabolic lesions. An electrocardiogram showed low voltages in the limb leads. Echocardiogram revealed severe left ventricular hypertrophy, a reduced left ventricle ejection fraction of 38.1%, and an elevated left ventricular filling pressure E/A of more than 2.04. Cardiovascular magnetic resonance imaging (MRI) showed the morphologic phenotype of increased left ventricle wall thickness, while 99technetium pyrophosphate (99mTc-PYP) planar scintigraphy showed a heart-to-contralateral ratio of 1.31.
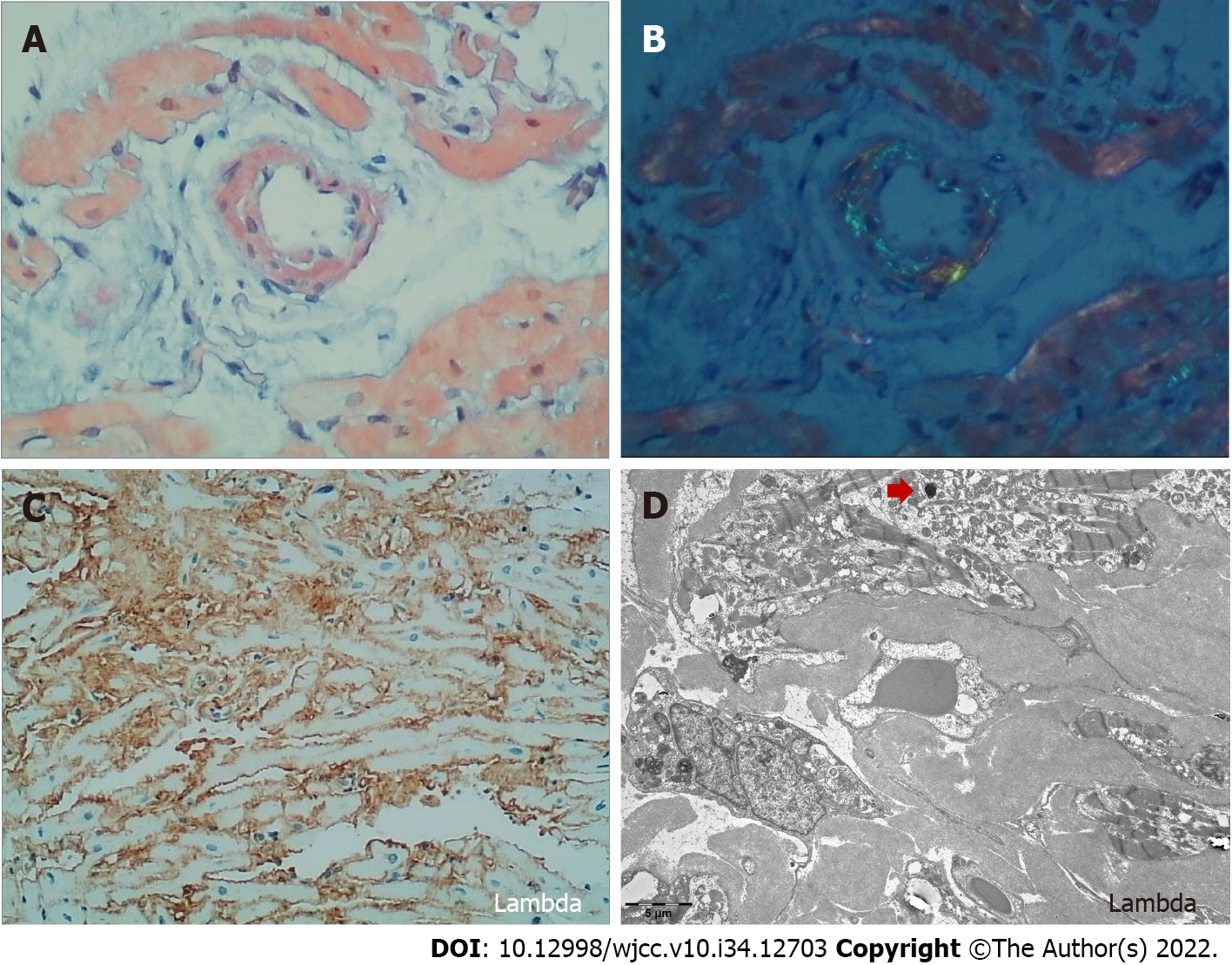
Cardiac amyloidosis was considered as a possible etiology of the cardiomyopathy due to the left ventricle thickness on echocardiogram and the heart-to-contralateral ratio of 1.31 on 99mTc-PYP. An endomyocardial biopsy was performed with electron microscopy, revealing fibroid deposits in the myocardium and after Congo-red staining, fibrous tissue with apple green birefringence visualized by polarized light microscopy (Figure 1). Immunohistochemistry analysis showed dominant positivity for monoclonal lambda light chains.
Taken together, these findings established a diagnosis of cardiac AL amyloidosis (stage 2, Mayo 2012 model).
The patient then underwent four courses of chemotherapy including bortezomib, daratumumab, and dexamethasone, which achieved a partial hematologic response without a cardiac response. Remarkably, despite there no longer being a detectable monoclonal component on serum immu
After two cycles, the patient showed good tolerance of the regimen without any notable side effects, and his brain natriuretic peptide and troponin T levels had reduced slightly to 304.1 pg/mL and 0.114 ng/mL, respectively. The results of echocardiography after treatment were as follows: Left ventricular hypertrophy; left ventricle ejection fraction of 37.4%; and E/A ratio of 1.94. These findings were similar to those at diagnosis, although the pleural effusion was eliminated with no recurrence (Figure 4).
This brief report describes a patient with AL amyloidosis diagnosed by an endomyocardial biopsy, in whom pomolidomide corrected severe pleural effusion after resistance to both bortezomib and daratumumab. The main points of this interesting case are as follows. First, the case emphasizes the crucial role of endomyocardial biopsy in the diagnosis of cardiac amyloidosis. Second, it shows the possibility that some patients may have progression of disease despite there no longer being a detectable monoclonal component on serum immunofixation or monoclonal plasma cells in a bone marrow aspirate. Third and last, the case demonstrates the efficacy of pomolidomide in treating relapsed/refractory AL amyloidosis and offers an opportunity for remission.
AL amyloidosis is a systemic disease characterized by deposition of ALs essentially in any organ or tissue, resulting in dysfunction of the affected organs. Because of the rapid progress and poor prognosis of AL amyloidosis, early detection and treatment are necessary. The diagnostic criteria for AL amyloidosis include clinical presentation of organ dysfunction, evidence of amyloid deposition, and dominant monoclonal immunoglobulin or free light chains. Histopathological detection of these changes remains the diagnostic gold standard[7].
To establish the diagnosis, detection of amyloidosis is required using a biopsy of subcutaneous fat, bone marrow, or salivary glands. However, biopsy of a clinically affected organ is the most sensitive method, in particular a heart biopsy, despite this procedure being associated with a risk of an adverse event[8]. In AL amyloidosis, positive staining for Congo red, an 8-14 nm wide fibrillar appearance by electron microscopy, and apple-green birefringence under polarized light microscopy are the common histopathologic features. Dominant positivity for monoclonal light chain kappa or lambda can be seen by immunohistochemistry, although in some cases identification of the amyloid subunit may be equivocal[5].
Mass spectrometry is therefore the standard for confirming protein composition in amyloid deposits and is superior to immunohistochemistry in typing the protein subunit[9]. In addition to histopathologic confirmation of amyloid deposits in tissue, cardiovascular imaging has taken on an increasingly important role in the diagnosis of cardiac amyloidosis. 99mTc-PYP has been found useful in distinguishing between AL cardiac amyloidosis and transthyretin amyloidosis (heart-to-contralateral ratio ≥ 1.5)[8]. Echocardiogram and cardiac MRI have also shown the ability to evaluate cardiac function and provide prognostic information to predict mortality. The potential utility of PET/CT is to identify cardiac amyloidosis and quantify the burden of amyloid deposition[10].
The major determinants of outcome in amyloidosis include the extent of cardiac involvement, response durability[11], and the burden of light chain deposition. Meanwhile, bone marrow plasma cell infiltration and cytogenetic risk have been shown to predict relapse and long-term survival[12]. According to previous clinical studies, AL amyloidosis patients with an initial difference between the involved and uninvolved serum FLC < 50 mg/L have a meaningful advantage in clinical outcome, in whom an early hematologic response within 3 mo indicates a favorable prognosis[13]. In our case, the patient had normal cytogenetics with low initial amyloidogenic FLC levels and had an early hematological remission without cardiac response. Although serum monoclonal immunoglobulin and bone marrow clonal plasma cells were negative following first-line treatment, the patient developed rapid progression of extra-cardiac infiltration including the pleura and pulmonary region. This imbalance between normal monoclonal light chain burden and apparent organ involvement could potentially be explained by distinctive selective and toxic monoclonal light chains in tissues[14]. However, the detailed mechanisms still remain unclear.
During the last two decades, application of novel drugs that have been developed have optimized the overall prognosis of AL amyloidosis. Bortezomib, a proteasome inhibitor, is an attractive option for AL amyloidosis patients, as there is evidence that it significantly improves overall response rate[15]. Bortezomib-based regimens have been used to initiate treatment for newly diagnosed patients with AL amyloidosis, in particular in combination with the anti-CD38 antibody daratumumab. In the ANDROMEDA study, daratumumab plus CyBorD (bortezomib-cyclophosphamide-dexamethasone) had a higher overall hematologic response rate (96%) and organ response rate (64%) compared to that of controls without daratumumab therapy[6]. However, these immunotherapeutic agents remain suboptimal for some patients. Immunomodulatory drugs such as thalidomide, lenalidomide, and pomalidomide exert direct and indirect anti-plasma cell activity by regulating the immune response, enhancing natural killer cell cytotoxicity directly or through T cell stimulation, and modulating the microenvironment[16].
Currently, most patients receive immunomodulatory drug-based rescue therapy, which can overcome resistance to daratumumab and bortezomib[8,16]. Because AL amyloidosis is commonly accompanied by significant organ dysfunction, treatment must be minimally toxic to avoid clinical deterioration. Thalidomide and lenalidomide have poor tolerance in patients with cardiac amyloidosis, with the efficacy of thalidomide also being limited. Pomalidomide, a next-generation immunomodulatory agent, induces a rapid hematologic response rate ranging from 44%-66%, associated with a renal response in 17%-44% of cases[17-19]. Even in heavily pretreated refractory patients, who previously received bortezomib (93%) and lenalidomide (81%), pomalidomide was an effective treatment, eliciting a hematologic response rate ranging from 37%-49%[17]. Moreover, pomalidomide toxicity is manageable, even in the fragile population of patients with cardiac or renal dysfunction. Pomalidomide will also most likely further improve the outcome of relapsed/refractory AL amyloidosis patients.
We report an AL amyloidosis patient diagnosed by an endomyocardial biopsy, to whom pomolidomide was effective after resistance to both bortezomib and daratumumab. We hope that our experience will provide a reference for physicians to improve early diagnosis and appropriate management for AL amyloidosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Charach L, Israel; Paydas S, Turkey S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Bianchi G, Kumar S. Systemic Amyloidosis Due to Clonal Plasma Cell Diseases. Hematol Oncol Clin North Am. 2020;34:1009-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Martinez-Naharro A, Hawkins PN, Fontana M. Cardiac amyloidosis. Clin Med (Lond). 2018;18:s30-s35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 160] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 3. | Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2:1046-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 270] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 4. | Sperry BW, Ikram A, Hachamovitch R, Valent J, Vranian MN, Phelan D, Hanna M. Efficacy of Chemotherapy for Light-Chain Amyloidosis in Patients Presenting With Symptomatic Heart Failure. J Am Coll Cardiol. 2016;67:2941-2948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Ryšavá R. AL amyloidosis: advances in diagnostics and treatment. Nephrol Dial Transplant. 2019;34:1460-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Palladini G, Kastritis E, Maurer MS, Zonder J, Minnema MC, Wechalekar AD, Jaccard A, Lee HC, Bumma N, Kaufman JL, Medvedova E, Kovacsovics T, Rosenzweig M, Sanchorawala V, Qin X, Vasey SY, Weiss BM, Vermeulen J, Merlini G, Comenzo RL. Daratumumab plus CyBorD for patients with newly diagnosed AL amyloidosis: safety run-in results of ANDROMEDA. Blood. 2020;136:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 147] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 7. | Siddiqi OK, Ruberg FL. Cardiac amyloidosis: An update on pathophysiology, diagnosis, and treatment. Trends Cardiovasc Med. 2018;28:10-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 231] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 8. | Gertz MA. Immunoglobulin light chain amyloidosis: 2022 update on diagnosis, prognosis, and treatment. Am J Hematol. 2022;97:818-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 9. | Barreca A, Bottasso E, Veneziano F, Giarin M, Nocifora A, Martinetti N, Attanasio A, Biancone L, Benevolo G, Roccatello D, Cassoni P, Papotti MG; Amyloidosis Group of the “Rete Interregionale Piemonte e Valle d’Aosta per le Malattie Rare”. Immunohistochemical typing of amyloid in fixed paraffin-embedded samples by an automatic procedure: Comparison with immunofluorescence data on fresh-frozen tissue. PLoS One. 2021;16:e0256306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Taiwo AA, Alapati L, Movahed A. Cardiac amyloidosis: A case report and review of literature. World J Clin Cases. 2019;7:742-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Ravichandran S, Law S, Mahmood S, Wisniowski B, Foard D, Fontana M, Martinez-Naharro A, Whelan C, Gillmore JD, Lachmann HJ, Hawkins PN, Wechalekar AD. Early relapse is an adverse prognostic marker in systemic immunoglobulin light chain (AL) Amyloidosis. Leukemia. 2022;36:1180-1184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Palladini G, Milani P, Merlini G. Predicting survival in light chain amyloidosis. Haematologica. 2019;104:1294-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Dittrich T, Bochtler T, Kimmich C, Becker N, Jauch A, Goldschmidt H, Ho AD, Hegenbart U, Schönland SO. AL amyloidosis patients with low amyloidogenic free light chain levels at first diagnosis have an excellent prognosis. Blood. 2017;130:632-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 14. | Merlini G, Palladini G. Light chain amyloidosis: the heart of the problem. Haematologica. 2013;98:1492-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Manwani R, Cohen O, Sharpley F, Mahmood S, Sachchithanantham S, Foard D, Lachmann HJ, Quarta C, Fontana M, Gillmore JD, Whelan C, Hawkins PN, Wechalekar AD. A prospective observational study of 915 patients with systemic AL amyloidosis treated with upfront bortezomib. Blood. 2019;134:2271-2280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 16. | Gavriatopoulou M, Kastritis E, Ntanasis-Stathopoulos I, Fotiou D, Roussou M, Migkou M, Ziogas DC, Kanellias N, Terpos E, Dimopoulos MA. The addition of IMiDs for patients with daratumumab-refractory multiple myeloma can overcome refractoriness to both agents. Blood. 2018;131:464-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Milani P, Sharpley F, Schönland SO, Basset M, Mahmood S, Nuvolone M, Kimmich C, Foli A, Sachchithanantham S, Merlini G, Wechalekar A, Palladini G, Hegenbart U. Pomalidomide and dexamethasone grant rapid haematologic responses in patients with relapsed and refractory AL amyloidosis: a European retrospective series of 153 patients. Amyloid. 2020;27:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Sharpley FA, Manwani R, Mahmood S, Sachchithanantham S, Lachmann H, Gilmore J, Whelan C, Hawkins P, Wechalekar A. Real world outcomes of pomalidomide for treatment of relapsed light chain amyloidosis. Br J Haematol. 2018;183:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Palladini G, Milani P, Foli A, Basset M, Russo F, Perlini S, Merlini G. A phase 2 trial of pomalidomide and dexamethasone rescue treatment in patients with AL amyloidosis. Blood. 2017;129:2120-2123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |