Published online Dec 6, 2022. doi: 10.12998/wjcc.v10.i34.12684
Peer-review started: July 18, 2022
First decision: October 12, 2022
Revised: October 20, 2022
Accepted: November 10, 2022
Article in press: November 10, 2022
Published online: December 6, 2022
Processing time: 137 Days and 9.5 Hours
In endovascular procedures including total percutaneous endovascular aneurysm repair (pEVAR), percutaneous access through the common femoral artery is most commonly performed. Access-site bleeding is a major concern in percutaneous techniques. Herein, we present a case of successful control of continuous oozing using a vascular closure device (VCD) and the application of Surgicel (Johnson & Johnson, United States) over the access tract.
An 82-year-old man presented with an unruptured abdominal aortic aneurysm measuring 83 mm × 75 mm. The patient had a medical history of atrial fibrillation and was receiving rivaroxaban (15 mg/d). Routine pEVAR was performed using the preclose technique with ProGlide (Abbott, Santa Clara, CA, United States). Significant amount of bleeding was observed at the end of the procedure after the deployment of the closure device at the access site. A sheet of Surgicel was applied to the suture thread using a surgical needle. Surgicel was applied to the surface of the artery along the access tract using a pusher, and hemostasis was immediately attained.
This simple technique is an excellent adjunct to control residual bleeding from the access site following VCD use.
Core Tip: Endovascular procedures, including percutaneous endovascular aneurysm repair, usually involve vascular access through the common femoral artery. Vascular closure devices (VCDs) are being increasingly used to achieve hemostasis. When continuous oozing is observed after the application of the VCD, Surgicel can be applied to the arterial surface along the VCD suture thread using a pusher. Surgicel is a simple and cost-effective hemostatic adjunct.
- Citation: Kim H, Lee K, Cho S, Joh JH. Rapid hemostasis of the residual inguinal access sites during endovascular procedures: A case report. World J Clin Cases 2022; 10(34): 12684-12689
- URL: https://www.wjgnet.com/2307-8960/full/v10/i34/12684.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i34.12684
Vascular surgery has radically shifted from traditional open surgery to percutaneous endovascular intervention in recent decades. In most endovascular procedures, including percutaneous endovascular aneurysm repair (pEVAR), the common femoral artery is the most commonly used vascular access site. Vascular closure devices (VCDs) are often used to avoid open closure of the puncture site, particularly when the procedure is associated with a larger sheath. During pEVAR, the preclose technique, which usually entails using two Perclose ProGlide closure devices (Abbot Vascular, Santa Clara, CA, United States), is widely used[1]. With successful and rapid hemostasis, the potential benefits of successful and rapid hemostasis include a shorter surgical duration, less need for transfusions, better management of patients on anticoagulants, a decrease in patient recovery time, and reduced wound exposure[2]. However, residual access-site bleeding is commonly encountered when using the suture-based closure mechanism.
The oxidized regenerated cellulose, Surgicel (Johnson & Johnson, New Brunswick, NJ, United States) is commonly used to control bleeding in various open vascular surgeries. Herein, we report a simple technique to achieve rapid hemostasis in residual access-site bleeding by using a Surgicel-assisted technique.
An 82-year-old man presented with a fist-sized pulsating mass in the periumbilical region.
Symptoms appeared 1 mo before presentation.
The patient had moderate-to-severe stenosis of both internal carotid arteries, stable angina, and atrial fibrillation. He was receiving rivaroxaban 15 mg/d and cilostazol 200 mg/d. He had recently developed cerebral infarction in the region supplied by the middle cerebral artery.
The patient had no family history of abdominal aortic aneurysms.
On physical examination, the patient’s vital signs were stable. A pulse was palpable over both the common femoral and pedal arteries.
All coagulation parameters were within the normal ranges.
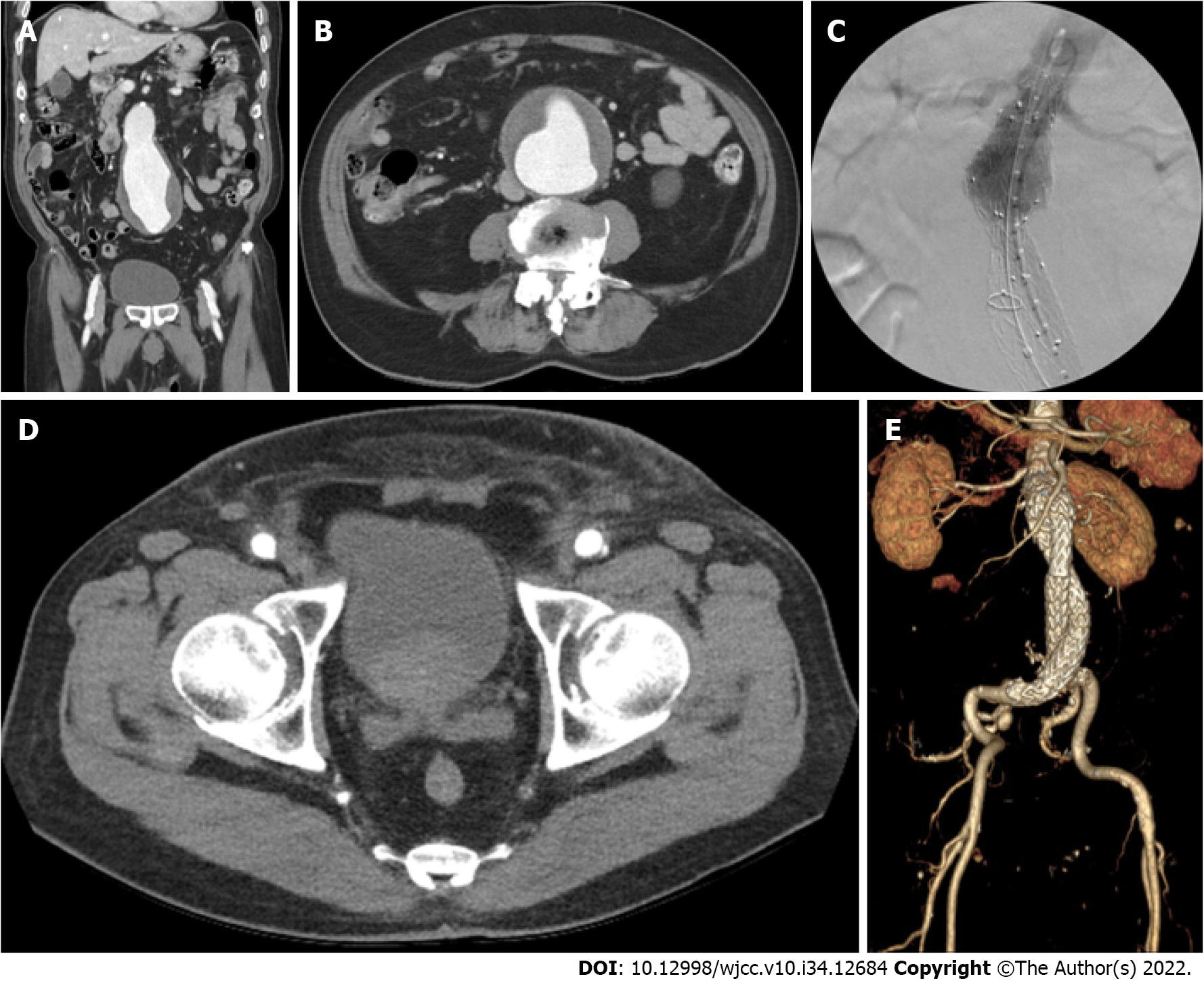
Contrast-enhanced abdominopelvic computed tomography (CT) revealed an unruptured abdominal aortic aneurysm measuring 83 mm × 75 mm (Figures 1A and B).
The final diagnosis was an unruptured abdominal aortic aneurysm.
Under general anesthesia, the bilateral common femoral arteries were accessed using a micropuncture set under ultrasound guidance. Two close ProGlides were applied to each common femoral artery, and heparin 3000 IU was administered after successfully implementing the preclose technique. The activated clotting time was 332 s. Routine EVAR was performed using an Endurant II (Medtronic Cardiovascular, Minneapolis, MN, United States) main body endograft measuring 36 mm × 14 mm × 103 mm. The main body was introduced through the left common femoral artery. A 16 mm × 20 mm × 124 mm Endurant II limb endograft was subsequently inserted via the right femoral artery after successful cannulation of the contralateral gate. Finally, a 16 mm × 20 mm × 156 mm Endurant II limb endograft was inserted through the left common femoral artery. Following molding balloon angioplasty using a Reliant balloon catheter (Medtronic Cardiovascular, Minneapolis, MN, United States), a completion angiogram revealed a type Ia endoleak. A 36 mm × 36 mm × 49 mm Endurant II aortic cuff was used to control the endoleak. Eventually, EVAR was successfully performed (Figure 1C). The total procedure time was 59 min.
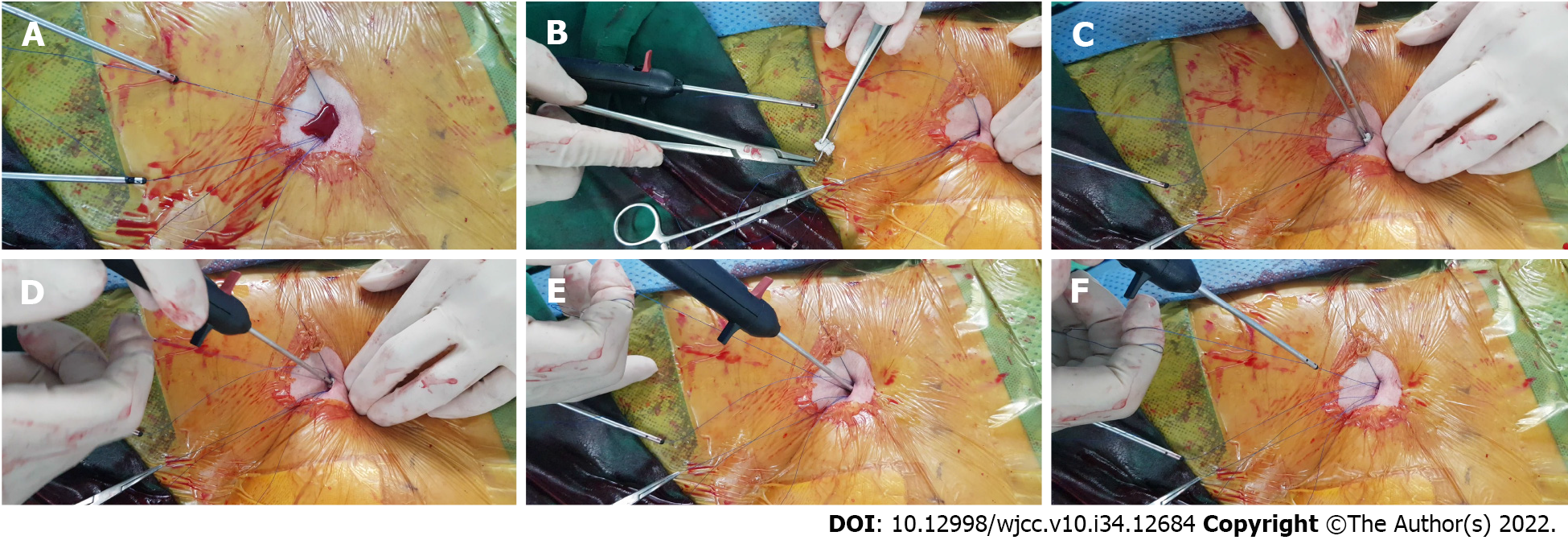
At the end of the procedure, residual access-site bleeding persisted on the patient’s right side. We applied a gel-foam sponge for several minutes; however, bleeding persisted. Therefore, we imple
A postoperative CT revealed no hematoma or other complications (Figures 1D and E). One month postoperatively, the patient exhibited no access-site complications.
EVAR is associated with lower perioperative mortality and early recovery[3,4], reinforcing its use as a primary treatment modality for abdominal aortic aneurysms. EVAR traditionally requires the cut-down of both common femoral arteries. The introduction of pEVAR using a VCD has further reduced the invasiveness of EVAR and is currently the preferred option for treating patients with aortic aneurysms at many centers[5]. However, the failure of closure devices after percutaneous access increases the risk of complications associated with pEVAR[6]. Similar to pEVAR, in other percutaneous peripheral interventions performed via access through the common femoral artery, perioperative complications are predominantly related to the access sites. Vascular surgeries are frequently performed under systemic heparinization, which may contribute to intraoperative bleeding or postoperative complications. In cases of VCD failure following percutaneous access, the currently used adjunctive procedures are external manual compression, additional VCD, fascial closure, or cut-down. These maneuvers are associated with patient and physician discomfort, increased medical costs, and potential complications[7,8].
Surgical, an oxidized cellulose, is a widely used hemostatic material. Cellulose acquires hemostatic and bactericidal properties after oxidization, and its low pH stimulates vessel constriction and platelet activation to stimulate the formation of a temporary platelet plug[9]. It is biodegradable and is completely absorbed within 4-8 wk[2]. Due to these characteristics, it is often used in open vascular surgery. However, it is not commonly used in percutaneous endovascular procedures because the vessel is not exposed.
In our case, preoperative anticoagulation with rivaroxaban, intraoperative heparin administration, and a short procedure time failed to achieve complete hemostasis after pEVAR. The reversal of heparin’s effect using protamine sulfate was considered inappropriate because of the patient’s history of recent cerebral infarction and atrial fibrillation. External manual compression with a gel-foam sponge was attempted; however, it was ineffective. Significant bleeding was observed, and we used a novel Surgicel-assisted hemostasis method to achieve focused compression and activate the local hemostatic cascade. Because a suture-based closure device was used, the suture thread and pusher were available, which could guide the Surgicel toward the arterial wall. Immediate hemostasis was successfully achieved, and removal was not required.
A previous literature review reported the use of a polytetrafluoroethylene pledget (CR Bard, Tempe, Ariz, United States)[10]. This method involved subcutaneous dissection and placement of an 18-gauge needle to allow the pledget to slip toward the arterial puncture site. However, in our case, the conformability of Surgicel allowed its delivery only with a pusher when attached to the suture thread. Moreover, Surgicel is an excellent choice owing to its bactericidal properties and biodegradability. It is more economical than other hemostatic materials or additional use of VCDs. Thus, Surgicel application at the access tract provides several potential benefits in endovascular procedures.
Some safety issues are associated with the use of Surgicel. Surgicel has the potential to swell and absorb 7-10 times its weight; therefore, it should not be used in closed spaces[2]. In our case, only a 5 mm × 25 mm piece was adequate. Another potential safety concern is the risk of hypersensitivity reactions, although it has rarely been reported[11]. The limitation of this technique is that it can only be used to reinforce suture-based closure devices and is unsuitable for clip- or plug-based closure devices. Furthermore, it requires a successful initial VCD deployment. However, we believe that this technique is a promising adjunct for percutaneous endovascular procedures.
Surgicel-assisted hemostasis is a simple and excellent adjunct for controlling residual access-site bleeding after VCD deployment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: Korean Medical Association, 57027; Korean Society for Surgery, 4668.
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dumont LS, Brasil; Feng J, China S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Baldino G, Persi F, Mortola P, Gori A. An Alternative Technique to Achieve Haemostasis During PEVAR Using Perclose ProGlide. EJVES Short Rep. 2018;41:8-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Vyas KS, Saha SP. Comparison of hemostatic agents used in vascular surgery. Expert Opin Biol Ther. 2013;13:1663-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Adkar SS, Turner MC, Leraas HJ, Gilmore BF, Nag U, Turley RS, Shortell CK, Mureebe L. Low mortality rates after endovascular aortic repair expand use to high-risk patients. J Vasc Surg. 2018;67:424-432.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | United Kingdom EVAR Trial Investigators, Greenhalgh RM, Brown LC, Powell JT, Thompson SG, Epstein D, Sculpher MJ. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med. 2010;362:1863-1871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1175] [Cited by in RCA: 1053] [Article Influence: 70.2] [Reference Citation Analysis (0)] |
| 5. | Gimzewska M, Jackson AI, Yeoh SE, Clarke M. Totally percutaneous versus surgical cut-down femoral artery access for elective bifurcated abdominal endovascular aneurysm repair. Cochrane Database Syst Rev. 2017;2:CD010185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Huff CM, Silver MJ, Ansel GM. Percutaneous Endovascular Aortic Aneurysm Repair for Abdominal Aortic Aneurysm. Curr Cardiol Rep. 2018;20:79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Noori VJ, Eldrup-Jørgensen J. A systematic review of vascular closure devices for femoral artery puncture sites. J Vasc Surg. 2018;68:887-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 8. | Lin SY, Lyu SY, Su TW, Chu SY, Chen CM, Hung CF, Chang CJ, Ko PJ. Predictive Factors for Additional ProGlide Deployment in Percutaneous Endovascular Aortic Repair. J Vasc Interv Radiol. 2017;28:570-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Zhong Y, Hu H, Min N, Wei Y, Li X. Application and outlook of topical hemostatic materials: a narrative review. Ann Transl Med. 2021;9:577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 10. | Theivacumar NS, Qureshi MI, Glasgow S, Najem M. Pledget reinforcement and traction compression as adjunctive techniques for suture-based closure of arterial cannulation sites in percutaneous endovascular aneurysm repair-initial experience. J Vasc Surg Cases Innov Tech. 2021;7:183-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Corti MC, Thomas AD, Sayegh MN, Vernon K, Sherman C, Trainor R. Surgicel-Induced Anaphylaxis Post Permacath Placement. Cureus. 2021;13:e16938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |