Published online Dec 6, 2022. doi: 10.12998/wjcc.v10.i34.12665
Peer-review started: July 10, 2022
First decision: August 22, 2022
Revised: September 10, 2022
Accepted: November 2, 2022
Article in press: November 2, 2022
Published online: December 6, 2022
Processing time: 145 Days and 1.1 Hours
Patellar dislocation may cause cartilage defects of various sizes. Large defects commonly require surgical treatment; however, conventional treatments are pro
A 15-year-old male with a large patellar cartilage defect due to patellar dislocation was treated via human umbilical cord blood-derived mesenchymal stem cell (hUCB-MSC) implantation. To our knowledge, this is the first report of this treatment for this purpose. The patient recovered well as indicated by good visual analog scale, International Knee Documentation Committee and McMaster Universities Osteoarthritis Index scores. Magnetic resonance imaging showed cartilage regeneration 18 mo postoperatively.
Umbilical cord blood-derived hUCB-MSCs may be a useful treatment option for the repair of large patellar cartilage defects.
Core Tip: Umbilical cord blood-derived mesenchymal stem cells consist of a unique population of progenitors co-expressing mesenchymal stem cells and neuronal markers capable of instantaneous differentiation. This report is of a 15-year-old male teen with a large patellar cartilage defect due to patellar dislocation who was treated with implantation of human umbilical cord blood-derived mesenchymal stem cells.
- Citation: Song JS, Hong KT, Song KJ, Kim SJ. Repair of a large patellar cartilage defect using human umbilical cord blood-derived mesenchymal stem cells: A case report. World J Clin Cases 2022; 10(34): 12665-12670
- URL: https://www.wjgnet.com/2307-8960/full/v10/i34/12665.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i34.12665
Patellar cartilage defects often accompany acute patellar dislocation[1]. Small cartilage defects can be treated conservatively or via microfracture. Large cartilage defects may cause problems, such as anterior knee pain and arthritis, and require cartilage repair treatment.
Treatment of large patellar cartilage defects via autologous chondrocyte implantation (ACI) or osteochondral autologous transplantation (OAT) has been reported[1,2]. ACI, a two-step procedure, is time-consuming and expensive, and OAT can cause problems at the harvest site of the osteochondral plug. To avoid these shortcomings, alternative treatment methods, especially those using mesenchymal stem cells (MSCs) have been evaluated recently[3].
We use human umbilical cord blood-derived MSCs (hUCB-MSCs) for cartilage repair. Compared with other MSCs, hUCB-MSCs have better cell activity and do not require invasive procedures for collection. Regardless of the cartilage defect size, the desired amount of the hUCB-MSCs can be prepared at any time[4,5]. To the best of our knowledge, there have been no reports of large patellar cartilage defects treated with hUCB-MSCs. This report presents such a case.
Left knee pain and swelling.
A 15-year-old male had fallen while running the day before.
There is no history of past illness.
There is no personal and family history.
Swelling and limitation of knee motion.
No specific findings.
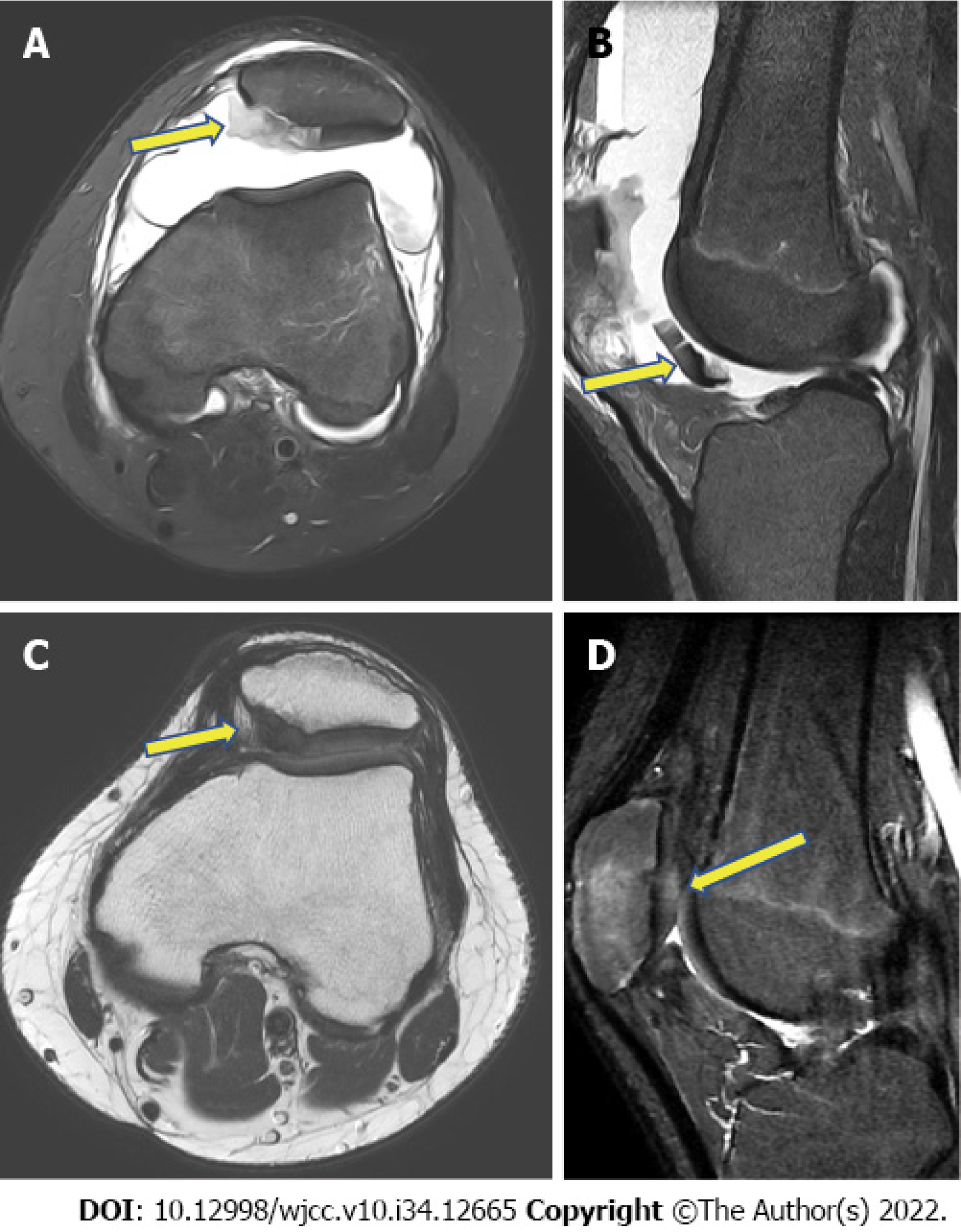
Radiography showed patellar dislocation. Magnetic resonance imaging (MRI) revealed a large amount of hemarthrosis, a medial patellofemoral ligament tear and a 4.92 cm2 (2.04 cm × 2.41 cm) patellar cartilage defect (Figure 1A and B).
Patellar dislocation with large patellar cartilage defects.
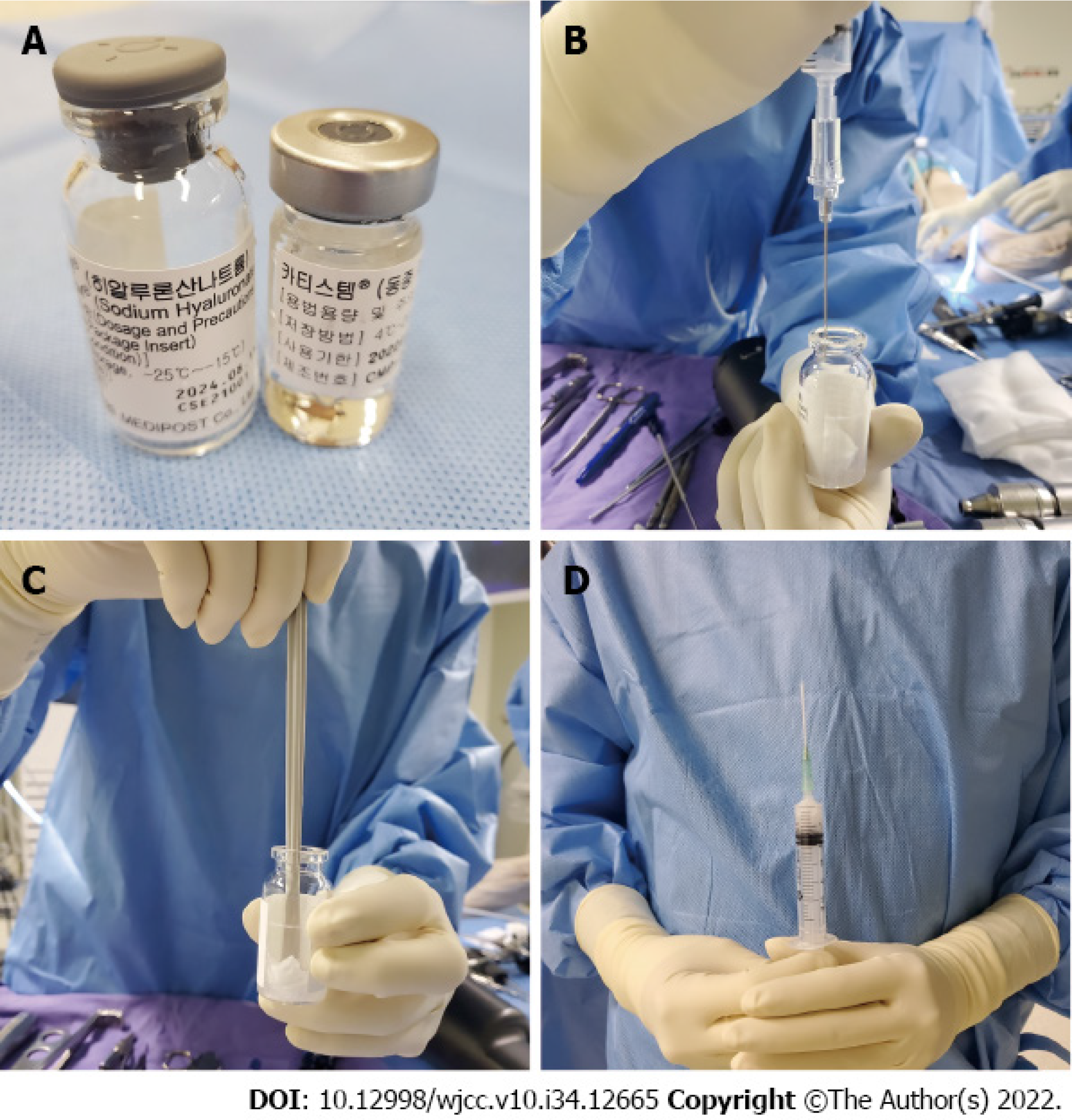
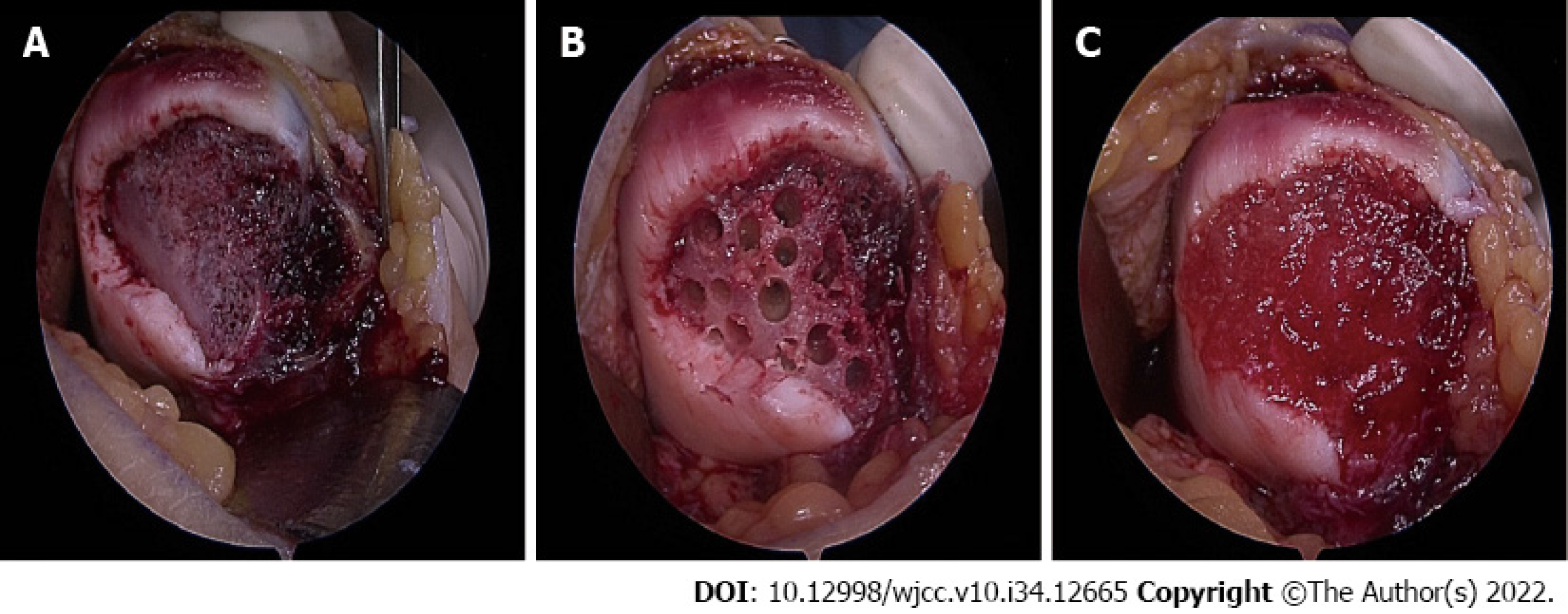
After routine arthroscopy for loose body removal and joint debridement, a skin incision was made along the medial side of the patella. The cartilage defect site was exposed via a 4-cm longitudinal arthrotomy (Figure 2A). Multiple holes were made in the patellar subchondral bone using a 5 mm drill bit (Figure 2B), and CARTISTEM was injected (Figure 3C). Subsequently, medial patellofemoral ligament repair was performed.
The patient was required to rest while wearing a knee brace for 3 d postoperatively. On postoperative day 4, he began performing a range of motion exercises using a continuous passive motion machine, quadriceps strengthening exercises and ankle pump exercises. On postoperative day 7, full weight-bearing walking with a hinged knee brace was permitted.
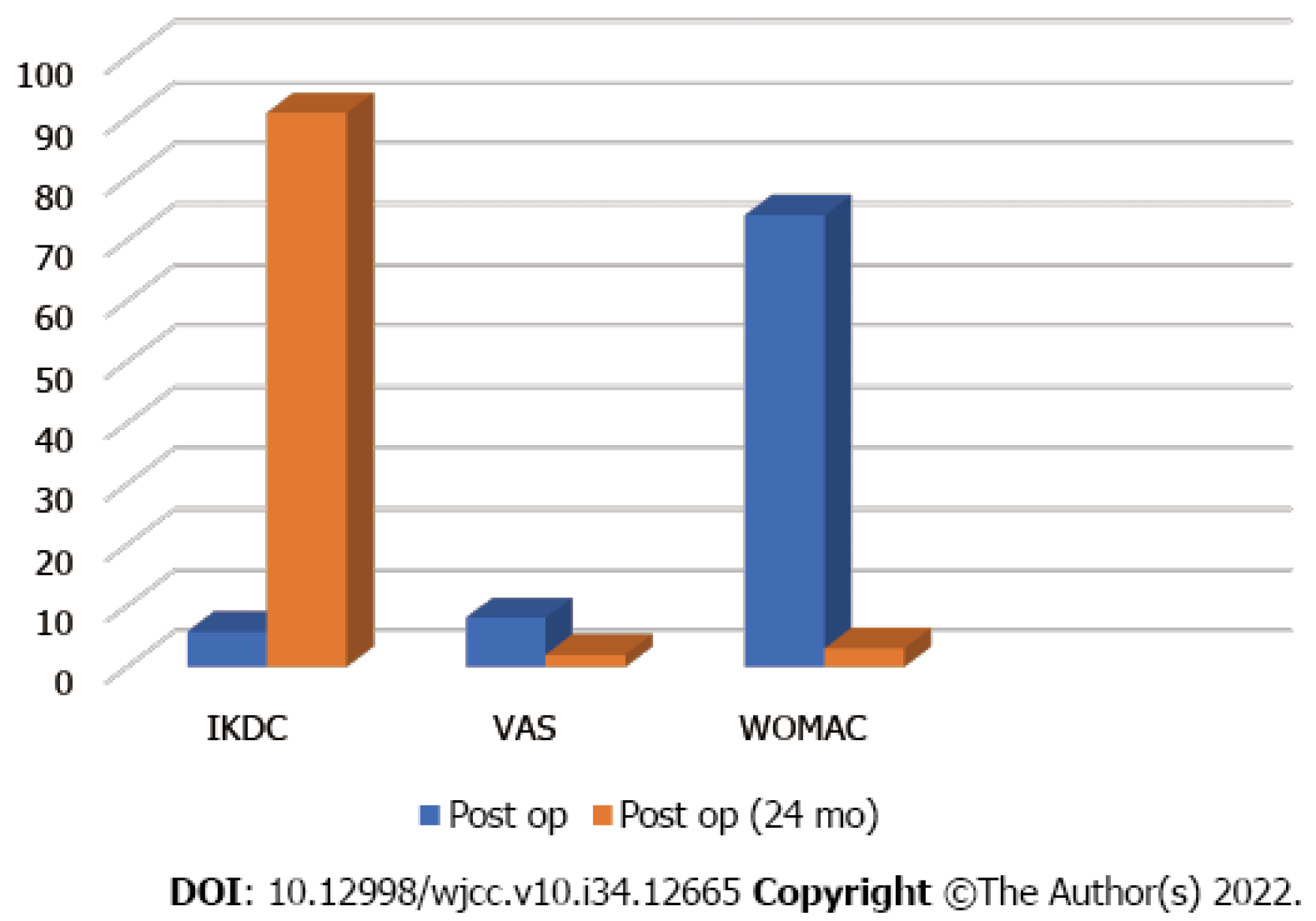
Cartilage regeneration was observed on follow-up MRI 2 years postoperatively (Figure 1C and D). Comparison of the International Knee Documentation Committee) score, the visual analog scale score, and the McMaster Universities Osteoarthritis Index score before and 2 years after surgery indicated improvement, from 5.7 to 90.8, 8 to 2, and 74 to 3, respectively (Figure 4).
Patellar cartilage defects are frequently associated with trauma-induced acute patellar dislocation. In the report by Song et al[6], 95% (37/39) of knees with acute patellar dislocation had patellar cartilage defects. The treatment of large patellar cartilage defects is challenging. Using ACI, Vasiliadis et al[7] achieved satisfactory results in 92 patients with patellar or trochlear cartilage damage (mean size of the defect: 5.5 cm2, average follow-up time: 12.6 years). After treatment, these patients had a high level of activity based on the Tegner score. Gracitelli et al[1] reported a survival rate of 78.1% at 5 years and 10 years after osteochondral allograft transplantation in 27 patients (28 knees) with patellar cartilage defects; eight knees (28.6%) showed allograft failure.
Despite their effectiveness, ACI and osteochondral transplantation are not without drawbacks: ACI is performed in two steps and can cause graft hypertrophy, and OAT can negatively impact the osteochondral plug harvest site and cause complications such as osteonecrosis[3]. Many studies have devised methods of restoring cartilage using MSCs to avoid these problems. Although initially extracted from the bone marrow or adipose tissue, MSCs are currently extracted from umbilical cord blood. hUCB-MSCs have several advantages over other types of MSCs. First, hUCB-MSCs are less immunogenic. Owing to the naïve nature of a newborn’s immune system, they do not require a close human leukocyte antigen match and thus can escape host immune surveillance. Therefore, they can be used regardless of sex, allergies and blood group. Second, they have a high expansion capacity compared with bone marrow-derived MSCs. Third, as an off-the-shelf product, they are readily accessible whenever required[3,5,6].
Treatment of juvenile osteochondral defects, especially large defects, using hUCB-MSCs has been previously reported[3]. Our patient had a large patellar cartilage defect with accompanying acute patellar dislocation. Cartilage regeneration was observed 18 mo after hUCB-MSC implantation, as indicated by MRI findings and clinical scores. To the best of our knowledge, this is the first detailed description of the results of hUCB-MSC treatment of a large patellar cartilage defect with patellar dislocation.
hUCB-MSCs are a potential treatment option for large patellar cartilage defects with patellar dislocation. Cultured cell therapy, including stem cells, could be more appropriate for large chondral defects.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen C, China; Yang B, China S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wu YXJ
| 1. | Gracitelli GC, Meric G, Pulido PA, Görtz S, De Young AJ, Bugbee WD. Fresh osteochondral allograft transplantation for isolated patellar cartilage injury. Am J Sports Med. 2015;43:879-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 2. | Nomura E, Inoue M, Kurimura M. Chondral and osteochondral injuries associated with acute patellar dislocation. Arthroscopy. 2003;19:717-721. [RCA] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 184] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 3. | Park YB, Song M, Lee CH, Kim JA, Ha CW. Cartilage repair by human umbilical cord blood-derived mesenchymal stem cells with different hydrogels in a rat model. J Orthop Res. 2015;33:1580-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Song JS, Hong KT, Kim NM, Jung JY, Park HS, Chun YS, Kim SJ. Cartilage regeneration in osteoarthritic knees treated with distal femoral osteotomy and intra-lesional implantation of allogenic human umbilical cord blood-derived mesenchymal stem cells: A report of two cases. Knee. 2019;26:1445-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Song JS, Hong KT, Kim NM, Jung JY, Park HS, Kim YC, Shetty AA, Kim SJ. Allogenic umbilical cord blood-derived mesenchymal stem cells implantation for the treatment of juvenile osteochondritis dissecans of the knee. J Clin Orthop Trauma. 2019;10:S20-S25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Song JS, Hong KT, Kim NM, Jung JY, Park HS, Lee SH, Cho YJ, Kim SJ. Implantation of allogenic umbilical cord blood-derived mesenchymal stem cells improves knee osteoarthritis outcomes: Two-year follow-up. Regen Ther. 2020;14:32-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 7. | Vasiliadis HS, Lindahl A, Georgoulis AD, Peterson L. Malalignment and cartilage lesions in the patellofemoral joint treated with autologous chondrocyte implantation. Knee Surg Sports Traumatol Arthrosc. 2011;19:452-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |