Published online Dec 6, 2022. doi: 10.12998/wjcc.v10.i34.12637
Peer-review started: June 30, 2022
First decision: August 4, 2022
Revised: September 16, 2022
Accepted: November 7, 2022
Article in press: November 7, 2022
Published online: December 6, 2022
Processing time: 154 Days and 22 Hours
An inflammatory myofibroblastic tumor (IMT) occurring in the central nervous system is very rare, and thus its pathogenesis is unknown. This case report and literature review aimed to explore the pathogenesis, clinical features, imaging findings, pathological characteristics, immunohistochemical characteristics, diagnoses, treatments, and risks of postoperative recurrence of IMT in the central nervous system.
A 67-year-old woman was admitted to the hospital with an exophthalmic protrusion and double vision in the left eye that had persisted for 3 mo. Magnetic resonance imaging (MRI) showed a 2.4 cm × 1.3 cm heterogeneous large mass in the bottom of the left anterior cranial fossa, which was closely related to the dura mater. Before surgery, we suspected the mass to be meningioma. The entire mass was successfully removed under neuronavigation and electrophysiological monitoring, and postoperative pathology indicated an IMT with extensive infiltration of chronic inflammatory cells and scattered multinucleated giant cells. Head MRI at the 3-mo follow-up showed that the tumor at the bottom of left anterior cranial fossa had been completely resected without recurrence.
From the histological, immunohistochemical, and genetic analyses, the present case suggests that the pathogenesis of IMT-CNS is related to autoimmunity.
Core Tip: Inflammatory myofibroblastic tumors (IMTs) rarely occur in the central nervous system (CNS), and the pathogenesis of IMT-CNS is unknown. We present a 67-year-old woman with IMT-CNS who presented with an exophthalmic protrusion and double vision in the left eye lasting for 3 mo. A 2.4 cm × 1.3 cm mass was found by magnetic resonance imaging at the left anterior cranial fossa base, and postoperative pathology indicated an IMT. Histological, immunohistochemical, and genetic analyses indicated that the IMT-CNS pathogenesis may be autoimmunity related. The main treatments for INT-CNS are gross tumor resection or rituximab infusion combined with high-dose prednisone.
- Citation: Su ZJ, Guo ZS, Wan HT, Hong XY. Inflammatory myofibroblastic tumor of the central nervous system: A case report. World J Clin Cases 2022; 10(34): 12637-12647
- URL: https://www.wjgnet.com/2307-8960/full/v10/i34/12637.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i34.12637
Inflammatory myofibroblastic tumors (IMTs) are characterized by proliferation of myofibroblastic spindle cells with mixed inflammatory infiltrates of plasma cells, lymphocytes, eosinophils, and histiocytes. While the most common site for these tumors is the lung in the form of a plasma cell granuloma, IMTs can occur in nearly every organ system, including the lung, liver, mesenteric system, gastrointestinal tract, retroperitoneum, urinary bladder, upper respiratory tract, and mediastinum. However, an IMT of the central nervous system (IMT-CNS) is an extremely rare disease[1,2].
IMTs were once referred to with diverse names such as inflammatory pseudotumor, plasma cell granuloma, pseudosarcomatous myofibroblastic proliferation, and inflammatory myofibrohistiocytic proliferation[3-5]. Then in 2002 the World Health Organization (WHO) classification of soft tissue tumors renamed these lesions as “inflammatory myofibroblastic tumors” and were allocated to the soft tissue tumor category. When the WHO revised the classification of soft tissue tumors in 2003 and again 2020, no changes were made regarding IMTs. However, in the latest 2021 WHO classification of CNS tumors, the content related to IMTs was revised to be consistent with that of soft tissue tumors. Recent studies have shown that nearly 50% of IMTs have clonal rearrangements of the anaplastic lymphoma kinase (ALK) receptor tyrosine kinase gene located on 2p23 and show immunohistochemical expression of the ALK protein[6].
Herein, we presented a case in which an IMT was found in the bottom left anterior cranial fossa. To better understand the pathological and radiological features of IMT-CNS as well as the relevant surgical treatment, adjuvant therapy, prognosis, and important different diagnoses, we also reviewed 49 cases of IMT-CNS reported in the literature since the year 2000.
A 67-year-old woman was admitted to the hospital for an exophthalmic protrusion and double vision in the left eye persisting for 3 mo.
The patient’s symptoms had started 3 mo prior with an exophthalmic protrusion and double vision in the left eye.
The patient had no notable medical history.
The patient had no notable personal and family history.
On physical examination, the patient had a temperature of 36.6 °C, heart rate of 93 bpm, respiratory rate of 16 breaths/min, blood pressure of 180/90 mmHg, and oxygen saturation in room air of 98%. The physical examination showed a fixed left eyeball, without any other pathological signs.
The preoperative complete blood count and coagulation results were all within normal ranges. Postoperative blood analysis revealed mild leukocytosis based on a leukocyte count of 11 × 109/L with predominantly neutrophils, a mildly reduced hemoglobin level of 113 g/L, a markedly elevated C-reactive protein level of 180 mg/dL, and a normal procalcitonin level of 0.056 ng/mL. The erythrocyte sedimentation rate was also normal. Arterial blood gas analysis showed mild lactic acid accumulation and metabolic acidosis. Other test results including blood glucose, urine analysis, stool analysis and coagulation were all within normal ranges.
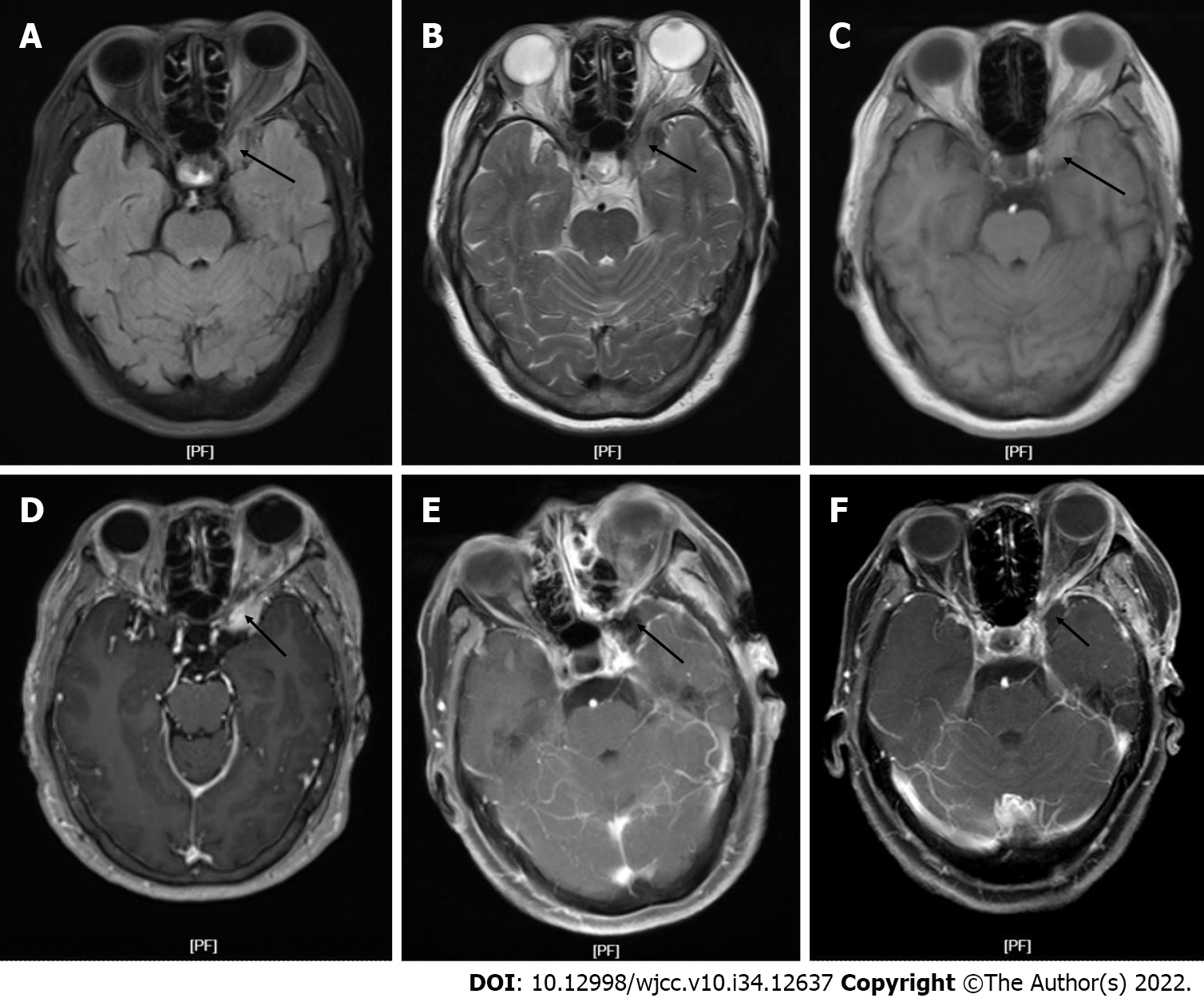
Computed tomography showed a hypointense mass in the bottom of the left anterior cranial fossa with a Computed tomography value of 45 HU. Magnetic resonance imaging (MRI) showed a large oval mass measuring 2.4 cm × 1.3 cm in the bottom of the left middle cranial fossa, with slight hyperintense signals on T1-weighted imaging (T1WI) and slightly hypointense signals on T2-weighted imaging (T2WI) (Figure 1A and B). Isointense signal was observed on the fluid attenuated inversion recovery image (Figure 1C). After gadolinium administration, obvious heterogenous enhancement was observed (Figure 1D). MRI showed significant compression displacement of the left optic nerve. No obvious restriction was observed on diffusion weighted imaging.
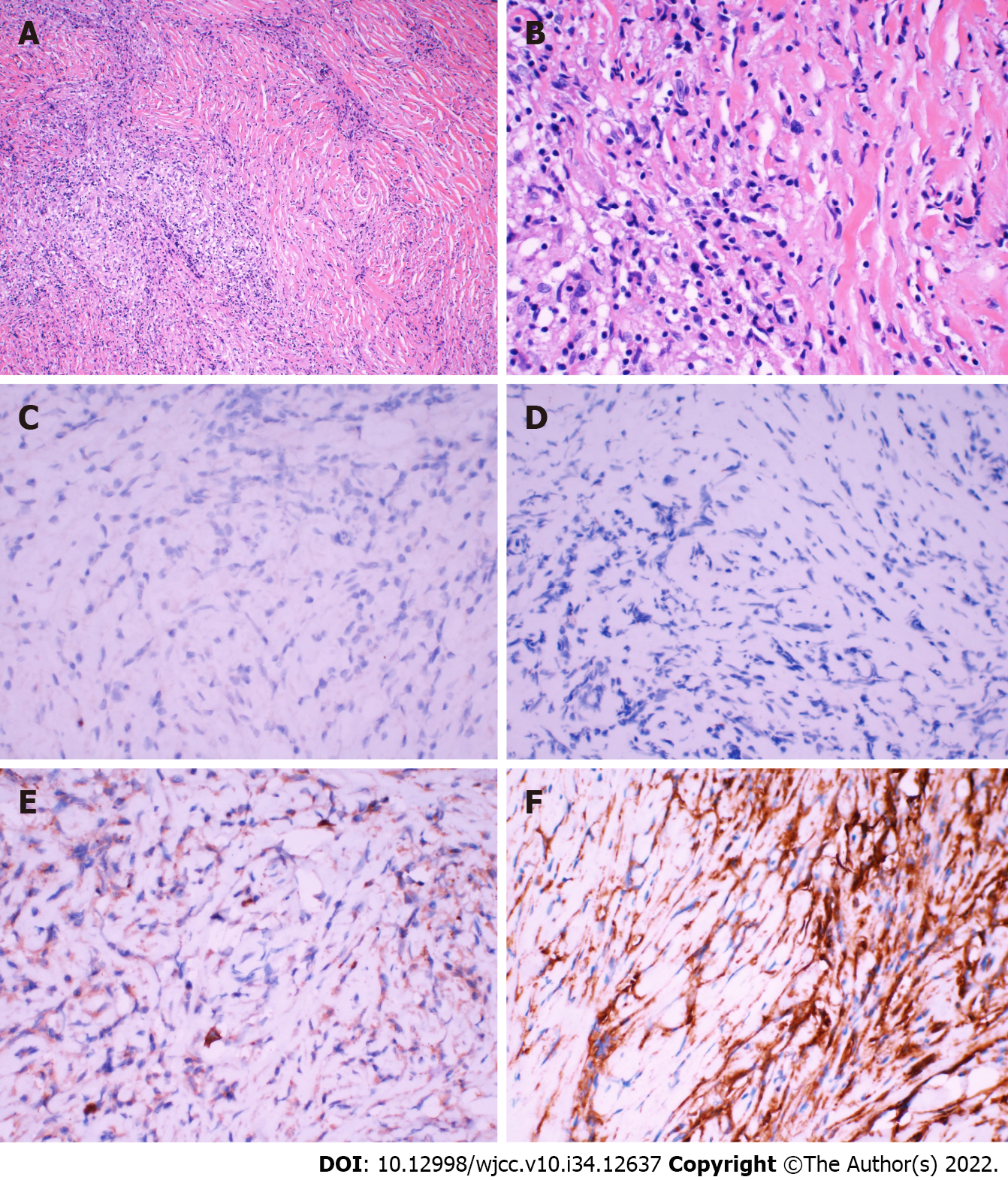
The results of pathological examination were suggestive of IMT. Hematoxylin and eosin-stained paraffin sections predominantly showed an IMT with extensive infiltration of chronic inflammatory cells and scattered multinucleated giant cells (Figure 2A and B). Immunohistochemical analysis indicated that the tumor was negative for ALK expression (Figure 2C). However, the specimen was positive for exponential moving average (Figure 2D), immunoglobulin G4 (IgG4, Figure 2E), vimentin (Figure 2F), smooth muscle actin, CD20, and Bcl-2 as well as negative for S-100, PR, and SSTR2. In addition, in situ hybridization showed negative results for Epstein-Barr virus (EBV)-encoded RNA.
A preoperative clinical and radiological diagnosis of meningioma was made, and surgery was performed with preoperative neuronavigation and intraoperative neurophysiological monitoring. The tumor was located at the base of the anterior cranial fossa 3.5 cm away from the bone window. The tumor appeared kermesinus and tough with a clear boundary from the surrounding tissues. The basal part was adhered tightly to the base of the anterior cranial fossa and sellar tubercle. The tumor filled the entire left wing of the sphenoid bone and completely wrapped around the pituitary stalk, left optic nerve, left internal carotid artery, and left posterior communicating artery. The tumor was pushing the left oculomotor nerve outward. The tumor blood supply decreased significantly after the tumor base was severed. During surgery, the tumor was completely removed. The patient then developed oculomotor nerve palsy after surgery. Hence, we suspected anoculomotor schwannoma. Postoperative computed tomography (Figure 1E) showed that the lesion was completely resected, with no signs of residual tumor.
This patient was followed up at 3 mo after surgery. Head MRI showed that the tumor at the bottom left anterior cranial fossa had been completed resected without recurrence (Figure 1F). As the tumor was completely wrapped around the left optic nerve before surgery, the nerve was inevitably injured during surgery. On general medical examination, the vision of the left eye had improved to a level only slightly lower than that preoperation. The function of the left abducens nerve had recovered. In addition, headache and ophthalmalgia were completely resolved.
IMT in the CNS, as observed in the present case, is very rare. For the purpose of characterizing the features of this disease, we performed a literature review of IMT-CNS cases. We recorded and analyzed the data for all cases that met the inclusion criteria of ALK immunohistochemical staining and treatment involving surgical resection since 2000 based on the timing of the WHO definition. After reviewing the literature related to IMT, we recorded the relevant data for 49 cases, which are presented in Table 1 in reverse chronological order.
| Ref. | Age in yr/sex | Duration of symptom in mo | Main symptoms and signs | Location | Contrast enhancement | Tumor diameter in cm | ALK expression | Main treatment | Chemotherapy | Prognosis |
| Present case | 67/F | 3 | Diplopia | Left middle cranial fossa | Intense | 2.4 | Negative | GTR | None | No recurrence |
| Wang et al[5], 2019 | 21/F | 0.5 | Blurred vision | Right frontal lobe | Intense | 10.0 | Negative | GTR | None | No recurrence |
| Wang et al[5], 2019 | 60/M | N/A | Gait disturbance | Cerebellar hemisphere | Intense | N/A | Negative | GTR | None | No recurrence |
| Wang et al[5], 2019 | 48/F | Several | Paranoid | Right temporal lobe | Intense | 5.3 | Negative | GTR | None | No recurrence |
| Wang et al[5], 2019 | 20/F | 0.75 | Headache | Right temporal lobe | Intense | 5.0 | Positive | GTR | None | 7 mo after 1st surgery |
| Wang et al[5], 2019 | 82/F | 6 | Memory decrease | Right temporal lobe | Intense | N/A | N/A | GTR | None | No recurrence |
| Wang et al[5], 2019 | 51/F | 1.5 | Headache | Cerebellar hemisphere | Intense | N/A | N/A | Biopsy | Steroid | N/A |
| Kuang et al[5], 2016 | 53/M | 12 | Blurred vision | Pineal | Intense | N/A | N/A | GTR | None | No recurrence |
| PascualGallego et al[7], 2013 | 47/F | N/A | Headache | Left temporooccipital lobe | Intense | N/A | Negative | GTR | None | 4 mo after 1st surgery |
| Denis et al[19], 2013 | 26/M | 3 | Headache, blurred vision | Left frontal lobe | Intense | 2.0 | Positive | GTR | None | 2 mo after 1st surgery |
| Denis et al[19], 2013 | 17/F | N/A | Seizures | Left frontal lobe | N/A | N/A | Positive | GTR | None | 2.5 yr after 1st surgery |
| Denis et al[19], 2013 | 65/F | N/A | Blurred vision | Occipital lobe | Intense | 5.0 | Negative | STR | None | No recurrence |
| Denis et al[19], 2013 | 43/M | 6 | Headache | Orbit | Intense | N/A | Negative | GTR | None | No recurrence |
| Denis et al[19], 2013 | 7/M | N/A | N/A | Right temporal fossa | N/A | 2.0 | Positive | GTR | None | 1.5 yr after 1st surgery |
| Denis et al[19], 2013 | 24/M | N/A | N/A | Left temporal lobe | N/A | N/A | Positive | GTR | None | 2 yr after 1st surgery |
| Kolenc et al[18], 2013 | 77/F | N/A | Right partial motoric seizures | Left temporal lobe | Intense | N/A | Negative | Biopsy | Corticosteroids | No recurrence |
| Moss et al[14], 2012 | 36/F | N/A | Diplopia | Left middle cranial fossa | Intense | N/A | Negative | STR | Corticosteroids | 24 mo after 1st surgery |
| Moss et al[14], 2012 | 50/F | 5 | Headache | Cavernous sinus | Intense | N/A | Negative | Steroid | None | N/A |
| Kato et al[28], 2011 | 60/M | N/A | Gait disturbance | Cerebellar | Intense | 1.8 | Negative | GTR | None | No recurrence |
| de Oliveira et al[27], 2009 | 7/M | 3 | Headache | Right temporal fossa | Intense | 4.0 | Positive | GTR | None | 24 mo after 1st surgery |
| Lui et al[2], 2009 | 60/F | N/A | Blurred vision | Cerebral falx | Intense | N/A | Negative | GTR | Radiation therapy | No recurrence |
| Lui et al[2], 2009 | 52/F | N/A | N/A | Right ventricle | Intense | N/A | Negative | GTR | None | No recurrence |
| Lui et al[2], 2009 | 45/M | N/A | Left-sided weakness | Right frontal lobe | N/A | N/A | Negative | GTR | None | No recurrence |
| Lui et al[2], 2009 | 26/F | 6 | Blurred vision | Left occipital lobe | Intense | N/A | Negative | Biopsy | Corticosteroids, thalidomide | No recurrence |
| Swain et al[6], 2008 | 8/M | N/A | Attention deficiency | Left temporal lobe | Intense | N/A | Negative | STR | None | No recurrence |
| Swain et al[6], 2008 | 28/F | N/A | Seizure | Left occipital lobe | Intense | 0.8 | Positive | GTR | None | No recurrence |
| Swain et al[6], 2008 | 30/M | N/A | Headache | Posterior fossa | N/A | N/A | Negative | GTR | Radiation therapy | No recurrence |
| Swain et al[6], 2008 | 31/F | 1 | Headache | Right frontal lobe | N/A | N/A | N/A | GTR | None | No recurrence |
| Swain et al[6], 2008 | 74/F | N/A | Headache, seizures | Right parietal lobe | N/A | 3.8 | GTR | None | No recurrence | |
| Swain et al[6], 2008 | 0.8/F | N/A | Seizures | Right temporal lobe | N/A | 8.4 | GTR | None | No recurrence | |
| Miyahara et al[20], 2008 | 73/M | 1 | Gait disturbance | Paracele | N/A | N/A | N/A | GTR | Steroid | Died of complications |
| Miyahara et al[20], 2008 | 48/M | N/A | Memory decrease | Paracele | N/A | N/A | N/A | GTR | Steroid | No recurrence |
| Miyahara et al[20], 2008 | 18/M | N/A | N/A | Paracele | N/A | N/A | N/A | GTR | Steroid | No recurrence |
| Miyahara et al[20], 2008 | 63/F | N/A | Headache | Paracele | N/A | N/A | N/A | GTR | Steroid | No recurrence |
| Miyahara et al[20], 2008 | 50/M | 0.3 | Blepharoptosis | Left orbit, cavernous sinus | Intense | 1.5 | Negative | Biopsy | Corticosteroids | Progressive reduction |
| Jeon et al[1], 2005 | 43/M | N/A | Dizziness | Orbit, falx, sup. sagittal sinus, tentorium | N/A | N/A | Negative | STR | Corticosteroids | 12 yr after 1st surgery |
| Jeon et al[1], 2005 | 31/M | N/A | Headache, ptosis | Cavernous sinus | Intense | 1.5 | Negative | GTR | None | No recurrence |
| Jeon et al[1], 2005 | 41/M | N/A | N/A | Temporal lobe | Intense | N/A | Negative | STR | None | No recurrence |
| Jeon et al[1], 2005 | 29/M | N/A | Aphasia | Temporal and frontal lobes | Intense | N/A | Negative | Steroid | None | No recurrence |
| Jeon et al[1], 2005 | 42/M | N/A | N/A | Subdura | Intense | N/A | Negative | GTR | None | No recurrence |
| Jeon et al[1], 2005 | 50/M | N/A | Seizures | Frontal lobe | Intense | 5.0 | Negative | STR | None | No recurrence |
| Jeon et al[1], 2005 | 52/F | N/A | Seizures | Occipital lobe | Intense | N/A | Negative | GTR | None | No recurrence |
| Jeon et al[1], 2005 | 24/F | N/A | Diplopia | Orbit | Intense | N/A | Negative | GTR | None | No recurrence |
| Jeon et al[1], 2005 | 65/F | N/A | N/A | Occipital lobe | Intense | 4.0 | Negative | GTR | None | 7 yr after 1st surgery |
| Roche et al[10], 2004 | 20/F | 0.3 | Headache | Left temporal lobe | Intense | N/A | N/A | STR | Corticosteroids | Dramatic decrease in the mass size |
| Hausler et al[11], 2003 | N/A/M | N/A | Seizures | Left temporal lobe | N/A | N/A | Negative | STR | None | No recurrence |
| Hausler et al[11], 2003 | 15/M | N/A | Seizures | Occipital lobe | Intense | N/A | N/A | STR | Radiation therapy | No recurrence |
| Hausler et al[11], 2003 | 17/F | N/A | Headache | Left frontal lobe | Intense | 6.0 | Positive | GTR | None | 20 mo after 1st surgery |
| Buccoliero et al[9], 2003 | 70/M | 6 | Partial loss of vision | The bottom of the frontal lobes | Intense | 2.0 | Negative | Biopsy | Corticosteroids, radiation therapy | No remission |
Among the 49 cases, 27 cases (55.1%) were treated by simple complete resection of the mass, 5 cases (10.2%) by simple partial resection of the mass, and the other 17 cases (34.7%) with comprehensive treatment. We found that the method of resection did not affect the recurrence of IMT (P = 0.521; Table 1). Postoperative immunohistochemical results showed that a total of 10 cases (20.4%) were ALK positive. A total of 9 cases (18.4%) experienced recurrence within 5 years after comprehensive treatment (Table 1). We observed that positive ALK expression in IMTs was associated with a higher recurrence rate (6/10) than negative ALK expression (1/14; P = 0.009).
IMT is a type of mesenchymal neoplasm, which frequently occurs in the lung[5,7]. IMT most commonly affects children and young adults, with a median age at first onset of 36 years[5]. The youngest reported age at onset was 3 mo[8] (age range: 3 mo to 82 years). Among the 49 cases reviewed, the mean patient age at onset was 41.0 years (range: 7–82 years), and there were 24 male and 25 female patients. Among these patients, the presenting sites were the hemicerebrum (n = 26), cerebellum (n = 3), basis cranii (n = 6), ventricle (n = 5), cavernous sinus (n = 3), and other CNS sites (n = 6; Table 1). By comparison, our patient was a 67-year-old woman who had an IMT in the bottom of the left anterior cranial fossa, and thus this case was extremely rare.
The exact pathogenesis of IMT remains unclear to date. Some authors have suggested that the disease is related to bacterial and viral infections such as EBV and human herpesvirus[6,9,10]. However, EBV and human herpesvirus type 8 were negative in the majority of cases. In the report by Haüsler et al[11], PCR analysis of the patient’s cerebrospinal fluid proved negative for Mycobacteria, herpes simplex virus, varicella-zoster virus, the human neurotropic polyomavirus JC, EBV, human herpesvirus type 6, and human herpesvirus type 8. The majority of our reviewed cases also showed no viral infection. However, these negative findings do not rule out a viral etiology, as it is possible the viral load may have been too low, viruses not being specifically investigated may be involved, and viruses may have triggered inflammatory or even neoplastic processes before being eliminated from the brain tissue[11-13]. Some patients had pulmonary nodules prior to the discovery of intracranial space-occupying lesions, and we believe that the occurrence of IMT may be associated with such lesions[4]. Because some researchers discovered polyclonal hypergammaglobulinemia, hyper-leukocytosis, and an increased sedimentation rate in some cases, it is also believed that IMT is an immune-related disorder[10,13,14]. Accordingly, corticosteroids have been used in IMT treatment. Post-traumatic or post-surgical mechanisms have also been proposed without convincing evidence[10,13].
Primary intracranial IMT is associated with a variety of clinical manifestations, mainly related to tumor location, size, relationship with surrounding tissues, and degree of edema. In the cases we reviewed, clinical symptoms included headache (n = 6, 35.3%), blurred vision (n = 5, 29.4%), ptosis (n = 2, 11.8%), seizure (n = 2, 11.8%), attention deficiency (n = 1, 5.9%), diplopia (n = 1, 5.9%), and dizziness (n = 1, 5.8%). The average duration of clinical symptoms was 2.7 mo (range, 9 d to 6 mo). The duration of the clinical presentation in our patient matched that reported in the literature. Unusual clinical signs including polyclonal hypergammaglobulinemia, hyperleukocytosis, and increased sedimentation rate have been reported in some studies[10]. Hence, it is advisable to screen for these during clinical evaluation. In our case report, the patient’s major complaint was exophthalmic protrusion and double vision, suggesting a mass in the bottom left middle cranial fossa.
After reviewing the previous literature, we found that IMT-CNS presents diverse results on T1WI, with most presenting isointense or hyperintense signals in T2WI[1,3,5,10,13-15]. The relative hypointensity of these lesions on T2WI, as seen in our case, can probably be attributed to the decreased free water content and is associated with a lack of mobile protons relative to a high degree of fibrosis and an elevated cellularity or high nucleus-to-cytoplasm ratio[16,17]. In most of the reviewed cases, heterogenous enhancement was observed after gadolinium administration. Our patient’s findings were consistent with the aforementioned findings. According to Häusler et al[11], the anatomical location is highly variable, with meningeal lesions being the most frequently encountered type. Some authors believe that IMT originates in the dura mater[2,13]. Lesions in approximately 28% of cases showed local thickening and/or radiologic enhancement of the dura mater[3,6,18], and this is consistent with our findings.
IMT can occur in virtually every organ system, but intracranial cases are extremely rare[1,9]. There is no gold standard diagnostic marker yet for IMT, and diagnosis is currently mainly dependent on a combination of histological examination, immunohistochemical analysis, and molecular genetic analyses. In general, IMTs consist of a large number of myofibroblastic spindle cells with mixed inflammatory infiltrates of plasma cells, lymphocytes, eosinophils, multinuclear giant cells, and histiocytes[1,6,19,20]. These cells coexist in a collagen matrix with differing degrees of vascularization[9,11].
Many other lesions such as lymphoplasmacyte-rich meningioma, schwannoma, lymphoma, and plasmacytoma have similar histological features as IMT. Lymphoma diagnosis can be ruled out by determining whether the cells are polyclonal. In addition, IMT is often closely associated with the dura, wherein the spindle cells are often mistaken for meningioma cells. This can be identified by concentric fibrosis in small vessels, which would not be present in meningioma[2,11,12].
Immunohistochemical analysis is an extremely important means of diagnosis of IMT. Fluorescence in situ hybridization with a probe flanking the ALK gene at 2p23 typically demonstrates a genetic rearrangement. While tumors with ALK gene rearrangements are readily considered a unique neoplastic category, histologically similar neoplastic lesions may lack this genetic alteration[6]. ALK expression is positive in nearly 50% of patients with IMT, especially in children, and is associated with local recurrence and distant metastasis[7]. However, in the literature we reviewed, only about 25% of cases were positive for ALK expression.
All other immunohistochemical markers are equally important. Spindle cells have the immunohistochemical characteristics of myofibroblasts and often express vimentin and smooth muscle actin[12]. Meningioma also shows infiltration by fibrous ground tissue and mononuclear cells, but the finding of meningeal whorls and positive exponential moving average were not observed in IMT-CNS[11]. S-100 is used to differentiate Rosai–Dorfman disease.
In recent years, IgG4 and programmed death receptor-1 have been proposed as markers to assist the diagnosis and treatment of IMT. IgG4-associated IMT was first detected in masses at sites other than the CNS. Some are part of a systemic disorder known as IgG4-related sclerosing disease. We found that intracranial IMTs had similar characteristics with IgG4-associated IMTs, except for the absence of granulomas and reactive lymphatic follicles[2]. Therefore, IgG4 may be useful for the diagnosis of IMT. Unfortunately, serum IgG4 levels have not been measured for most reported cases to date.
Cottrell et al[21] recently studied the relationship between programmed cell death ligand 1 expression and IMT. They found that in IMT programmed cell death ligand 1 is highly expressed in both tumor and immune cells and that it was also commonly expressed in ALK-negative cases (88%), whereas adaptive programmed cell death ligand 1 expression was present in the majority of tumors.
Our review of previous cases showed that immune-related mechanisms play an important role in the pathogenesis of IMT; thus, we should aim to detect the levels of relevant antibodies in the serum and cerebrospinal fluid as well as measure the erythrocyte sedimentation rate and other laboratory indicators to assist in the diagnosis of IMT.
Due to the rarity of IMT-CNS, no gold standard therapy has yet been identified. Currently, complete surgical resection of the tumor is the preferred treatment. In addition to surgical treatment, several other treatment options have emerged in recent years. Many researchers have treated IMT with corticosteroids for autoimmune pathogenesis, and the clinical and radioimaging findings showed improvement in most cases after such treatment[2,3,9,12]. Radiation therapy is rarely used in the treatment of IMT, and its efficacy is uncertain[12]. Several reportedly active regimens have been described in the literature, including vincristine plus methotrexate, vincristine plus etoposide, cisplatin/carboplatin-based regimes, ifosfamide-based regimens, and doxorubicin in combination with other drugs and taxanes[22-24].
Mycophenolate mofetil is commonly used in the treatment of IgG4-related immune diseases, and Moss et al[14] proposed the use of mycophenolate mofetil in IgG4-positive IMT patients. In 2 cases, the side effects of corticosteroids were greatly improved after mycophenolate mofetil treatment, and clinical symptoms were relieved. In some cases, rituximab also helped shrink the IMT and improve the patient’s symptoms[4]. The exact mechanism of action of rituximab is unknown, but it is believed to induce apoptosis via complement and antibody-dependent cell-mediated cytotoxicity[25].
Although IMT is a benign disease, the risk of recurrence is high[11]. The histological features of the recurrent tumor have been consistent with IMT. However, the morphology of the recurrent tumor showed a more sarcomatous pattern[26]. In this review, we sought to identity the high-risk factors related to IMT recurrence. We divided 47 cases of IMT-CNS into groups according to the resection method, ALK expression, and sex and counted the number of cases with tumor recurrence in each group. Seven patients treated with total tumor resection experienced recurrence within 5 years, and 5 patients with subtotal tumor resection experienced recurrence. Postoperative immunohistochemistry showed that only 1 ALK-positive case did not experience recurrence within 5 years, while only 1 ALK-negative case experienced recurrence within 5 years. We applied the Fisher exact probability method to analyze these data, using SPSS software (version 23.0; IBM Corp., Armonk, NY, United States). We found that ALK expression had an effect on recurrence of IMT-CNS (P < 0.05; Table 2).
| Surgery type | Recurrence in 5 yr | No recurrence in 5 yr | Total |
| GTR | 1 | 13 | 14 |
| STR | 1 | 5 | 6 |
| Total | 2 | 18 | 20 |
The expression of some immunohistochemical factors is related to drug sensitivity, which affects disease prognosis[11,19]. In general, gross total resection should be used as a surgical target. Interestingly, statistical analysis showed that the method of resection had no effect on the recurrence of IMT (P > 0.05; Table 3). We think this may be related to the fact that IMT is a low-grade aggressive tumor. However, we should still use total resection as the first option whenever possible. Immunohistochemistry and other data should be used to determine the follow-up treatment to reduce disease recurrence. Finally, we believe that as our understanding of IMT continues to grow, a prognosis based on the biological profile of tumors is possible.
| Staining result | Recurrence in 5 yr | No recurrence in 5 yr | Total |
| ALK (+) | 6 | 4 | 10 |
| ALK (-) | 1 | 13 | 14 |
| Total | 7 | 17 | 24 |
Intracranial IMT is a very rare disease. We summarized and analyzed 49 cases of intracranial IMT and found that the incidence rates in male and female patients were similar. Headache is the most common initial symptom in clinical manifestations, but it is also closely related to the location of tumor growth. MRI is an important means of IMT diagnosis, and expression of ALK as an important molecular marker of IMT has been widely used in recent years. Complete surgical resection of the tumor is the preferred treatment, and continued treatment after operation cannot be ignored. For tumors that are difficult to remove, glucocorticoids, radiotherapy, and other treatments can be administered to limit tumor growth or even eliminate tumors. ALK expression was closely related to the recurrence of IMT. Further analyses of more cases of IMT-CNS and long-term follow-up investigations are needed. We believe our case report provides an additional reference for clinicians for the pathogenetic diagnosis and treatment of IMT-CNS.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cao X, China; Shalli K, United Kingdom S-Editor: Liu GL L-Editor: Filipodia P-Editor: Liu GL
| 1. | Jeon YK, Chang KH, Suh YL, Jung HW, Park SH. Inflammatory myofibroblastic tumor of the central nervous system: clinicopathologic analysis of 10 cases. J Neuropathol Exp Neurol. 2005;64:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Lui PC, Fan YS, Wong SS, Chan AN, Wong G, Chau TK, Tse GM, Cheng Y, Poon WS, Ng HK. Inflammatory pseudotumors of the central nervous system. Hum Pathol. 2009;40:1611-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | McKinney AM, Short J, Lucato L, SantaCruz K, McKinney Z, Kim Y. Inflammatory myofibroblastic tumor of the orbit with associated enhancement of the meninges and multiple cranial nerves. AJNR Am J Neuroradiol. 2006;27:2217-2220. [PubMed] |
| 4. | Patel A, Kocoglu MH, Kaul A. Therapeutic strategies for durable response in plasma cell granulomas in the central nervous system. Ann Hematol. 2019;98:1027-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Wang X, Chen Y, Wu X, Zhang H. Intracranial Inflammatory Myofibroblastic Tumor with Negative Expression of Anaplastic Lymphoma Kinase: A Case Report and Review of the Literature. World Neurosurg. 2019;125:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Swain RS, Tihan T, Horvai AE, Di Vizio D, Loda M, Burger PC, Scheithauer BW, Kim GE. Inflammatory myofibroblastic tumor of the central nervous system and its relationship to inflammatory pseudotumor. Hum Pathol. 2008;39:410-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Pascual-Gallego M, Yus-Fuertes M, Jorquera M, Gonzalez-Mat A, Ortega L, Martinez-Martinez A, Zimman H. Recurrent meningeal inflammatory myofibroblastic tumor: a case report and literature review. Neurol India. 2013;61:644-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1029] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 9. | Buccoliero AM, Caldarella A, Santucci M, Ammannati F, Mennonna P, Taddei A, Taddei GL. Plasma cell granuloma--an enigmatic lesion: description of an extensive intracranial case and review of the literature. Arch Pathol Lab Med. 2003;127:e220-e223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Roche PH, Figarella-Branger D, Pellet W. Mixed meningeal and brain plasma-cell granuloma: an example of an unusual evolution. Acta Neurochir (Wien). 2004;146:69-72; discussion 72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Häusler M, Schaade L, Ramaekers VT, Doenges M, Heimann G, Sellhaus B. Inflammatory pseudotumors of the central nervous system: report of 3 cases and a literature review. Hum Pathol. 2003;34:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Saint-Blancard P, Harket A, Tine I, Daumas-Duport C, de Soultrait FR. [A rare lesion of the central nervous system: inflammatory pseudotumor]. Neurochirurgie. 2008;54:37-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Buccoliero AM, Caldarella A, Santucci M, Ammannati F, Mennonna P, Taddei A, Taddei GL. Plasma cell granuloma--an enigmatic lesion: description of an extensive intracranial case and review of the literature. Arch Pathol Lab Med. 2003;127:e220-e223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Moss HE, Mejico LJ, de la Roza G, Coyne TM, Galetta SL, Liu GT. IgG4-related inflammatory pseudotumor of the central nervous system responsive to mycophenolate mofetil. J Neurol Sci. 2012;318:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Kuang PD, Li QH, Liu ZY, Tang JL, Dong F, Wang Y, Zhu XL. Inflammatory pseudotumor of the pineal region: First reported case. Oncol Lett. 2016;11:2127-2130. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Han MH, Chi JG, Kim MS, Chang KH, Kim KH, Yeon KM, Han MC. Fibrosing inflammatory pseudotumors involving the skull base: MR and CT manifestations with histopathologic comparison. AJNR Am J Neuroradiol. 1996;17:515-521. [PubMed] |
| 17. | Nakayama K, Inoue Y, Aiba T, Kono K, Wakasa K, Yamada R. Unusual CT and MR findings of inflammatory pseudotumor in the parapharyngeal space: case report. AJNR Am J Neuroradiol. 2001;22:1394-1397. [PubMed] |
| 18. | Kolenc D, Dotlić S, Adamec I, Zadro I, Stambuk C, Ozretić D, Habek M. Isolated plasma cell granuloma of the meninges. Neurol Sci. 2013;34:2245-2247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Denis DJ, Elayoubi K, Weil AG, Berthelet F, Bojanowski MW. Inflammatory myofibroblastic tumors of the central nervous system that express anaplastic lymphoma kinase have a high recurrence rate. Surg Neurol Int. 2013;4:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Miyahara K, Fujitsu K, Yagishita S, Takemoto Y, Ichikawa T, Matsunaga S, Takeda Y, Niino H, Shiina T. Inflammatory pseudotumor of the choroid plexus. Case report. J Neurosurg. 2008;108:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Cottrell TR, Duong AT, Gocke CD, Xu H, Ogurtsova A, Taube JM, Belchis DA. PD-L1 expression in inflammatory myofibroblastic tumors. Mod Pathol. 2018;31:1155-1163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Kovach SJ, Fischer AC, Katzman PJ, Salloum RM, Ettinghausen SE, Madeb R, Koniaris LG. Inflammatory myofibroblastic tumors. J Surg Oncol. 2006;94:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 203] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 23. | Dishop MK, Warner BW, Dehner LP, Kriss VM, Greenwood MF, Geil JD, Moscow JA. Successful treatment of inflammatory myofibroblastic tumor with malignant transformation by surgical resection and chemotherapy. J Pediatr Hematol Oncol. 2003;25:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Trojan A, Stallmach T, Kollias S, Pestalozzi BC. Inflammatory myofibroblastic tumor with CNS involvement. Onkologie. 2001;24:368-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Garcia BA, Tinsley S, Schellenberger T, Bobustuc GC. Recurrent inflammatory pseudotumor of the jaw with perineural intracranial invasion demonstrating sustained response to Rituximab. Med Oncol. 2012;29:2452-2455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Chuah YY, Tashi T, Shy CG, Shyu JS, Dong MJ, Hsueh EJ. Intracranial Inflammatory Myofibroblastic Tumor with Sarcomatous Local Recurrence. World Neurosurg. 2016;93:484.e1-484.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | de Oliveira RS, Amato MC, Brassesco MS, Valera ET, Jucá CE, Neder L, Tone LG, Machado HR. Clinical and cytogenetic analysis of an intracranial inflammatory myofibroblastic tumor induced by a ventriculoperitoneal shunt. J Neurosurg Pediatr. 2009;4:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Kato K, Moteki Y, Nakagawa M, Kadoyama S, Ujiie H. Inflammatory myofibroblastic tumor of the cerebellar hemisphere--case report. Neurol Med Chir (Tokyo). 2011;51:79-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |