Published online Dec 6, 2022. doi: 10.12998/wjcc.v10.i34.12623
Peer-review started: June 27, 2022
First decision: September 5, 2022
Revised: September 17, 2022
Accepted: November 7, 2022
Article in press: November 7, 2022
Published online: December 6, 2022
Processing time: 158 Days and 3.4 Hours
Chronic intestinal pseudo-obstruction (CIPO) is a syndrome of intestinal motor dysfunction caused by intestinal nerve, muscle, and/or Cajal stromal cell lesions. CIPO is a serious category of gastrointestinal dynamic dysfunction, which can eventually lead to the death of patients with intestinal failure. Due to considerable phenotypic heterogeneity, the estimated incidence of CIPO is 1/476190 and 1/416666 in men and women, respectively. According to the etiology, CIPO can be divided into idiopathic and secondary, of which the latter is the most common, often secondary to tumor, virus infection, connective tissue disease, neurological diseases, and endocrine diseases. Idiopathic CIPO in the intestinal tract is divided into visceral myopathy, neuropathy, and stromal cell lesions according to the location. Surgery is usually not recommended for CIPO, because it often does not benefit patients with CIPO, and postoperative intestinal obstruction is likely to occur, which may even worsen the condition.
Here, we describe the case of a 43-year-old male Han Chinese patient with a 15-year history of recurrent abdominal distention with no clear cause. The results of physical, biochemical, and other relevant examinations showed no clear abnormalities. Contrast-enhanced computed tomography (CT) indicated a large duodenum, clear expansion of the intestinal lumen, and CIPO. Whole exome sequencing (WES) of the patient and his mother confirmed the diagnosis of primary familial visceral myopathy type 2 chronic pseudoileus with a rare heterozygous gene mutation in MYH11. This is the second reported case of CIPO with a heterozygous MYH11 [NM_001040113.1: c.5819delC (p.Pro1940Hisfs*91)] mutation.
This case report indicates that physicians can perform routine clinical examinations, CT, and WES to achieve a diagnosis and treatment of CIPO in early disease stages.
Core Tip: Chronic intestinal pseudo-obstruction is a rare abdominal disease with high morbidity and mortality. A patient developed abdominal symptoms with no mechanical intestinal obstruction, characterized by symptoms of chronic intestinal obstruction; whole exome sequencing (WES) was performed and revealed a rare autosomal dominant mutation associated with primary familial visceral myopathy type 2, MYH11, NM_001040113.1:c.5819delC (p.Pro1940Hisfs*91), which is a rare heterozygous mutation. In this case, mechanical ileus and secondary causes of pseudoileus were excluded, and the location, nature, and extent of the lesions were determined by small bowel computed tomography examination, and the etiology was determined by WES.
- Citation: Li N, Song YM, Zhang XD, Zhao XS, He XY, Yu LF, Zou DW. Pseudoileus caused by primary visceral myopathy in a Han Chinese patient with a rare MYH11 mutation: A case report. World J Clin Cases 2022; 10(34): 12623-12630
- URL: https://www.wjgnet.com/2307-8960/full/v10/i34/12623.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i34.12623
Chronic intestinal pseudo-obstruction (CIPO) is an intestinal motor dysfunction syndrome caused by intestinal nerve, muscle, and/or Cajal stromal cell lesions[1,2]. CIPO causes severe gastrointestinal dynamic dysfunction, which can eventually lead to death due to intestinal failure[3]. CIPO exhibits considerable phenotypic heterogeneity and has an estimated incidence of 1/476190 and 1/416666 in men and women, respectively[4]. According to its etiology, CIPO can be divided into idiopathic and secondary types. Secondary CIPO, the most common form, often occurs secondarily to tumors, viral infections, connective tissue diseases, neurological diseases, and endocrine diseases[5]. Idiopathic CIPO in the intestinal tract is divided into visceral myopathy, neuropathy, and stromal cell lesions according to its location[4]. Surgery is usually not recommended for CIPO, because it often does not benefit patients, and postoperative intestinal obstruction is likely to occur and potentially worsen the condition.
Autosomal dominant mutations in the smooth muscle actin gene ACTG2 occur in 44%-50% of patients with CIPO[6]. Moreover, studies have identified homozygous mutations in MYLK6, MYH117-9, LMOD110, MYL911, and RAD2112, as well as X-linked mutations in FLNA13 in CIPO cases in recent years[7-11]. Among these, mutations in the MYH11 gene have been associated with effects on smooth muscle cell contractile function, signaling, and cell motility; visceral myopathy type 2; familial thoracic aortic and aortic dissection type 4; giant bladder-small colon-bowel motility syndrome type 2; lung cancer, large bowel cancer, breast cancer, bladder cancer, and myeloid leukemia; and other diseases[12,13]. The MYH11 gene maps to the middle of the short arm of chromosome 16[14]. Gauthier et al[8] have performed exome sequencing in a newborn with megacystis-microcolon-intestinal hypoperistalsis syndrome (MMIHS) and identified a homozygous variant (c.3598A>T:p.Lys1200Ter) in MYH11, thus suggesting that loss-of-function variants in MYH11 cause MMIHS. Compound heterozygous mutations in MYH11 have been found in several familial CIPO cases. Cospain et al[15] have reported a case of mucopolysaccharidosis type I in a patient with early-onset CIPO, who had a 1.7-Mb heterozygous deletion of the chromosomal region 16p13.11p12.3, comprising MYH11. Furthermore, a rare dominant mutation in MYH11 has been found in one extended family with 13 affected members[16].
CIPO often lacks specific laboratory findings, biomarkers, and symptoms, and its symptoms are similar to those of other peristaltic disorders[17]. Consequently, a long time period usually elapses before an accurate CIPO diagnosis is obtained, thus sometimes resulting in unnecessary surgery[18]. Because visceral myopathy is relatively common in CIPO, the clinical diagnosis of CIPO depends primarily on endoscopic or imaging examination. Next-generation sequencing has greatly increased the chances of identifying known and new causal genes for CIPO. As whole exome sequencing (WES) has become more widely used in clinical settings, the number of patients benefiting from applications of this method is growing rapidly.
A 43-year-old man was admitted to the Gastroenterology Department of Ruijin Hospital affiliated to Shanghai Jiao Tong University in October 2021 because of CIPO. His main clinical manifestation was lower abdominal distension with no clear cause, which had started 15 years prior.
A diagnosis of intestinal obstruction and superior mesenteric artery compression syndrome was suggested in a local hospital, where adhesion reduction and superior jejunal partial resection were recommended.
In the prior 10 years, the patient experienced continued abdominal distension; frequent anal defecation; absence of nausea; vomiting; and abdominal pain. In July 2021, he was diagnosed with small bowel obstruction, duodenal stasis, mild malnutrition, and urinary retention; symptomatic treatment and hospitalization were proposed. Since the onset of the disease, the patient had a clear mind and acceptable mental stomach; defecated three or four times per day with unformed stools; and had no abnormal urination or significant weight loss.
The patient was married and had two children, both of whom were healthy. His mother privately reported a notable and concerning history of duodenal enlargement.
The patient was clearly thinking and energetic. He was thin and had abdominal distension, and the length of his abdomen was 110 cm. A longitudinal old surgical scar on the upper abdomen was about 10 cm long, without intestinal shape and peristaltic waves, tenderness, rebound pain, or muscle tension. He had drumming in his abdomen, bowel hyperactivity (more than 10 beats/min), and dullness of negative activity. Physical examination showed that the body temperature of the patient was 36.7 °C, pulse 80 beats/min, breathing rate 18 times/min, blood pressure 87/52 mmHg, height 160 cm, weight 44 kg, and body mass index (BMI) 17.19 kg/m2 (Table 1).
| Physical examination | Data |
| Body temperature | 36.7 °C |
| Pulse rate | 80 beats/min |
| Breathing rate | 18 times/min |
| Blood pressure | 87/52 mmHg |
| Height | 160 cm |
| Weight | 44 kg |
| BMI | 17.19 kg/m2 |
Routine blood tests indicated a red blood cell count of 3.62 × 1012/L, and a hemoglobin level of 116 g/L. Blood gas analysis showed a pH value of 7.29, partial pressure of oxygen of 16.63 kpa, partial pressure of CO2 of 4.00 kpa, oxygen saturation of 98.4%, hydrogen ion concentration of 51.3 nmol/L, standard bicarbonate of 15.9 mmol/L, actual bicarbonate of 14.1 mmol/L, and standard residual base of 10.8 mmol/L. The 24-h urinary protein was 1168 mg/24 h; the 24-h urine potassium was 19.49 mmol/24 h; the 24-h urinary calcium was 7.74 mmol/24 h; and the 24-h urine phosphorus was 12.18 mmol/24 h (Table 2). Examinations of the endocrine system, immune system, connective tissue, and tumor index showed no clear abnormalities.
| Biochemical parameter | Data |
| Red blood cell count | 3.62 × 1012/L |
| Hemoglobin | 116 g/L |
| Blood pH | 7.29 |
| Partial pressure of oxygen | 6.63 kpa |
| Partial pressure of CO2 | 4.00 kpa |
| Oxygen saturation | 98.4% |
| Hydrogen ion concentration | 51.3 nmol/L |
| Standard bicarbonate | 15.9 mmol/L |
| Actual bicarbonate | 14.1 mmol/L |
| Standard residual base | 10.8 mmol/L |
| 24-h urinary protein | 1168 mg/24 h |
| 24-h urine potassium | 19.49 mmol/24 h |
| 24-h urinary calcium | 7.74 mmol/24 h |
| 24-h urine phosphorus | 12.18 mmol/24 h |
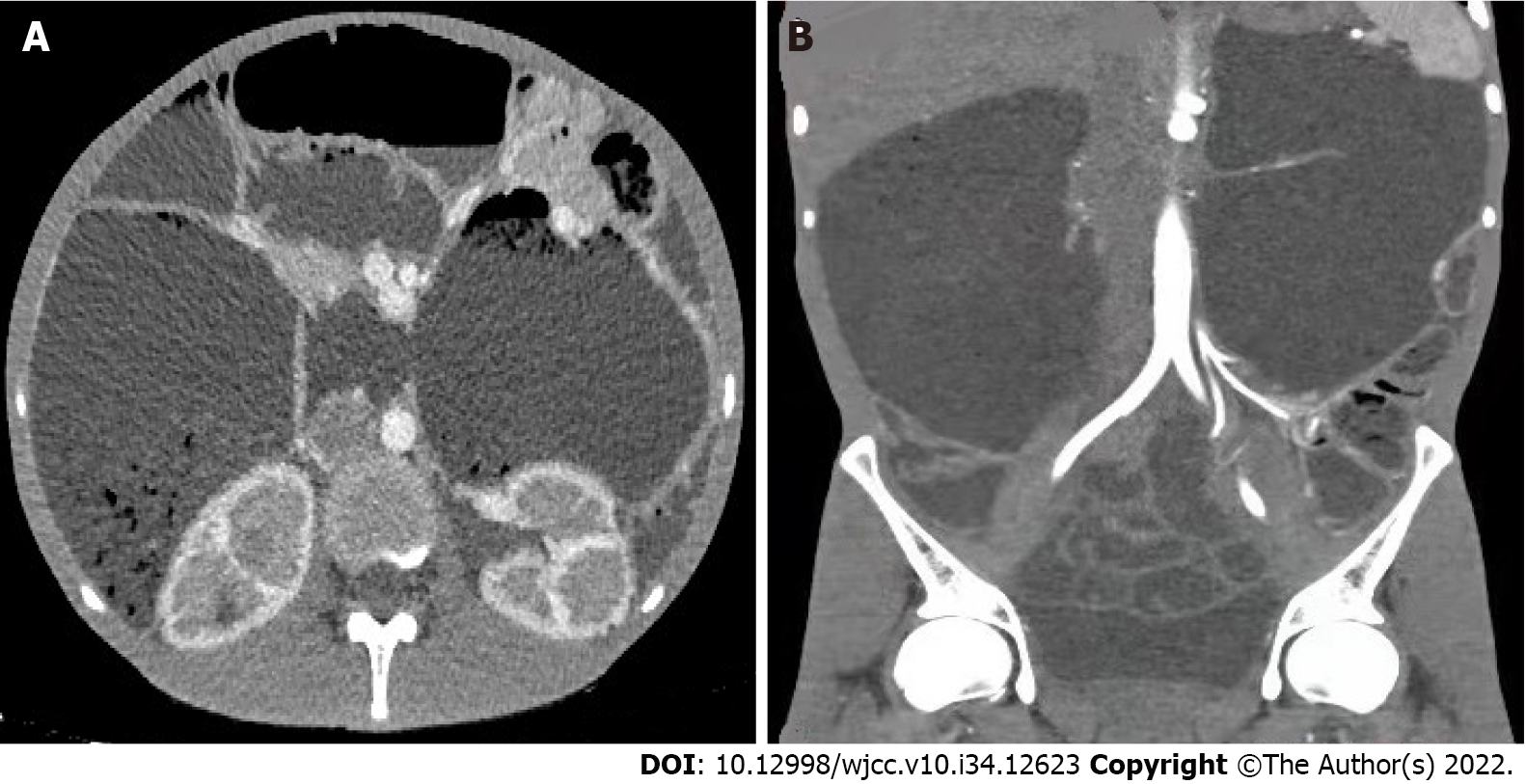
Contrast-enhanced computed tomography (CT) (Figure 1) showed that the duodenum was large, and the intestinal tube was significantly dilated with a diameter of approximately 12.68 cm. The mucosal folds were normal, no clear obstruction point was identified, and the intestinal wall did not show thickening. Moreover, no edema or inflammation was observed around the intestine, and the lymph nodes were not enlarged. The observed changes suggested chronic pseudo-intestinal obstruction, and the renal medulla showed delayed enhancement.
The patient was diagnosed with CIPO.
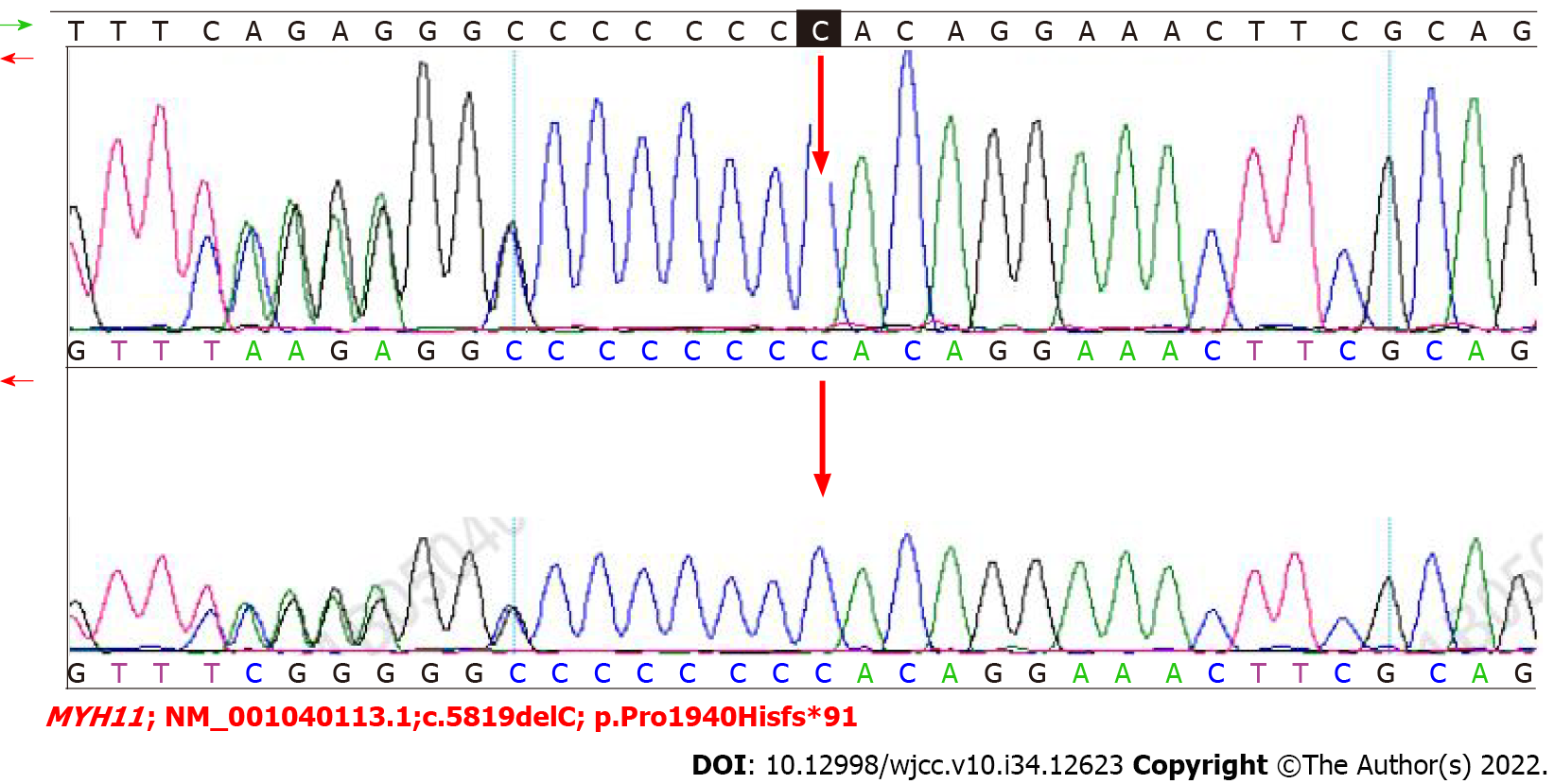
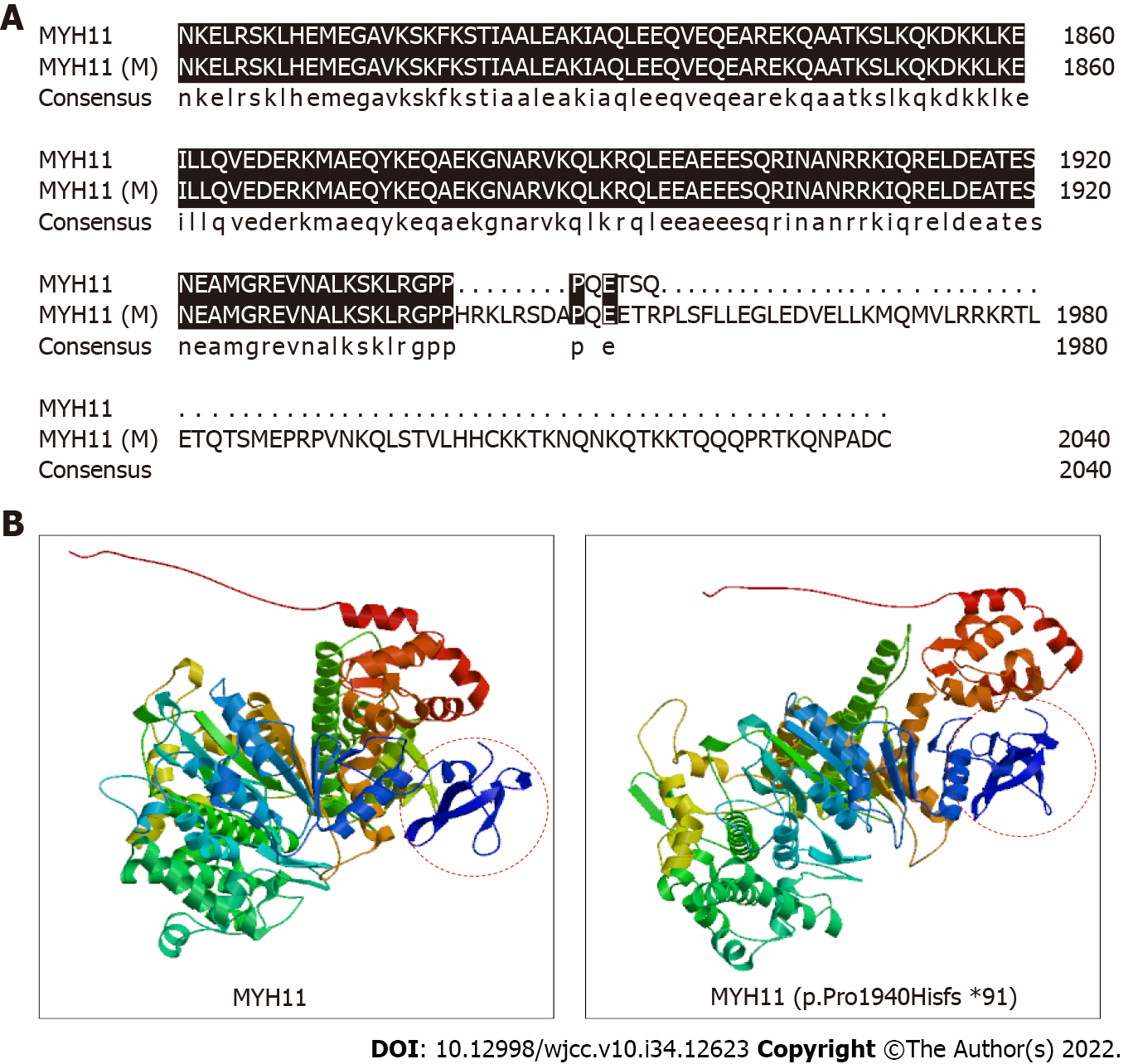
We performed WES to detect the presence of any mutation in relevant disease-causing genes. A 2 mL blood sample in an ethylenediamine tetra-acetic acid-coated tube was sent to the Shenzhen BGI Medical Test Laboratory for sequencing, which was performed through capture high-throughput technology and detected nearly 20000 genes in the human genome. Sanger sequencing was used to verify the mutations. A suspected disease-causing mutation in MYH11, located on chromosome chr16:15802687; NM_001040113.1:c.5819delC (p.Pro1940Hisfs*91), was identified, which is a heterozygous gene mutation associated with primary familial visceral myopathy type 2 chronic pseudoileus[4]. The heterozygous mutation, a frameshift mutation caused by the deletion of one C nucleotide at position 5819 of the gene coding sequence, changes the proline codon at position 1940 to histidine, and then produces a stop codon at position 91, thus elongating the C terminal sequence of the MYH11 protein and causing local changes in the three-dimensional structure of the protein (Figure 2). We subsequently collected blood samples from the patient’s mother and verified the mutation by Sanger sequencing, which confirmed that both the patient and his mother carried this heterozygous mutation (Figure 3).
After being diagnosed, the patient received nutritional support, and his digestive tract motor function was restored. However, his abdominal distension symptoms did not substantially improve. No effective non-surgical treatment for this disease is currently available. In the acute period, fasting, gastrointestinal decompression, and correction of electrolyte disorders should be the main treatments. In the remission period, nutritional support, recovery of digestive tract movement function, and prevention and treatment of infection should be emphasized to improve the quality of life.
Through nutritional support, the recovery of digestive tract movement function, and other treatments, the patient’s abdominal distension and frequent anal defecation were relieved. He had one or two regular bowel movements per day without abnormal urination or substantial weight loss, and his quality of life was significantly improved.
CIPO is a rare abdominal disease with high morbidity and mortality. Our patient developed abdominal symptoms with no mechanical intestinal obstruction, which were characterized by chronic intestinal obstruction, such as abdominal pain, distension, and vomiting[4]. CIPO often lacks specific laboratory findings, biomarkers, and symptoms, and its symptoms are similar to those of other peristaltic disorders. Owing to the lack of specific findings, a long time period usually elapses before patients obtain an accurate CIPO diagnosis, and unnecessary surgery is sometimes performed[5]. In our case, according to the CT findings of a large duodenum and chronic pseudo-obstruction, we determined that the lesions were located mainly in the duodenum.
WES, a precision medical technology developed in recent years, captures DNA sequences of nearly 20000 coding genes in the genome with high throughput, and uses comparative bioinformatics analysis to determine the microbial species and abundance information contained in the samples. WES can detect most disease variants and is gradually being adopted in clinical genetic testing and diagnosis, particularly in the diagnosis of rare genetic diseases[19,20]. The development of sequencing and gene editing technologies is expected to lead to therapeutic breakthroughs in gene therapy in the future.
In addition to the pseudo-intestinal obstruction, our patient had symptoms of urinary retention, which might have been associated with the abnormal smooth muscle cell function caused by the MYH11 gene mutation. In clinical settings, visceral myopathic pseudointestinal obstruction caused by MYH11 gene mutation is rarely encountered. In a previous study, two frameshift mutations in MHY11 have been reported. The first was a 2-bp deletion in exon 22 (c.2809_2810del, p.Arg937Glyfs*7, paternal), whereas the second mutation in exon 26 was a 49-bp deletion (c.3422_3470del, p.Lys1141Thrfs*20, maternal)[8]. Kloth et al[21] have reported a patient with MMIHS with a novel heterozygous missense variant (c.379C>T) in MYH11. In another case, a MHY11 frameshift mutation has been detected in exon 42 (NM_001040113.1:c.5819delC, p.Pro1940HisfsTer91)[16]. In our case, WES revealed a rare autosomal dominant mutation in MYH11, NM_001040113.1:c.5819delC (p.Pro1940Hisfs*91), which was consistent with the results of a previously reported case[16]. Our study strengthens the understanding of CIPO etiology and provides genetic evidence supporting the diagnosis of CIPO.
Surgical treatment is usually not recommended for CIPO, because surgery often does not benefit patients, and postoperative intestinal obstruction is likely to occur and may aggravate the condition. If surgical treatment is necessary, careful and rigorous evaluation is required. Clinically, gastrostomy, jejunal catheterization, or jejunostomy can effectively decrease abdominal distension and vomiting, provide an important means of providing enteral nutrition, and substantially increase the transport capacity of the digestive tract, thus decreasing hospitalization and operation rates[22]. The disease is complex, and its treatment is difficult, thus requiring multidisciplinary teams from gastroenterology, gastrointestinal surgery, nutrition, imaging, transplant surgery, psychology, and other departments to formulate treatment plans.
In our patient, mechanical ileus and secondary causes of pseudoileus were excluded, and the location, nature, and extent of the lesions were examined through small bowel CT. WES was used to identify a rare mutation in MYH11 [NM_001040113.1:c.5819delC (p.Pro1940Hisfs*91)]. This case report may help clinicians understand the genetic basis of the etiology of CIPO.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mankotia DS, India; Su YY, Taiwan S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
| 1. | Downes TJ, Cheruvu MS, Karunaratne TB, De Giorgio R, Farmer AD. Pathophysiology, Diagnosis, and Management of Chronic Intestinal Pseudo-Obstruction. J Clin Gastroenterol. 2018;52:477-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Pironi L, Sasdelli AS. Management of the Patient with Chronic Intestinal Pseudo-Obstruction and Intestinal Failure. Gastroenterol Clin North Am. 2019;48:513-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Stanghellini V, Cogliandro RF, de Giorgio R, Barbara G, Salvioli B, Corinaldesi R. Chronic intestinal pseudo-obstruction: manifestations, natural history and management. Neurogastroenterol Motil. 2007;19:440-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Iida H, Ohkubo H, Inamori M, Nakajima A, Sato H. Epidemiology and clinical experience of chronic intestinal pseudo-obstruction in Japan: a nationwide epidemiologic survey. J Epidemiol. 2013;23:288-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Amiot A, Cazals-Hatem D, Joly F, Lavergne-Slove A, Peuchmaur M, Bouhnik Y, Bedossa P, Messing B. The role of immunohistochemistry in idiopathic chronic intestinal pseudoobstruction (CIPO): a case-control study. Am J Surg Pathol. 2009;33:749-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Milunsky A, Baldwin C, Zhang X, Primack D, Curnow A, Milunsky J. Diagnosis of Chronic Intestinal Pseudo-obstruction and Megacystis by Sequencing the ACTG2 Gene. J Pediatr Gastroenterol Nutr. 2017;65:384-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Halim D, Brosens E, Muller F, Wangler MF, Beaudet AL, Lupski JR, Akdemir ZHC, Doukas M, Stoop HJ, de Graaf BM, Brouwer RWW, van Ijcken WFJ, Oury JF, Rosenblatt J, Burns AJ, Tibboel D, Hofstra RMW, Alves MM. Loss-of-Function Variants in MYLK Cause Recessive Megacystis Microcolon Intestinal Hypoperistalsis Syndrome. Am J Hum Genet. 2017;101:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Gauthier J, Ouled Amar Bencheikh B, Hamdan FF, Harrison SM, Baker LA, Couture F, Thiffault I, Ouazzani R, Samuels ME, Mitchell GA, Rouleau GA, Michaud JL, Soucy JF. A homozygous loss-of-function variant in MYH11 in a case with megacystis-microcolon-intestinal hypoperistalsis syndrome. Eur J Hum Genet. 2015;23:1266-1268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Bonora E, Bianco F, Cordeddu L, Bamshad M, Francescatto L, Dowless D, Stanghellini V, Cogliandro RF, Lindberg G, Mungan Z, Cefle K, Ozcelik T, Palanduz S, Ozturk S, Gedikbasi A, Gori A, Pippucci T, Graziano C, Volta U, Caio G, Barbara G, D'Amato M, Seri M, Katsanis N, Romeo G, De Giorgio R. Mutations in RAD21 disrupt regulation of APOB in patients with chronic intestinal pseudo-obstruction. Gastroenterology. 2015;148:771-782.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Moreno CA, Sobreira N, Pugh E, Zhang P, Steel G, Torres FR, Cavalcanti DP. Homozygous deletion in MYL9 expands the molecular basis of megacystis-microcolon-intestinal hypoperistalsis syndrome. Eur J Hum Genet. 2018;26:669-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 11. | Halim D, Wilson MP, Oliver D, Brosens E, Verheij JB, Han Y, Nanda V, Lyu Q, Doukas M, Stoop H, Brouwer RW, van IJcken WF, Slivano OJ, Burns AJ, Christie CK, de Mesy Bentley KL, Brooks AS, Tibboel D, Xu S, Jin ZG, Djuwantono T, Yan W, Alves MM, Hofstra RM, Miano JM. Loss of LMOD1 impairs smooth muscle cytocontractility and causes megacystis microcolon intestinal hypoperistalsis syndrome in humans and mice. Proc Natl Acad Sci U S A. 2017;114:E2739-E2747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 12. | Biernacki MA, Foster KA, Woodward KB, Coon ME, Cummings C, Cunningham TM, Dossa RG, Brault M, Stokke J, Olsen TM, Gardner K, Estey E, Meshinchi S, Rongvaux A, Bleakley M. CBFB-MYH11 fusion neoantigen enables T cell recognition and killing of acute myeloid leukemia. J Clin Invest. 2020;130:5127-5141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 13. | Derycke L, Stove C, Vercoutter-Edouart AS, De Wever O, Dollé L, Colpaert N, Depypere H, Michalski JC, Bracke M. The role of non-muscle myosin IIA in aggregation and invasion of human MCF-7 breast cancer cells. Int J Dev Biol. 2011;55:835-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Deng Z, Liu P, Marlton P, Claxton DF, Lane S, Callen DF, Collins FS, Siciliano MJ. Smooth muscle myosin heavy chain locus (MYH11) maps to 16p13.13-p13.12 and establishes a new region of conserved synteny between human 16p and mouse 16. Genomics. 1993;18:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Cospain A, Dubourg C, Gastineau S, Pichard S, Gandemer V, Bonneau J, de Tayrac M, Moreau C, Odent S, Pasquier L, Damaj L, Lavillaureix A. Incidental diagnosis of mucopolysaccharidosis type I in an infant with chronic intestinal pseudoobstruction by exome sequencing. Mol Genet Metab Rep. 2020;24:100621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Dong W, Baldwin C, Choi J, Milunsky JM, Zhang J, Bilguvar K, Lifton RP, Milunsky A. Identification of a dominant MYH11 causal variant in chronic intestinal pseudo-obstruction: Results of whole-exome sequencing. Clin Genet. 2019;96:473-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Di Nardo G, Di Lorenzo C, Lauro A, Stanghellini V, Thapar N, Karunaratne TB, Volta U, De Giorgio R. Chronic intestinal pseudo-obstruction in children and adults: diagnosis and therapeutic options. Neurogastroenterol Motil. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 18. | Xiong X, Li J, Liu C, Xu F. Visceral myopathy diagnosed by a de novo ACTG2 mutation in a patient with chronic intestinal pseudo-obstruction-a case report. Transl Pediatr. 2021;10:679-685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Dos Santos W, de Andrade ES, Garcia FAO, Campacci N, Sábato CDS, Melendez ME, Reis RM, Galvão HCR, Palmero EI. Whole-Exome Sequencing Identifies Pathogenic Germline Variants in Patients with Lynch-Like Syndrome. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 20. | Kim SY, Jang SS, Kim H, Hwang H, Choi JE, Chae JH, Kim KJ, Lim BC. Genetic diagnosis of infantile-onset epilepsy in the clinic: Application of whole-exome sequencing following epilepsy gene panel testing. Clin Genet. 2021;99:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Kloth K, Renner S, Burmester G, Steinemann D, Pabst B, Lorenz B, Simon R, Kolbe V, Hempel M, Rosenberger G. 16p13.11 microdeletion uncovers loss-of-function of a MYH11 missense variant in a patient with megacystis-microcolon-intestinal-hypoperistalsis syndrome. Clin Genet. 2019;96:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Billiauws L, Corcos O, Joly F. Dysmotility disorders: a nutritional approach. Curr Opin Clin Nutr Metab Care. 2014;17:483-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |