Published online Dec 6, 2022. doi: 10.12998/wjcc.v10.i34.12559
Peer-review started: September 30, 2022
First decision: October 13, 2022
Revised: October 18, 2022
Accepted: November 7, 2022
Article in press: November 7, 2022
Published online: December 6, 2022
Processing time: 63 Days and 11.3 Hours
There are difficulties in diagnosing nosocomial transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in hospital settings. Furthermore, mortality of cases of nosocomial infection (NI) with SARS-CoV-2 is higher than that of the general infected population. In the early stage of the pandemic in Taiwan, as patients were not tested for SARS-CoV-2 at admission, NIs often go undetected. Strictly applying the systematic polymerase chain reaction (PCR) screening, as a standard infection control measure was subse
To assess NI incidence of SARS-CoV-2 among hospital staff, hospitalized patients, and caregivers, and the transmission routes of clusters of infection.
This descriptive retrospective analysis at our hospital from May 15 to August 15, 2021 included data on 132 SARS-CoV-2 NIs cases among hospital staff, inpatients, and caregivers who previously tested negative but subsequently identified with a positive SARS-CoV-2 reverse transcriptase-PCR (RT-PCR) test results, or a hospital staff who tested positive following routine SARS-CoV-2 RT-PCR test. Chi-square tests were performed to compare the differences between hospital staff and private caregivers, and between clusters and sporadic infections.
Overall, 9149 patients and 2005 hospital staff members underwent routine SARS-CoV-2 RT-PCR testing, resulting in 12 confirmed cluster and 23 sporadic infections. Among the index cases of the clusters, three (25%) cases were among hospital staff and nine (75%) cases were among other contacts. Among sporadic infections, 21 (91%) cases were among hospital staff and two (9%) cases were among other contacts (P < 0.001). There was an average of 8.08 infections per cluster. The secondary cases of cluster infection were inpatients (45%), hospital staff (30%), and caregivers (25%). Private caregivers constituted 27% and 4% of the clusters and sporadic infections, respectively (P = 0.024); 92.3% of them were infected in the clusters. The mortality rate was 0.0%.
The incidence of SARS-CoV-2 infection was relatively high among private caregivers, indicating a need for infection control education in this group, such as social distancing, frequent hand-washing, and wearing PPE.
Core Tip: Patients were more likely to acquire a nosocomial infection than hospital staff and caregivers. Private caregivers tended to be part of clusters of infection, due to social interaction. The incidence of severe acute respiratory syndrome coronavirus 2 infection was relatively high among private caregivers, indicating a need for infection control education in this group.
- Citation: Cheng CC, Fann LY, Chou YC, Liu CC, Hu HY, Chu D. Nosocomial infection and spread of SARS-CoV-2 infection among hospital staff, patients and caregivers. World J Clin Cases 2022; 10(34): 12559-12565
- URL: https://www.wjgnet.com/2307-8960/full/v10/i34/12559.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i34.12559
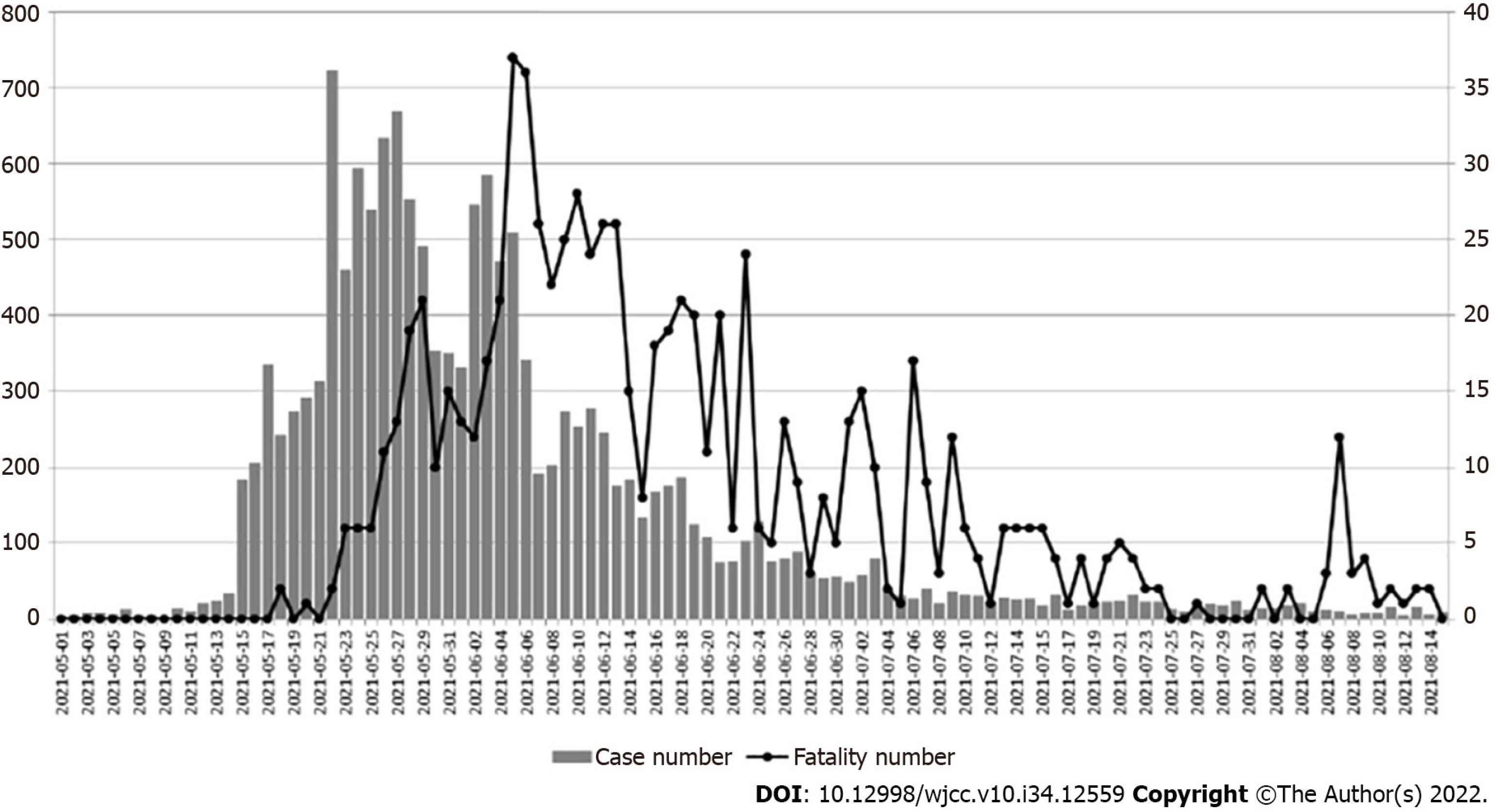
Since the outbreak of coronavirus disease (COVID-19) in December 2019, the disease has been prevalent in over 200 countries and regions around the world and claimed more than 1840000 Lives[1,2]. A large proportion of the infection is asymptomatic. In a study at our hospital (Ren-Ai branch of Taipei City Hospital) during the first outbreak in Taiwan[3], approximately 43% to 46% of people diagnosed with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were asymptomatic. Thus, there are risks and difficulties in the diagnosis of nosocomial transmission of the SARS-CoV-2 infection in hospital settings. Furthermore, the mortality of the cases with nosocomial infection of SARS-CoV-2 is higher than that of the general infected population. In a study of 11 hospitals in Italy and the United Kingdom[4], the mortality rate of nosocomial infection cases was 27%, and their median survival time was 14 days, compared to the approximately 2% mortality rate of the general population[5]. Considering the condition of the nosocomial infection, a high rate of co-morbidities was observed in nosocomial infection cases. The study of Quebec and British Columbia[6] in Canada demonstrated that a high rate of nosocomial transmission of COVID-19 is associated with increased mortality in the cancer population, reinforcing the importance of treating patients with cancer in COVID-free units. In a study in France, all the nosocomial infection cases of SARS-CoV-2 presented with co-morbidities[7]. Thus, controlling nosocomial transmission is crucial to reducing COVID-19 morbidity and mortality. Infection surveillance and risk factor analysis are important for the prevention and treatment of nosocomial infections (NIs). During the first outbreak in Taiwan in 2021, cases surged rapidly; as shown in Figure 1, this local outbreak starting in May 2021 made the Central Epidemic Command Center raise the national endemic alert tier (which included four levels of alert), from level one to level two on May 11, 2021, and to level three from May 15 to July 26, 2021. As the cases staggeringly increased, the case fatality rate increased as well, yet was slightly delayed temporarily. As of July 25, there were 14262 Local cases (versus 15511 cases, including imported cases) and 778 fatalities in total. The prevalence of SARS-CoV-2 in Taiwan was 0.07%, and the case fatality rate was 5.03% at that time. In the early stage of the pandemic in Taiwan, as patients were not tested for SARS-CoV-2 at admission, it was possible for NIs to go undetected. The strict application of systematic polymerase chain reaction (PCR) screening, as part of the standard infection control measures, was subsequently implemented to reduce the incidence of NIs. Evidence on risk factors for SARS-CoV-2 NIs among healthcare workers (HCWs) and caregivers is limited. The goal of this study was to assess the incidence of nosocomial SARS-CoV-2 infection among hospital staff, hospitalized patients, and caregivers, and the transmission routes of clusters of infection.
We conducted a retrospective analysis of SARS-CoV-2 NIs at our hospital from May 15 to August 15, 2021. We collected data on 132 cases of SARS-CoV-2 NIs among hospital staff, inpatients, and caregivers (including inpatients’ family members and private caregivers). The study was approved by the hospital’s institutional review board (IRB), with IRB number TCHIRB-11101009-E. All investigations were carried out in compliance with relevant laws and guidelines, and with the ethical standards of the Declaration of Helsinki. An NI was defined as an infection occurring in a patient or caregiver who developed COVID-19 symptoms after admission and was discovered at the hospital with a positive SARS-CoV-2 reverse transcriptase-PCR (RT-PCR) test; or a member of the hospital staff who tested positive following a routine SARS-CoV-2 RT-PCR test. Before admission, all the patients admitted to the general wards in Taipei City Hospital had tested negative for SARS-CoV-2 following RT-PCR test. All the data we collected were recorded and classified in detail according to the onset of the symptoms, duration, image findings and laboratory results. The first confirmed positive case in a cluster was regarded as the index case. After the index case was identified, the infection control department conducted an outbreak investigation and performed PCR testing of contacts of the index case. If additional cases were found among contacts, it was defined as a cluster; otherwise, it was regarded as a sporadic infection. We performed descriptive analyses of clusters and sporadic infections among hospital staff and other contacts and presented them separately as index cases and cases infected by index cases. Hospital staff included physicians, nurses, and other HCWs. Other contacts included inpatients, family members, and private caregivers. Private caregivers included caregivers provided by companies contracted by the hospital and caregivers employed by patients. Chi-square tests were performed to compare the differences between hospital staff and private caregivers, and between clusters and sporadic infections. The threshold for statistical significance was set at 5%. All analyses were performed using SAS (version 9.4; SAS Institute, Inc., Cary, NC, United States).
A total of 9149 patients were admitted to the hospital and 2005 hospital staff underwent routine SARS-CoV-2 RT-PCR testing during the study period. During this period, there were 12 confirmed clusters and 23 sporadic infections (Table 1). Among the index cases of clusters, three (25%) cases were among hospital staff, and nine (75%) cases were among other contacts. Among the sporadic infections, 21 (91%) cases were among hospital staff and two (9%) cases were among other contacts (P < 0.001). There was an average of 8.08 infections per cluster. The sources of secondary cases were inpatients (45%), hospital staff members (30%), and caregivers (25%). Table 2 shows the incidence of clusters among hospital staff and private caregivers. Physicians constituted 11.9% and 8.3% of the clusters and sporadic infections, respectively (P = 0.650). Nurses constituted 14.3% and 33.3% of the clusters and sporadic infections, respectively (P = 0.069). Other hospital staff members (other than physicians and nurses) constituted 45.2% and 54.2%of the clusters and sporadic infections, respectively (P = 0.485). Private caregivers constituted 28.6% and 4.2%of the clusters and sporadic infections, respectively (P = 0.016). The incidence of NI among hospital staff was 53/2005 (2.64%), while the incidence among inpatient was 50/9149 (0.5%). In addition, the mortality rate was 0.0%.
The nosocomial spread of SARS-CoV-2 may cause clusters of infection among high-risk individuals. Controlling the spread is critical for reducing COVID-19 morbidity and mortality. In Taipei City Hospital, during the period from May 15 to August 15, 2021, we implemented the following protective measures. First, we mandated every hospital staff, private caregiver, and patient to wear masks all the time. Second, we equipped every ward with 75% alcohol disinfectant as hand sanitizer. Third, the hospital staff performed RT-PCR to detect for those suspected to be infected with SARS-CoV-2, while staff wearing isolation gown. Finally, we provided the patients with information and knowledge of self-protection against contracting SARS-CoV-2 infection. To prevent the secondary cases in our hospital, infected HCWs and private caregivers were instructed to suspend work and undergo contact tracing. All the infected HCWs, private caregivers and patients remained in isolation for up to 14 days and under observation for 7 days until two successive negative RT-PCR test results were obtained. Furthermore, to prevent the spread of NIs, all the people at risk of infection, such as those admitted in the same ward with or taking care of patients with a confirmed diagnosis, had to be quarantined. Systematic PCR screening of patients upon admission and HCWs to identify asymptomatic individuals can reduce the spread of SARS-CoV-2 in healthcare settings[8]. Among HCWs, the use of personal protective equipment (PPE) and good hygiene practices, and social distancing are critical for preventing SARS-CoV-2 Nis[9]. Casanova et al[10] found that coronavirus can survive for more than 24 h on an N95 respirator, and at least for 4 h on other PPE, which makes it important to train HCWs on how to wear and remove PPE correctly. The correct order of wearing PPE is as follows: Hairnet, gown (with a back closure, which may require two people), filter mask, goggles, and finally two layers of gloves, with the inner layer covering up the wrist[11]. According to a review[12], it is suggested that social distancing must be at least 1.5 m indoors and 1.0 m outdoors in Taiwan. The World Health Organization also suggested that social distancing should be at least 1.0 m and that people should wear a mask if they could not keep proper social distance. In addition, strict application of standard infection control measures is important for the management of SARS-CoV-2 NIs, including containing clusters and preventing patient-to-HCW transmission[13,14]. Rapid screening and extensive testing, prompt quarantine, contact tracing, and social distancing have contributed to preventing community transmissions[15]. Thus, infection control strategies to prevent NIs must correspond with the community disease prevalence. Routine surveillance for asymptomatic infections among HCWs, patients, and caregivers is important during an epidemic[16]. Transmission in healthcare settings can result in many infections among HCWs and patients. Understanding infection dynamics has important implications for methods employed in hospitals to prevent NIs. SARS-CoV-2 infection can be introduced to the ward by asymptomatic and minimally symptomatic HCWs. Transmission of infection from pre-symptomatic, asymptomatic, and minimally symptomatic individuals means that universal precautions are required to minimize transmissions in hospital settings[17]. The study results reinforce the importance of periodic screening of HCWs for asymptomatic infection and routine surveillance of patients and caregivers during epidemic periods. There are two limitations to this study. First, data on personal characteristics were not available. Second, the study was conducted at a single hospital in Taiwan; therefore, the generalizability of the results may be limited. This study has three main findings. First, NIs among HCWs were mainly sporadic, whereas patients, their family members, and caregivers were more likely to be part of a cluster. This highlights the importance of mandatory screening of patients upon admission and confirms the effectiveness of protective awareness and regular screening among HCWs. Infections among HCWs were usually detected early and subsequent clusters of infection were prevented. Second, in the NI clusters, patients were more likely to acquire COVID-19 than hospital staff, private caregivers, and family members. This may be because patients tend to have a relatively poor immune system and underlying diseases. Third, private caregivers tended to be part of clusters of infection. Social interaction, switching to different employers, and returning to the dispatch center may have contributed to clusters among private caregivers. Thus, to prevent clusters of SARS-CoV2 infection in hospitals, private caregivers need to be educated by providing information about social distancing, frequent hand-washing, and wearing of PPE.
The incidence of SARS-CoV-2 infection was relatively high among private caregivers, indicating a need for infection control education in this group, such as social distancing, frequent hand-washing, and wearing PPE.
There are difficulties in diagnosing nosocomial transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in hospital settings. Furthermore, mortality of cases of nosocomial infection (NI) with SARS-CoV-2 is higher than that of the general infected population. In the early stage of the pandemic in Taiwan, as patients were not tested for SARS-CoV-2 at admission, NIs often go undetected. Strictly applying the systematic polymerase chain reaction (PCR) screening, as a standard infection control measure was subsequently implemented to reduce NI incidence.
Evidence on risk factors for SARS-CoV-2 NIs among healthcare workers (HCWs) and caregivers is limited.
To assess SARS-CoV-2 NI incidence among hospital staff, hospitalized patients, and caregivers, and the transmission routes of clusters of infection.
This descriptive retrospective analysis at our hospital from May 15 to August 15, 2021 included data on 132 SARS-CoV-2 NIs cases among hospital staff, inpatients, and caregivers who previously tested negative but subsequently identified with a positive SARS-CoV-2 reverse transcriptase-PCR (RT-PCR) test results, or a hospital staff who tested positive following routine SARS-CoV-2 RT-PCR test. Chi-square tests were performed to compare the differences between hospital staff and private caregivers, and between clusters and sporadic infections.
Overall, 9149 patients and 2005 hospital staff members underwent routine SARS-CoV-2 RT-PCR testing, resulting in 12 confirmed cluster and 23 sporadic infections. Among the index cases of the clusters, three (25%) cases were among hospital staff and nine (75%) cases were among other contacts. Among sporadic infections, 21 (91%) cases were among hospital staff and two (9%) cases were among other contacts (P < 0.001). There was an average of 8.08 infections per cluster. The secondary cases of cluster infection were inpatients (45%), hospital staff (30%), and caregivers (25%). Private caregivers constituted 27% and 4% of the clusters and sporadic infections, respectively (P = 0.024); 92.3% of them were infected in the clusters. The mortality rate was 0.0%.
The incidence of SARS-CoV-2 infection was relatively high among private caregivers, indicating a need for infection control education in this group, such as social distancing, frequent hand-washing, and wearing PPE.
The incidence of SARS-CoV-2 infection was relatively high among private caregivers, indicating a need for infection control education in this group. The study results reinforce the importance of periodic screening of HCWs and routine surveillance of patients and caregivers and for the direction of future research of nosocomial infections.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Taipei City Hospital, 0000000316458457.
Specialty type: Health care sciences and services
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Li J, China; Lomeli SM, Mexico; Paparoupa M, Germany S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Viswanathan M, Kahwati L, Jahn B, Giger K, Dobrescu AI, Hill C, Klerings I, Meixner J, Persad E, Teufer B, Gartlehner G. Universal screening for SARS-CoV-2 infection: a rapid review. Cochrane Database Syst Rev. 2020;9:CD013718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 2. | World Health Organization (WHO). WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/. Accessed January 19, 2021.. [RCA] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 293] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 3. | Cheng CC, Liu CC, Yen JC, Chiu TF, Liu YN, Hsueh TY, Hsiao SH. Systematic Screening for SARS-CoV-2 to Detect Asymptomatic Infections: An Epitome of Taiwan's Outbreak. Can J Infect Dis Med Microbiol. 2022;2022:6441339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Carter B, Collins JT, Barlow-Pay F, Rickard F, Bruce E, Verduri A, Quinn TJ, Mitchell E, Price A, Vilches-Moraga A, Stechman MJ, Short R, Einarsson A, Braude P, Moug S, Myint PK, Hewitt J, Pearce L, McCarthy K; COPE Study Collaborators. Nosocomial COVID-19 infection: examining the risk of mortality. The COPE-Nosocomial Study (COVID in Older PEople). J Hosp Infect. 2020;106:376-384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 5. | Chow EJ, Schwartz NG, Tobolowsky FA, Zacks RLT, Huntington-Frazier M, Reddy SC, Rao AK. Symptom Screening at Illness Onset of Health Care Personnel With SARS-CoV-2 Infection in King County, Washington. JAMA. 2020;323:2087-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 127] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 6. | Elkrief A, Desilets A, Papneja N, Cvetkovic L, Groleau C, Lakehal YA, Shbat L, Richard C, Malo J, Belkaid W, Cook E, Doucet S, Tran TH, Jao K, Daaboul N, Bhang E, Loree JM, Miller WH Jr, Vinh DC, Bouganim N, Batist G, Letendre C, Routy B. High mortality among hospital-acquired COVID-19 infection in patients with cancer: A multicentre observational cohort study. Eur J Cancer. 2020;139:181-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 7. | Luong-Nguyen M, Hermand H, Abdalla S, Cabrit N, Hobeika C, Brouquet A, Goéré D, Sauvanet A. [Nosocomial infection with SARS-Cov-2 within departments of digestive surgery]. J Chir Visc. 2020;157:S13-S19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Harada S, Uno S, Ando T, Iida M, Takano Y, Ishibashi Y, Uwamino Y, Nishimura T, Takeda A, Uchida S, Hirata A, Sata M, Matsumoto M, Takeuchi A, Obara H, Yokoyama H, Fukunaga K, Amagai M, Kitagawa Y, Takebayashi T, Hasegawa N. Control of a Nosocomial Outbreak of COVID-19 in a University Hospital. Open Forum Infect Dis. 2020;7: ofaa512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Lai X, Zhou Q, Zhang X, Tan L. What influences the infection of COVID-19 in healthcare workers? J Infect DevCtries. 2020; 14:1231-1237.. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Casanova L, Rutala WA, Weber DJ, Sobsey MD. Coronavirus survival on healthcare personal protective equipment. Infect Control Hosp Epidemiol. 2010;31:560-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Repici A, Maselli R, Colombo M, Gabbiadini R, Spadaccini M, Anderloni A, Carrara S, Fugazza A, Di Leo M, Galtieri PA, Pellegatta G, Ferrara EC, Azzolini E, Lagioia M. Coronavirus (COVID-19) outbreak: what the department of endoscopy should know. Gastrointest Endosc. 2020;92:192-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 373] [Cited by in RCA: 380] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 12. | Chou MC, Chung CT, Chiu TF, Chang YS, Cheng CC. Effectiveness of social distancing to prevent COVID-19 transmission. Taipei City Medical Journal. 2021;18:374-384. [DOI] [Full Text] |
| 13. | Höring S, Fussen R, Neusser J, Kleines M, Laurentius T, Bollheimer LC, Keller D, Lemmen S. Management of a Hospital-Wide COVID-19 Outbreak Affecting Patients and Healthcare Workers. SN Compr Clin Med. 2020;2:2540-2545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Wee LE, Hsieh JYC, Phua GC, Tan Y, Conceicao EP, Wijaya L, Tan TT, Tan BH. Respiratory surveillance wards as a strategy to reduce nosocomial transmission of COVID-19 through early detection: The experience of a tertiary-care hospital in Singapore. Infect Control Hosp Epidemiol. 2020;41:820-825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 15. | Duy C, Nong VM, Van Ngo A, Doan TT, Nguyen TQ, Truong PT, Olson L, Larsson M. Nosocomial Coronavirus Disease Outbreak Containment, Hanoi, Vietnam, March-April 2020. Emerg Infect Dis. 2021;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Lee U, Kim SE, Lee SY, Wi HN, Choi O, Park JW, Kim D, Kim YJ, Shin HY, Kim M, Kim EJ, Kang SJ, Jung SI, Park KH. Source Analysis and Effective Control of a COVID-19 Outbreak in a University Teaching Hospital during a Period of Increasing Community Prevalence of COVID-19. J Korean Med Sci. 2021;36: e179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Asad H, Johnston C, Blyth I, Holborow A, Bone A, Porter L, Tidswell P, Healy B. Health Care Workers and Patients as Trojan Horses: a COVID19 ward outbreak. Infect Prev Pract. 2020;2:100073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |