Published online Dec 6, 2022. doi: 10.12998/wjcc.v10.i34.12500
Peer-review started: July 23, 2022
First decision: September 26, 2022
Revised: October 9, 2022
Accepted: November 7, 2022
Article in press: November 7, 2022
Published online: December 6, 2022
Processing time: 132 Days and 1.4 Hours
Helicobacter pylori (H. pylori) is the most important infectious agent and plays an important role in the progression of chronic gastritis and the development of gastric cancer.
To identify efficient therapeutic agents or strategies that can treat H. pylori infection.
We performed literature analysis, experimental validation, and network pharmacology. First, traditional Chinese medicine (TCM) prescriptions for the treatment of H. pylori infection were obtained from the China National Knowledge Infrastructure, China Biology Medicine, China Science and Technology Journal Database, and WanFang databases. In addition, we conducted a relevant search by Reference Citation Analysis (RCA) (https://www.referencecitationanaly
The TCM treatment of H. pylori was mainly based on reinforcing the healthy Qi and eliminating pathogenic factors by simultaneously applying pungent dispersing, bitter descending, cold and warm drugs. The combination of Coptis, Pinellia, and Scutellaria (CPS) was identified as the core drug combination from 207 prescriptions and 168 herbs. This drug combination eradicated H. pylori, alleviated the gastric pathology induced by H. pylori infection, and reduced the expression levels of tumor necrosis factor-α (P = 0.024) and interleukin-1β (P = 0.001). Moreover, a total of 35 compounds and 2807 targets of CPS were identified using online databases. Nine key compounds (tenaxin I, neobaicalein, norwogonin, skullcapflavone II, baicalein, 5,8,2'-trihydroxy-7-methoxyflavone, acacetin, panicolin, and wogonin) and nine hub target proteins (EGFR, PTGS2, STAT3, MAPK3, MAPK8, HSP90AA1, MAPK1, MMP9, and MTOR) were further explored. Seventy-seven signaling pathways were correlated with H. pylori-induced inflammation and carcinogenesis.
In summary, we showed that CPS is the core drug combination for treating H. pylori infection. Animal experiments demonstrated that CPS has bacteriostatic properties and can reduce the release of inflammatory cytokines in the gastric mucosa. Network pharmacology predictions further revealed that CPS showed complex chemical compositions with multi-target and multi-pathway regulatory mechanisms. Although the results derived from network pharmacology are not necessarily comprehensive, they still expand our understanding of CPS for treating H. pylori infection.
Core Tip: Infection with Helicobacter pylori (H. pylori) is associated with severe digestive diseases, while successful eradication of this pathogen is known to prevent the occurrence of peptic ulcer disease and gastric cancer. Traditional Chinese medicine (TCM) has unique advantages for treating H. pylori infection, which can reduce drug resistance and increase the eradication rate of H. pylori. Here, we attempted to identify medication rules, effective materials, and the molecular mechanisms underlying the therapeutic effect of TCM against H. pylori using data mining, in vivo experiment, and network pharmacology. Coptis, Pinellia, and Scutellaria (CPS) were identified as the core drug combination for H. pylori eradication. We evidenced that CPS eradicated H. pylori, alleviated the gastric pathology, and reduced circulating tumor necrosis factor-α and interleukin-1β levels. Nine key compounds and nine hub target proteins were further determined as the key active ingredients and therapeutic targets of CPS against H. pylori, respectively. Altogether, our data strongly suggest that the efficacy of CPS in the treatment of H. pylori is worthy of affirmation.
- Citation: Yu Z, Sheng WD, Yin X, Bin Y. Coptis, Pinellia, and Scutellaria as a promising new drug combination for treatment of Helicobacter pylori infection . World J Clin Cases 2022; 10(34): 12500-12514
- URL: https://www.wjgnet.com/2307-8960/full/v10/i34/12500.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i34.12500
Worldwide, the most common cause of chronic gastritis is infection with Helicobacter pylori (H. pylori)[1]. H. pylori-induced gastritis is considered an independent disease and is commonly referred to as H. pylori-associated gastritis (HAG)[2,3]. H. pylori infection leads to progressive damage to the gastric mucosa by its virulence factors, host proinflammatory cytokines release, and reactive oxygen species[4]. Mucosal damage can progress to gastritis, peptic ulcers, gastric mucosa-associated lymphoid tissue lymphoma, and even gastric carcinoma[5]. According to cancer statistics provided by GLOBOCAN 2018, H. pylori is the most important infectious organism and has been responsible for 810000 new cancer cases in 2018[6]. Considering that H. pylori is the major cause of gastric carcinoma, the World Health Organization classifies H. pylori as a class I carcinogen[7,8]. The discovery of H. pylori led to increased research to uncover the etiology, natural history, and prognosis of HAG[9,10]. The Kyoto Global Consensus Report on HAG recommended that all individuals with H. pylori infection should receive eradication therapy to prevent gastric cancer[1]. However, it has been well established that eradicating H. pylori is challenging[11]. The main treatment for H. pylori infection is antibiotics, usually the combination of two types of antibiotics in conjunction with a proton pump inhibitor[12]. Antibiotic resistance of H. pylori is increasing due to the extensive use (or abuse) of antibiotics as the leading cause of treatment failure[13,14]. Current treatments are not sufficiently effective; therefore, there are significant attempts to identify more effective drugs or substances that can stop or inhibit H. pylori infection.
Over the last few decades, there has been a significant increase in the use of traditional Chinese medicine (TCM) as a form of complementary and alternative medicine[15]. TCM has unique advantages for treating H. pylori infection and has been widely used due to its remarkable efficacy. Consequently, a large body of clinical and experimental literature has accumulated[16-18]. Therefore, it is possible to apply data mining techniques to the existing literature and analyze specific herbal prescriptions to identify potential associations and core herbs. Here, we attempted to identify medication rules, effective materials, and the molecular mechanisms underlying the therapeutic effect of TCM against H. pylori using data mining and network pharmacology. Our findings will provide a theoretical basis for further research.
Data source and normalization: We collated data by searching key databases, including the China National Knowledge Infrastructure, China Biology Medicine disc, China Science and Technology Journal Database, and WanFang databases for articles related to the use of TCM for H. pylori infection. We eliminated studies that did not meet our specific requirements, including reviews, proven reports, and animal experiments. Next, we extracted syndrome types, prescriptions, and herbs from the literature. Traditional Chinese Medicine Inheritance Support System V2.5 (TCMISS V2.5) was used to perform prescription analysis. This system was designed especially for data mining and integrates statistical methods such as association rules analysis and complex system entropy methods. Herbs were classified according to the standard name and Chinese medicine classification provided by People's Republic of China Pharmacopoeia (2015 Edition)[19]. In addition, we conducted a relevant search by Reference Citation Analysis (RCA) (https://www.referencecitationanalysis.com).
Data processing and analysis: Frequency analysis, association rules algorithms, and complex system entropy clustering analysis methods were integrated with TCMISS V2.5 and were applied for data analysis and mining. Details related to herbs and their characteristics, flavors, meridian tropisms, and medication modes were summarized. High-frequency herbs, along with the association rules method, were used to identify core drug combinations.
Materials: BALB/c mice of either sex (aged 5 wk; n = 40) were purchased from Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China; qualification certificate number: SCXK (XIANG) 2016-0002). Animals had free access to sterilized standard food and water and were kept in 12:12 h light to dark cycles and standard conditions. The H. pylori Sydney strain was provided by Dr. Wu Canrong of the Hunan University of Traditional Chinese Medicine (Changsha). CPS was prepared from three herbs (Coptis 6 g, Pinellia 10 g, and Scutellaria 10 g) and used in the following manner. CPS was extracted with water. The concentration of the water extract was 374.99 mg/mL. Quadruple therapy (QT) included esomeprazole (20 mg bid, Chongqing, China), amoxicillin (1000 mg bid, Shanxi, China), clarithromycin (500 mg bid, Guangdong, China), and colloidal bismuth pectin (300 mg bid, Hunan, China). The QT suspension was given by oral gavage, and the suspension concentration was 26.25 mg/mL.
H. pylori culture: The H. pylori Sydney strain (SS1, a CagA+ and VacA+ strain) was inoculated on 10% sheep blood-containing Brucella agar (HOPEBIO, QingDao, China) plates and placed in a culture box (ZheJiang, China) with the following settings: 37℃, 5% O2, 10% CO2, and 85% N2. After 72 h of culture, H. pylori was identified by the unaided visualization of bacterial morphology[20].
Forty mice were randomly assigned to a negative control (NC) group (n = 10) and an infection model group (n = 30). All mice were fasted for 12 h before inoculation. On day 1, mice in the infection model group were intra-gastrically inoculated with a suspension of H. pylori containing 1 × 109 CFUs/mL using gastric intubation needles. The NC group was given an equal volume (200 μL) of normal saline. Inoculation was performed every 2 d and five times in total. H. pylori colonized for 2 wk after the last inoculation. On day 23, three mice from each group were randomly anesthetized with an intraperitoneal injection of pentobarbital and sacrificed by cervical dislocation. Stomach tissues from the greater curvature were harvested and washed in ice-cold normal saline. The rapid urease test (RUT) and Warthin-Starry silver staining were performed to identify infection and confirm the successful establishment of the infection. The animal experiment was approved by the Animal Ethics Committee of Hunan University of Traditional Chinese Medicine (ZYFY20190620). After induction, mice in the infection model were randomly assigned to a vehicle-treated group, a QT-treated group, and a CPS-treated group (n = 9). Treatment began on day 24, and mice (n = 7-9) received a daily dose of CPS (3749.86 mg/kg), QT (262.49 mg/kg), or vehicle (0.9% normal saline) for 2 wk and were subsequently sacrificed.
Measurements of serum cytokine levels: Serum levels of key cytokines tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) were measured using enzyme linked immunosorbent assay (ELISA) kits for IL-1β (Boster Wuhan, China) and TNF-α (Boster Wuhan, China). Measurements were done according to the manufacturer’s recommendations.
Histopathological examinations: Mice were sacrificed, and their stomachs were excised and kept in 10% phosphate buffered saline-buffered formalin. Fixed gastric tissues were embedded in paraffin, longitudinally cut into 3 μm sections, and stained with hematoxylin and eosin (H&E).
Active compounds and targets of CPS: TCMISP (http://Lsp.nwsuaf.edu.cn/tcmsp.pHp) can be used to analyze the drug molecule-target network and drug-target-disease network. It can help unfold the underlying mechanisms of herbs[21]. The active compounds of CPS and their targets were obtained in TCMSP. The obtained two-dimensional structures of the bioactive compounds in the PubChem (https://pubchem.ncbi.nlm.nih.gov/) database were then imported into Swiss Target Prediction (http://www.swisstargetprediction.ch/) to identify their targets.
Identifying gene targets associated with HAG: The term “Helicobacter pylori-associated gastritis” was used as a search term in the GeneCards database (http://www.genecards.org/) and OMIM database (http://www.omim.org/). Then genes, signaling pathways, and other items of key information related to HAG were identified. The targets generated by the intersection between HAG-related targets and the predicted targets of CPS were then identified as HAG targets of CPS.
Construction of a protein-protein interaction network: The HAG targets of CPS were submitted to the STRING database (https://string-db.org/), which has information related to protein interactions. Key protein information was then imported into Cytoscape to construct a protein-protein interaction (PPI) network and merge with an herbs-compounds-targets network[22]. The size of a node represents its degree value. The larger diameter of the node shows greater degrees.
Gene ontology and pathway enrichment analysis: To evaluate the role of targets by bioinformatics annotation, the HAG targets of CPS were imported into the DAVID database (https://david.ncifcrf.
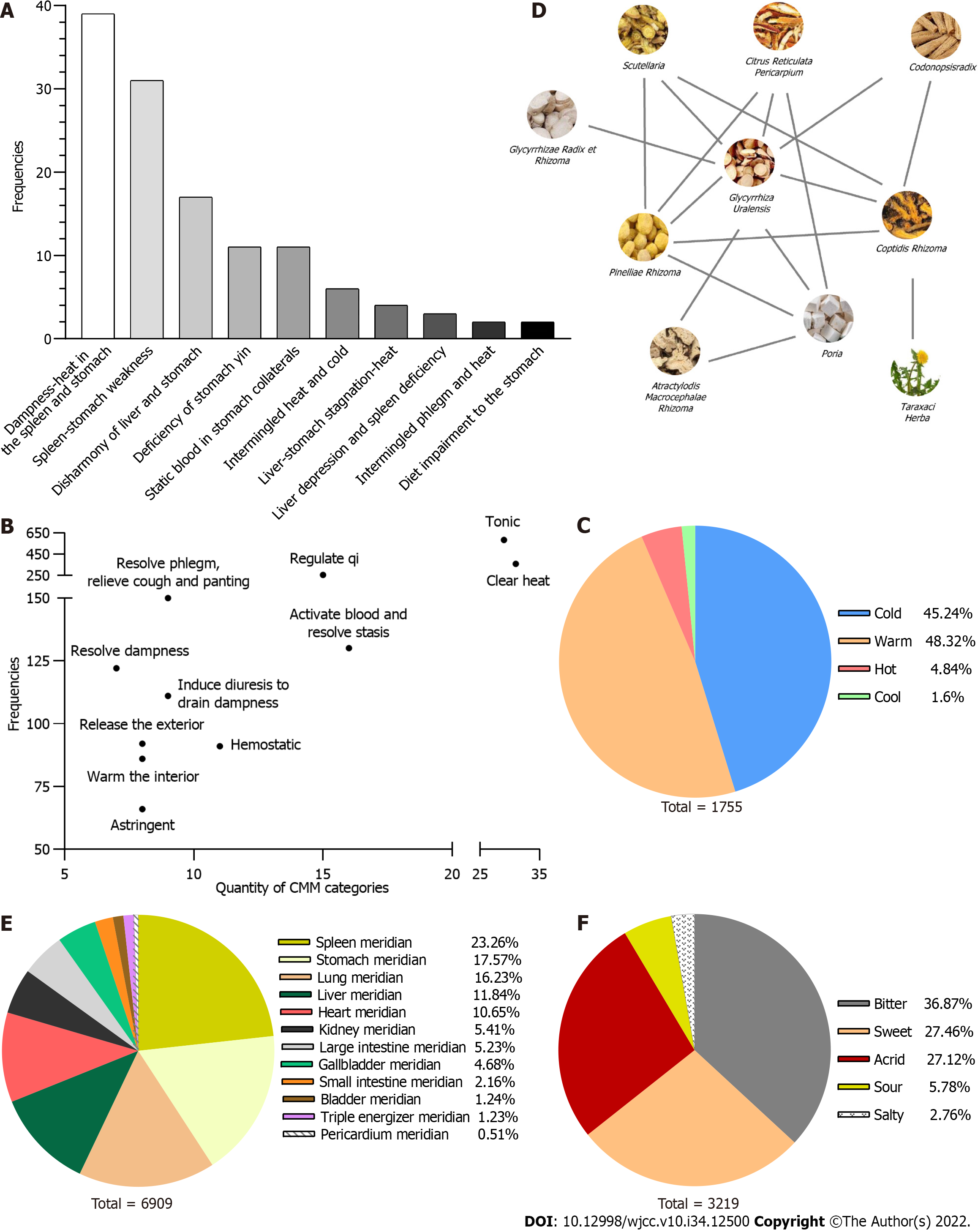
Distribution of TCM syndrome types: Of 178 articles identified in the first step, we identified 77 clear TCM syndrome types. Ten TCM syndrome types were evident in 77 articles; spleen-stomach damp-heat syndrome, spleen-stomach weakness syndrome, and liver-stomach discordance syndrome appeared more frequently, with a cumulative frequency of 69.05% (Figure 1A).
Features and properties of herbs for HAG: TCMISS V2.5 was employed to analyze the frequencies of all herbs. In total, 168 herbs with a total of 2139 using times were identified. Glycyrrhizae Radix et Rhizoma, Coptidis Rhizoma, and Pinelliae Rhizoma had the top three frequencies (Table 1). According to their properties for HAG, the herbs were classified into 19 categories, and their frequencies of use are shown in Figure 1B. The nature and flavor of the 168 herbs were mostly cold, warm, bitter, and spicy. Their meridian tropism into the spleen, stomach, and lungs accounted for the largest proportion of applications with a cumulative frequency of 57.06% (Figure 1C, E and F).
| Herb | Frequency | Herb | Frequency | Herb | Frequency |
| Glycyrrhizae Radix et Rhizoma | 154 | Aucklandiae Radix | 40 | Citri Sarcodactylis Fructus | 14 |
| Coptidis Rhizoma | 121 | Magnoliae Officinalis Cortex | 40 | Gardeniae Fructus | 14 |
| Pinelliae Rhizoma | 100 | Salviae Miltiorrhizae Radix et Rhizoma | 38 | Rehmanniae Radix | 13 |
| Paeoniae Radix Alba | 82 | Notoginseng Radix et Rhizoma | 36 | Hordei Fructus Germinatus | 13 |
| Scutellariae Radix | 79 | Bletillae Rhizoma | 35 | Perillae Fructus | 13 |
| Pericarpium Citri Reticulatae | 78 | Jujubae Fructus | 35 | Toosendan Fructus | 13 |
| Atractylodis Macrocephalae Rhizoma | 75 | Aurantii Fructus | 33 | Lilii Bulbus | 12 |
| Codonopsis Radix | 73 | Euodiae Fructus | 24 | Linderae Radix | 12 |
| Poria | 73 | Aurantii Fructus Immaturus | 23 | Faeces Trogopterpri | 12 |
| Taraxaci Herba | 65 | Cyperi Rhizoma | 22 | Atractylodis Rhizoma | 12 |
| Astragali Radix | 56 | Cinnamomi Ramulus | 19 | Angelicae Sinensis Radix | 11 |
| Sepiae Endoconcha | 54 | Coicis Semen | 18 | Crataegi Fructus | 11 |
| Zingiberis Rhizoma | 49 | Rhei Radix et Rhizoma | 16 | Typhae Pollen | 11 |
| Corydalis Rhizoma | 43 | Arcae Concha | 15 | Amomi Fructus Rotundus | 11 |
| Amomi Fructus | 41 | Hedyotis Diffusa | 14 | Pseudostellariae Radix | 11 |
| Bupleuri Radix | 41 | Fritillariae Thunbergii Bulbus | 14 | Ginseng Radix et Rhizoma | 10 |
Drug combinations and association rules: The support degree was set to ≥ 45[11]. We identified 21 drug combinations using this setting, as shown in Table 2. The top four drug combinations were {Coptidis Rhizoma, Glycyrrhizae Radix et Rhizoma}, {Pinelliae Rhizoma, Coptidis Rhizoma}, {Pinelliae Rhizoma, Glycyrrhizae Radix et Rhizoma}, and {Scutellariae Radix, Coptidis Rhizoma}. These 21 drug combinations were matched by 10 herbs: Coptidis Rhizoma, Pinelliae Rhizoma, Scutellariae Radix, Glycyrrhizae Radix et Rhizoma, Pericarpium Citri Reticulatae, Atractylodis Macrocephalae Rhizoma, Codonopsis, Radix Poria, Taraxaci Herba, and Paeoniae Radix Alba. Figure 1D shows a map of the drug combination relationships. The confidence level was set to ≥ 70%. This led to the identification of 20 correlation rules (Table 3). Asso
| Drug combination | Frequency | Drug combination | Frequency |
| Coptidis Rhizoma, Glycyrrhizae Radix et Rhizoma | 88 | Glycyrrhizae Radix et Rhizoma, Poria | 55 |
| Pinelliae Rhizoma, Glycyrrhizae Radix et Rhizoma | 76 | Scutellariae Radix, Coptidis Rhizoma, Glycyrrhizae Radix et Rhizoma | 54 |
| Pinelliae Rhizoma, Glycyrrhizae Radix et Rhizoma | 74 | Pinelliae Rhizoma, Scutellariae Radix and Coptidis Rhizoma | 52 |
| Scutellariae Radix, Coptidis Rhizoma | 69 | Pericarpium Citri Reticulatae, Poria | 47 |
| Glycyrrhizae Radix et Rhizoma, Paeoniae Radix Alba | 68 | Coptidis Rhizoma, Taraxaci Herba | 46 |
| Scutellariae Radix, Glycyrrhizae Radix et Rhizoma | 61 | Pinelliae Rhizoma, Pericarpium Citri Reticulatae | 46 |
| Codonopsis Radix, Glycyrrhizae Radix et Rhizoma | 60 | Pinelliae Rhizoma, Poria | 46 |
| Pinelliae Rhizoma, Coptidis Rhizoma and Glycyrrhizae Radix et Rhizoma | 57 | Pinelliae Rhizoma, Scutellariae Radix, Glycyrrhizae Radix et Rhizoma | 45 |
| Pinelliae Rhizoma, Scutellariae Radix | 57 | Atractylodis Macrocephalae Rhizoma, Poria | 45 |
| Pericarpium Citri Reticulatae, Glycyrrhizae Radix et Rhizoma | 56 | Coptidis Rhizoma, Codonopsis Radix | 45 |
| Atractylodis Macrocephalae Rhizoma, Glycyrrhizae Radix et Rhizoma | 56 | - | - |
| Association rules | Confidence | Association rules | Confidence |
| Pinelliae Rhizoma - Scutellariae Radix → Coptidis Rhizoma | 0.9123 | Pinelliae Rhizoma - Glycyrrhizae Radix et Rhizoma → Coptidis Rhizoma | 0.7703 |
| Scutellariae Radix - Glycyrrhizae Radix et Rhizoma → Coptidis Rhizoma | 0.8852 | Atractylodis Macrocephalae Rhizoma → Glycyrrhizae Radix et Rhizoma | 0.7568 |
| Scutellariae Radix → Coptidis Rhizoma | 0.8734 | Pinelliae Rhizoma → Glycyrrhizae Radix et Rhizoma | 0.7551 |
| Codonopsis Radix → Glycyrrhizae Radix et Rhizoma | 0.8219 | Scutellariae Radix - Coptidis Rhizoma → Pinelliae Rhizoma | 0.7536 |
| Paeoniae Radix Alba → Glycyrrhizae Radix et Rhizoma | 0.8000 | Pinelliae Rhizoma - Coptidis Rhizoma → Glycyrrhizae Radix et Rhizoma | 0.7500 |
| Pinelliae Rhizoma - Scutellariae Radix → Glycyrrhizae Radix et Rhizoma | 0.7895 | Scutellariae Radix - Glycyrrhizae Radix et Rhizoma → Pinelliae Rhizoma | 0.7377 |
| Scutellariae Radix - Coptidis Rhizoma → Glycyrrhizae Radix et Rhizoma | 0.7826 | Coptidis Rhizoma → Glycyrrhizae Radix et Rhizoma | 0.7273 |
| Pinelliae Rhizoma → Coptidis Rhizoma | 0.7755 | Scutellariae Radix → Pinelliae Rhizoma | 0.7215 |
| Poria → Glycyrrhizae Radix et Rhizoma | 0.7746 | Pericarpium Citri Reticulatae → Glycyrrhizae Radix et Rhizoma | 0.7089 |
| Scutellariae Radix → Glycyrrhizae Radix et Rhizoma | 0.7722 | Taraxaci Herba → Coptidis Rhizoma | 0.7077 |
These drug combinations and association rules were related to four herbs: Coptidis Rhizoma, Glycyrrhizae Radix et Rhizoma, Pinelliae Rhizoma zi, and Scutellariae Radix. Since Glycyrrhizae Radix et Rhizoma is used as a courier medicinal in prescriptions, we demonstrated that Coptis, Pinellia, and Scutellaria represented the core drug combination for HAG treatment.
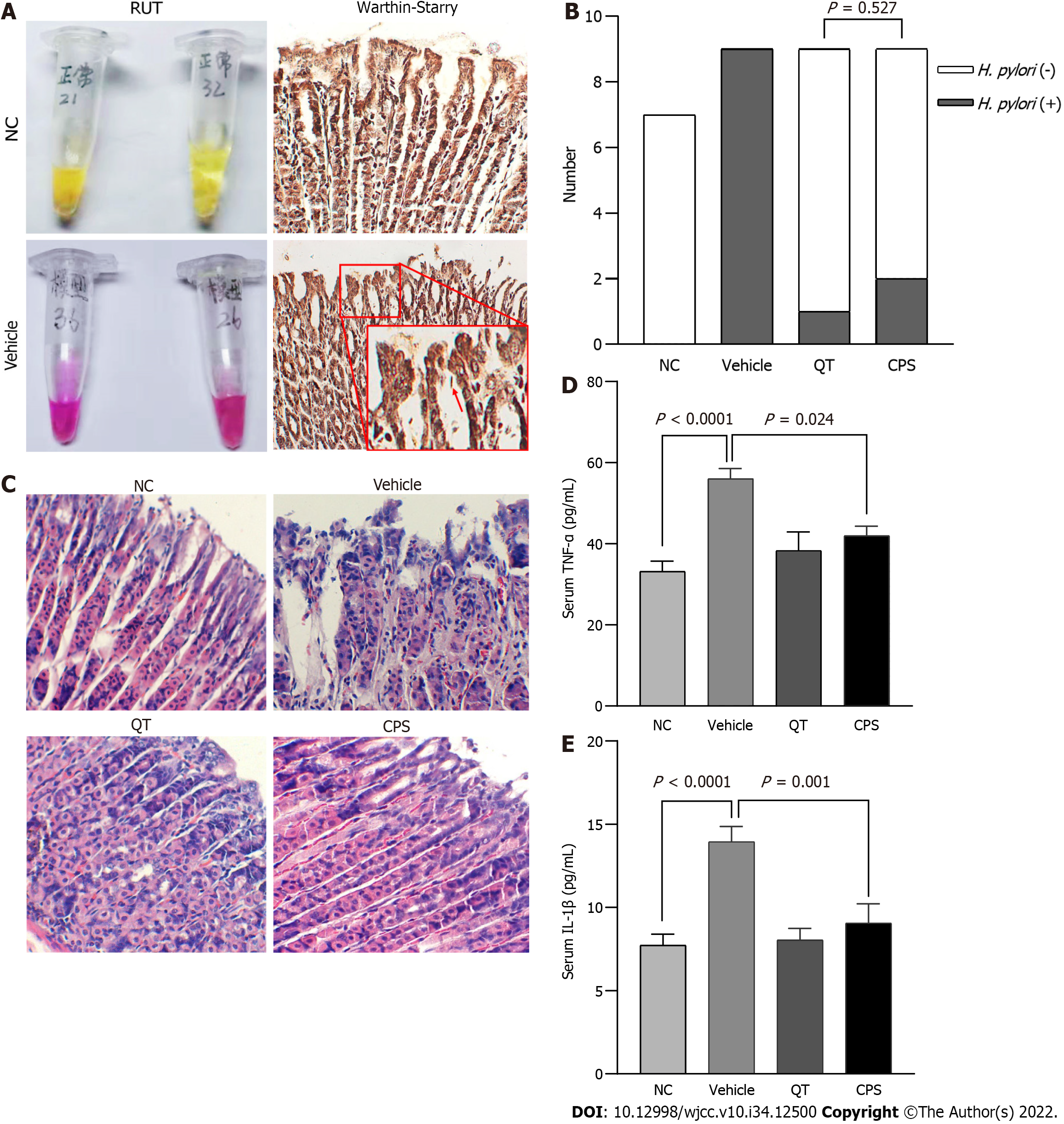
Confirmation and analysis of the model: The results of RUT showed that within 30 min, the colors of the reaction solution in the NC group were yellow. In contrast, the solutions in the infection model group all became red (Figure 2A). These results demonstrated that bacteria produced urease in the stomachs of infected mice. In addition, Warthin-Starry silver staining showed that silver ions had been adsorbed and reduced to arc or spiral black metallic silver by the argyrophilic bacterium in the gastric mucosa of infected mice (Figure 2A). Collectively, RUT and silver staining demonstrated that mice in the infection model group had been successfully infected with H. pylori.
Eradication rate of H.pylori: To understand the role of CPS in eradicating H. pylori, we assessed the eradication rate of H. pylori after 2 wk of treatment. RUT results showed that the H. pylori eradication rates for QT-treated mice and CPS-treated mice exceeded 80%. There was no statistical difference between the groups in this regard (Figure 2B). These data indicate that CPS had a positive eradication effect on H. pylori.
CPS inhibits the inflammatory response induced by H.pylori: CPS was administered after H. pylori infection. Then, the anti-inflammatory effects of CPS on HAG mice were investigated by H&E staining and ELISA. Pathological analysis showed that the gastric mucosa of the NC mice was intact, characterized by normal glands without inflammatory cell infiltration and congestion. The vehicle-treated mice showed crypt destruction, abnormal glands, and variable amounts of lymphocytic infiltration in the epithelium and lamina propria (Figure 2C). ELISA analysis further confirmed high expression levels of TNF-α and IL-1β after H. pylori infection. CPS significantly reduced TNF-α levels (Figure 2D-E). Collectively, these results demonstrate that CPS significantly inhibited the inflammatory response induced by H. pylori.
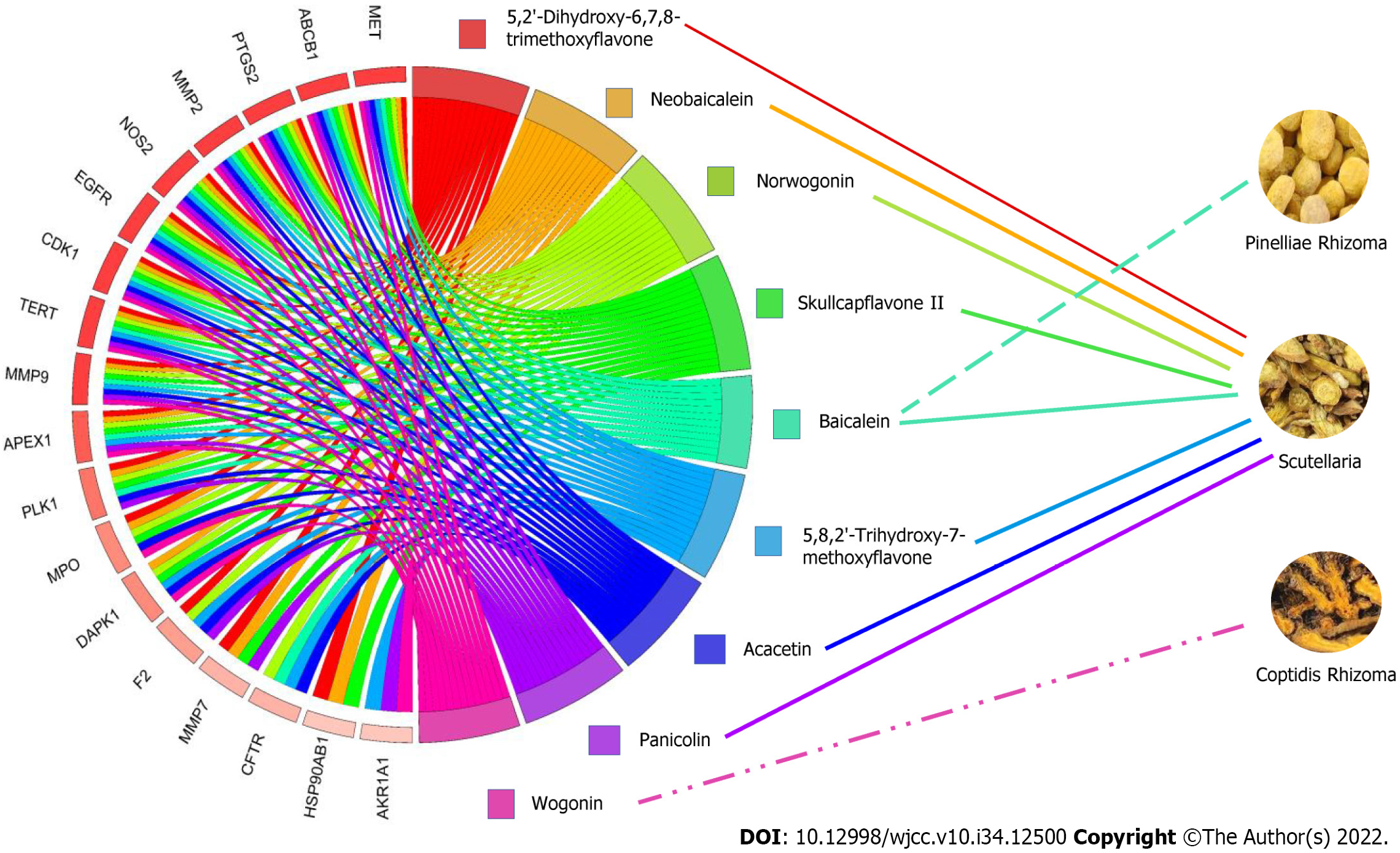
Identification of CPS active compounds and predicted targets: The 40 main active compounds (including 5 duplicates) and 2806 targets of CPS were predicted by TCMSP, SwissTarget Predicted, Gene Cards, and OMIM. We identified 7, 9, and 24 compounds, along with 399, 499, and 1908 predicted targets in Coptis, Pinellia, and Scutellaria, respectively (Table 4). To demonstrate the relationship between herbs, compounds, and targets, we constructed a network diagram showing CPS, nine compounds with a degree ≥ 14, and their predicted targets (Figure 3).
| Herb | Compounds |
| Coptidis Rhizoma | Berberine; Berberrubine; (R)-Canadine; Berlambine; Worenine; Coptisine; Epiberberine |
| Pinelliae Rhizoma | Coniferin; Cavidine; Cycloartenol; Gondoic acid; 24-Ethylcholest-4-en-3-one; 10,13-eicosadienoic; Baicalein; Beta-sitosterol; Stigmasterol |
| Scutellariae Radix | Acacetin; Wogonin; Rivularin; Salvigenin; Norwogonin; Panicolin; Sitosterol; Oroxylina; Skullcapflavone II; Neobaicalein; Moslosooflavone; 11,13-Eicosadienoic acid; Methyl ester; Dihydrooroxylin A; Dihydrobaicalin_qt; 5,2‘,6’-Trihydroxy-7,8-dimethoxyflavone; 5,8,2‘-Trihydroxy-7-methoxyflavone; Dihydrooroxylin; (2R)-7-hydroxy-5-methoxy-2-phenylchroman-4-one; Tenaxin I; Coptisine; Epiberberine; Baicalein; Beta-sitosterol; Stigmasterol |
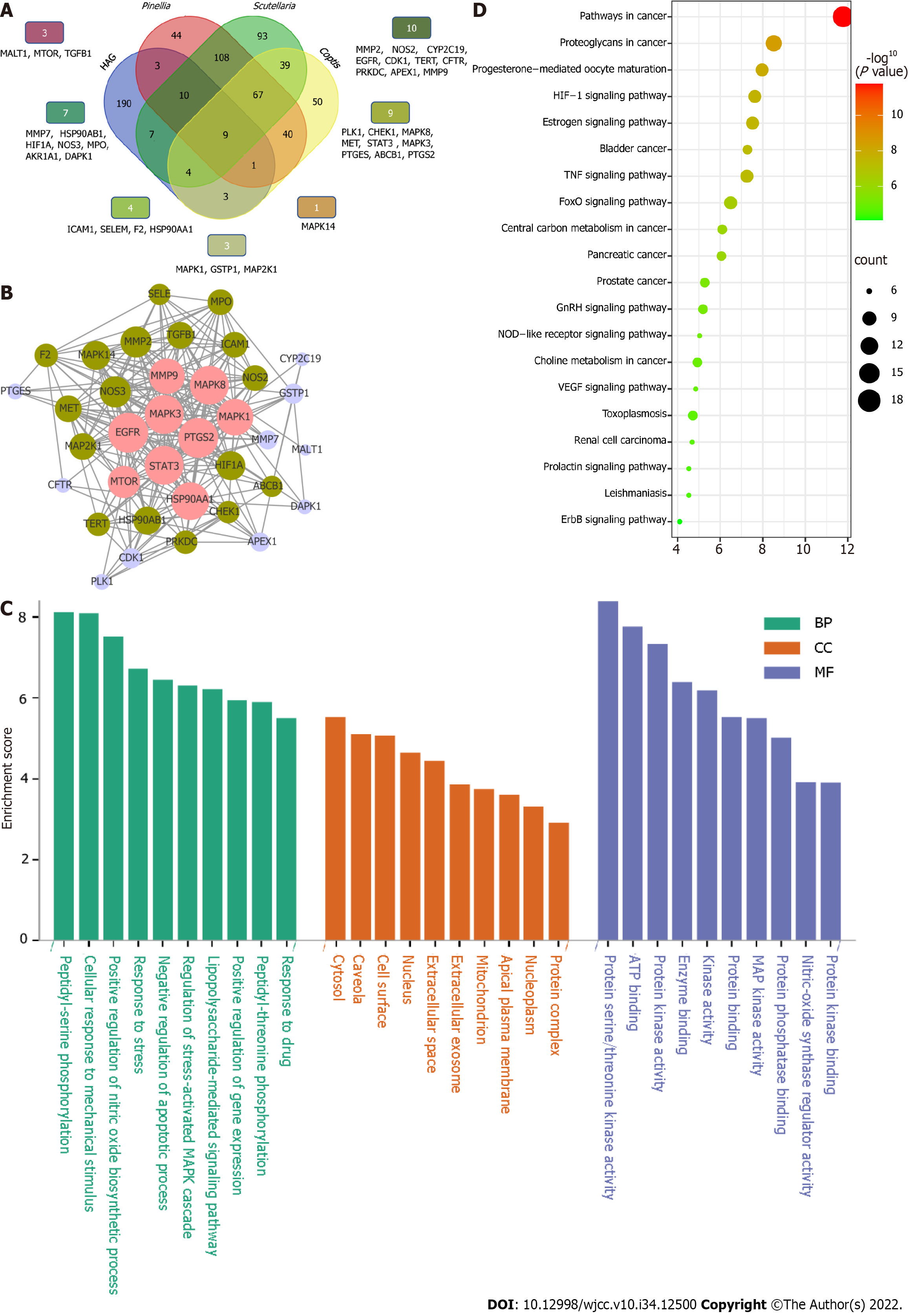
Identification of potential targets of CPS involved in HAG: In total, we identified 277 proteins that may be closely related to the occurrence and development of HAG. A total of 37 potential targets of CPS acting on HAG were identified by intersection analysis (Figure 4A). Next, we used STRING to generate a PPI network diagram for interactions between 37 potential targets of CPS involved in HAG. The PPI network diagram contained 37 nodes, 262 edges, and 9 target proteins with a degree ≥ 20: EGFR, PTGS2, STAT3, MAPK3, MAPK8, HSP90AA1, MAPK1, MMP9, and MTOR. Collectively, these results indicated that CPS might have a therapeutic effect on HAG by affecting these target proteins (Figure 4B).
Functional analysis of predicted targets of CPS: The 37 predicted targets of CPS were imported into the DAVID database. We set the significance to P < 0.05 for analysis. In this study, 119 GO terms were obtained, including 58 biological processes, 25 cellular components, and 36 molecular functions. The top 10 GO enrichment analyses were performed individually and were ranked by a combined score (Figure 4C). The targets of CPS acting on HAG were distributed in various cellular components, mainly in the cytoplasm. They participated in biological processes by various binding methods, including peptidyl-serine phosphorylation, positive regulation of the nitric oxide biosynthesis, regulation of the stress-activated MAPK cascade, and negative regulation of apoptosis. In addition, 77 of the most representative gene enrichments were involved in signaling pathways, such as cancer-related pathways, HIF-1 signaling pathway, estrogen signaling pathway, TNF signaling pathway, FoxO signaling pathway, and central carbon metabolism. The top 20 KEGG pathways were then visualized as a bubble plot (Figure 4D).
H. pylori is a major driver of gastric cancer[24]. Over the last three decades, there has been a broad paradigm shift in our understanding of gastric cancer prevention. Efforts to identify agents or strategies capable of eradicating H. pylori remain a top priority in preventing gastric cancer.
TCM has potential advantages for treating H. pylori infection. TCM increases the eradication rate and reduces drug resistance[25]. According to statistics, the success rate of treatment with TCM for H. pylori infection can reach 95.45%[26]. According to TCM theory, H. pylori is caused by "damp-heat pathogenic Qi" or "toxins from pathogenic bacteria". Splenic and gastric dysfunction due to H. pylori infection often causes phlegm-turbidity, heat stagnation, and blood stasis. They can also lead to several pathological changes, including gastric atrophy and intestinal metaplasia. A previous cross-sectional study conducted in four hospitals in China found that phlegm turbidity and heat play a crucial role in the pathogenesis of chronic atrophic gastritis[27]. Using metabonomics technology, Xu et al[28] demon
Based on the frequent use of herbs and association rules, the core drug combination was Coptis, Pinellia, and Scutellaria in this study. There are many examples of the remarkable therapeutic effects of Coptis, Pinellia, and Scutellaria supported by previous studies. Ma Feng et al conducted a susceptibility test for 50 selected traditional Chinese herbal medicines using a broth dilution method. They found that water extract from Rhizoma Coptis and Scutellaria could significantly inhibit the activity of H. pylori[31]. A recent meta-analysis showed that the Banxia Xiexin Decoction, using Pinellia as a monarch drug, had similar effects to standard therapy for eradicating H. pylori[32].
The active compounds of medicinal plants were extracted and analyzed using various analytical techniques to rationalize the use of TCM. Identifying the effective ingredients in these drugs may provide a basis for understanding their mechanisms of action and illuminate future research directions. Coptis contains several protoberberine alkaloids of berberine, palmatine, coptisine, epiberberine, and aporphinoid alkaloid of magnoflorine as the major pharmacologically active constituents[33,34]. These findings were consistent with the results of the present study. In vitro, Coptisine, epiberberine, and palmatine inhibited urease activity in a concentration-dependent manner, thereby effectively inhibiting H. pylori activity[35,36]. In vivo, berberine and palmatine exhibited H. pylori-inhibiting and antiulcerogenic properties by eliminating radical oxygen species[37]. Baicalin, baicalein, wogonin, and oroxylin were identified as the main active components of Radix Scutellariae[38,39]. However, only a few reports have been published discovering the active components of Radix Scutellariae. Lai et al[40] compared the metabolic pharmacokinetics of baicalin and baicalein in rats and found that baicalin absorption was slower and less efficient than baicalein absorption. Baicalin was not screened in this study. These results may be due to differences in study design. The flavonoid compounds (baicalin and baicalein) mainly found in medicinal plants have anti-inflammatory properties[41]. Chen et al[12] revealed that baicalin and baicalein both suppressed the expression of the VacA gene in H. pylori and interfered with the growth and virulence of H. pylori. These herbs also decreased H. pylori-induced expression of interleukin-8[42]. Pinellia is rich in flavonoids such as baicalin and baicalein[43]. An antimicrobial cerebroside, pinelloside, was previously isolated from Pinellia. This compound could inhibit the growth of Bacillus subtilis, Staphylococcus aureus, Aspergillus niger, and Candida albicans[44]. The present study demonstrated the inhibitory potential of the aqueous extract of CPS against H. pylori-induced gastric mucosal inflammation.
The constituents in the compound prescription showed delayed absorption and elimination and had a longer residence time in the body than a single herb decoction[45]. Therefore, compound prescriptions are more efficient and durable, making them promising candidates for in vivo studies. However, the multi-compound and multi-target approaches represent a significant obstacle to studying compound prescriptions. Network pharmacology provides a new research method for studying the network regulation effects of TCM[46].
EGFR, PTGS2, STAT3, MAPK3, MAPK8, HSP90AA1, MAPK1, MMP9, and MTOR were identified as the hub target proteins of CPS for HAG treatment. These targets mainly mediate H. pylori-induced inflammation and carcinogenesis. Multiple signaling pathways and receptors, including the ERK, JNK, JAK/STAT, and EGFR pathways, can be activated early post-infection and are involved in chemokine induction[47,48]. Matrix degradation by MMPs is critical for tumor cell invasion and metastasis. Pan et al indicated that H. pylori infection induces MMP9 expression by activating the ERK signaling pathway and increasing proliferation, growth, migration, and invasion of gastric cancer cells[49]. Aberrant activation of the PI3K/Akt/mTOR pathway modulates autophagy, epithelial-mesenchymal transition, apoptosis, chemoresistance, and metastasis in many human cancers and regulates the migration of gastric cancer cells[50].
In addition to identifying key inflammatory and oncogenic pathways, we also performed a KEGG pathway enrichment analysis. These analyses revealed that the CPS was related to pathways in cancer, proteoglycan synthesis in cancer, progesterone-mediated oocyte maturation, HIF-1 signaling pathway, estrogen signaling pathway, bladder cancer, and TNF signaling pathway. The antibacterial properties of estrogen and progestogen have been unfolded over recent years. A previous study found that feeding mice with estrogen alleviated H. pylori-induced gastric pathology[51]. In addition to estrogen, another steroid hormone, progestogen, was also found to exert bacteriolytic effects against H. pylori, stronger than those exhibited by estrogen[52]. In another study, Fong et al revealed the potential protective effect of oral contraceptives against H. pylori infection using the US National Health and Nutrition Examination Survey dataset[53]. A recent study found that the nuclear receptor estrogen-related receptor gamma (ESRRG) can affect H. pylori infection-driven gastric cancer. Mechanistically, activation of B cells (NF-κB) and p65 by H. pylori abrogates ESRRG-mediated activation of TFF1, a tumor suppressor[54]. Accumulating evidence links H. pylori infection with extra-gastric diseases, such as neurological, dermatological, hematological, ocular, cardiovascular, metabolic, and allergic diseases, although causal relationships must be considered[55,56]. The specific role of H. pylori in the patho
The literature search shows that CPS was the core drug for treating H. Pylori infection. Using an in vivo model, we revealed that CPS has bacteriostatic properties and can reduce inflammatory cytokine release and inflammation in the gastric mucosa. Network pharmacology predictions showed that CPS has complex chemical compositions and multi-target, multi-pathway regulatory mechanisms. Although our network pharmacology results are not extensive, our results still highlight the role of CPS in treating H. pylori. We also described the close association between an H. pylori-induced persistent chronic inflammatory state and gastric cancer progression and extra-gastric diseases. These findings can provide a basis to investigate the mechanisms of action for CPS in the future.
Infection with Helicobacter pylori (H. pylori) is associated with severe digestive diseases, while successful eradication of this pathogen is known to prevent the occurrence of peptic ulcer disease and gastric cancer.
H. pylori eradication has become more challenging over the past decade due to increasing antimicrobial resistance. Traditional Chinese medicine (TCM) has unique advantages for treating H. pylori infection, which can reduce drug resistance and increase the eradication rate of H. pylori.
To effectively treat H. pylori infection in the future, we need efficient therapeutic agents or strategies.
Here, we attempted to identify medication rules, effective materials, and the molecular mechanisms underlying the therapeutic effect of TCM against H. pylori using data mining, in vivo experiment, and network pharmacology.
Coptis, Pinellia, and Scutellaria (CPS) were identified as the core drug combination for H. pylori eradication. We evidenced that CPS eradicated H. pylori, alleviated the gastric pathology, and reduced circulating tumor necrosis factor-α and interleukin-1β levels. Nine key compounds and nine hub target proteins were further determined as the key active ingredients and therapeutic targets of CPS against H. pylori, respectively.
Altogether, our data strongly suggest that the efficacy of CPS in the treatment of H. pylori is worthy of affirmation.
We will further explore the mechanism of CPS in treating H. pylori infection through in vitro and in vivo experiments based on the close association between an H. pylori-induced persistent chronic inflammatory state and gastric cancer progression and extra-gastric diseases.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gupta L, Indonesia; Krzyzek P, Poland S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Zhao S
| 1. | Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N, Malfertheiner P; faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1322] [Cited by in RCA: 1185] [Article Influence: 118.5] [Reference Citation Analysis (0)] |
| 2. | Song X, He Y, Liu M, Yang Y, Yuan Y, Yan J, Zhang M, Huang J, Zhang S, Mo F. Mechanism underlying Polygonum capitatum effect on Helicobacter pylori-associated gastritis based on network pharmacology. Bioorg Chem. 2021;114:105044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Sokolova O, Naumann M. Matrix Metalloproteinases in Helicobacter pylori-Associated Gastritis and Gastric Cancer. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Toh JWT, Wilson RB. Pathways of Gastric Carcinogenesis, Helicobacter pylori Virulence and Interactions with Antioxidant Systems, Vitamin C and Phytochemicals. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 5. | Farzi N, Yadegar A, Aghdaei HA, Yamaoka Y, Zali MR. Genetic diversity and functional analysis of oipA gene in association with other virulence factors among Helicobacter pylori isolates from Iranian patients with different gastric diseases. Infect Genet Evol. 2018;60:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8:e180-e190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1318] [Article Influence: 263.6] [Reference Citation Analysis (0)] |
| 7. | Kumar S, Metz DC, Ellenberg S, Kaplan DE, Goldberg DS. Risk Factors and Incidence of Gastric Cancer After Detection of Helicobacter pylori Infection: A Large Cohort Study. Gastroenterology. 2020;158:527-536.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 191] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 8. | Yadegar A, Mohabati Mobarez A, Zali MR. Genetic diversity and amino acid sequence polymorphism in Helicobacter pylori CagL hypervariable motif and its association with virulence markers and gastroduodenal diseases. Cancer Med. 2019;8:1619-1632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Yang H, Hu B. Immunological Perspective: Helicobacter pylori Infection and Gastritis. Mediators Inflamm. 2022;2022:2944156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 10. | Yang H, Zhou X, Hu B. The 'reversibility' of chronic atrophic gastritis after the eradication of Helicobacter pylori. Postgrad Med. 2022;134:474-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Marcus EA, Sachs G, Scott DR. Eradication of Helicobacter pylori Infection. Curr Gastroenterol Rep. 2016;18:33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Chen BR, Li WM, Li TL, Chan YL, Wu CJ. Fucoidan from Sargassum hemiphyllum inhibits infection and inflammation of Helicobacter pylori. Sci Rep. 2022;12:429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | Hussein RA, Al-Ouqaili MTS, Majeed YH. Detection of clarithromycin resistance and 23SrRNA point mutations in clinical isolates of Helicobacter pylori isolates: Phenotypic and molecular methods. Saudi J Biol Sci. 2022;29:513-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Liu Y, Wang S, Yang F, Chi W, Ding L, Liu T, Zhu F, Ji D, Zhou J, Fang Y, Zhang J, Xiang P, Zhang Y, Zhao H. Antimicrobial resistance patterns and genetic elements associated with the antibiotic resistance of Helicobacter pylori strains from Shanghai. Gut Pathog. 2022;14:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 736] [Article Influence: 49.1] [Reference Citation Analysis (1)] |
| 16. | Yen CH, Chiu HF, Huang SY, Lu YY, Han YC, Shen YC, Venkatakrishnan K, Wang CK. Beneficial effect of Burdock complex on asymptomatic Helicobacter pylori-infected subjects: A randomized, double-blind placebo-controlled clinical trial. Helicobacter. 2018;23:e12469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Huang YQ, Huang GR, Wu MH, Tang HY, Huang ZS, Zhou XH, Yu WQ, Su JW, Mo XQ, Chen BP, Zhao LJ, Huang XF, Wei HY, Wei LD. Inhibitory effects of emodin, baicalin, schizandrin and berberine on hefA gene: treatment of Helicobacter pylori-induced multidrug resistance. World J Gastroenterol. 2015;21:4225-4231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 18. | Zhang BL, Fan CQ, Dong L, Wang FD, Yue JM. Structural modification of a specific antimicrobial lead against Helicobacter pylori discovered from traditional Chinese medicine and a structure-activity relationship study. Eur J Med Chem. 2010;45:5258-5264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | National Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China, China Medical Science and Technology Press, 2015 edition, 2010. |
| 20. | Ren SZ, Guo JS, Zeng GR, Wu CR. Preventive and therapeutic effects of Anwei pills on the H. pylori infected gastritisin the Balb/c mice model. Zhongguo Yiyuan Yaoxuezazhi. 2012;32:117-120. [DOI] [Full Text] |
| 21. | Ru J, Li P, Wang J, Zhou W, Li B, Huang C, Guo Z, Tao W, Yang Y, Xu X, Li Y, Wang Y, Yang L. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1623] [Cited by in RCA: 3148] [Article Influence: 286.2] [Reference Citation Analysis (0)] |
| 22. | Law JN, Akers K, Tasnina N, Santina CMD, Deutsch S, Kshirsagar M, Klein-Seetharaman J, Crovella M, Rajagopalan P, Kasif S, Murali TM. Interpretable network propagation with application to expanding the repertoire of human proteins that interact with SARS-CoV-2. Gigascience. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Otasek D, Morris JH, Bouças J, Pico AR, Demchak B. Cytoscape Automation: empowering workflow-based network analysis. Genome Biol. 2019;20:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 938] [Cited by in RCA: 1032] [Article Influence: 172.0] [Reference Citation Analysis (0)] |
| 24. | Shi Y, Wang P, Guo Y, Liang X, Li Y, Ding S. Helicobacter pylori-Induced DNA Damage Is a Potential Driver for Human Gastric Cancer AGS Cells. DNA Cell Biol. 2019;38:272-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Li Y, Li X, Tan Z. An overview of traditional Chinese medicine therapy for Helicobacter pylori-related gastritis. Helicobacter. 2021;26:e12799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 26. | Ning WH. Therapeutic effect of traditional Chinese medicine on gastropathy caused by Helicobacter pylori infection. Gen Stomatol. 2019;6:14-24. [DOI] [Full Text] |
| 27. | Zhang Y, Liu Y, Li Y, Zhao X, Zhuo L, Zhou A, Zhang L, Su Z, Chen C, Du S, Liu D, Ding X. Hierarchical and Complex System Entropy Clustering Analysis Based Validation for Traditional Chinese Medicine Syndrome Patterns of Chronic Atrophic Gastritis. J Altern Complement Med. 2019;25:1130-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Xu XH, Sun YN, Yang Q, Zhou SH, Wan Y, Gao QH, Lv WL. Study on Mechanism Transformation of Spleen and Stomach Dampness and Heat Syndrome in Chronic Gastritis Based on Metabonomics Technique. Shijie Kexuejishu-Zhongyiyao Xiandaihua. 2021;23:2607-2615. [DOI] [Full Text] |
| 29. | Li RJ, Dai YY, Qin C, Huang GR, Qin YC, Huang YY, Huang ZS, Luo XK, Huang YQ. Application of traditional Chinese medicine in treatment of Helicobacter pylori infection. World J Clin Cases. 2021;9:10781-10791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (3)] |
| 30. | Zhang BH, Tang XD, Wang FY, Li ZH, Li BS. Research Progress on Anti-Helicobacter Pylori Mechanism of Chinese Herbs. Zhonghua Zhongyiyaoxue Zazhi. 2015;33:555-557. [DOI] [Full Text] |
| 31. | Ma F, Chen Y, Li J, Qing HP, Wang JD, Zhang YL, Long BG, Bai Y. Screening test for anti-Helicobacter pylori activity of traditional Chinese herbal medicines. World J Gastroenterol. 2010;16:5629-5634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 32. | Han M, Clery A, Liu JP, Li XM, Zhang J, Dong C, Feng S, Xia Y. Banxia Xiexin Decoction for patients with peptic ulcer or chronic gastritis infected with Helicobacter pylori. Zhongyi Kexue Zazhi. 2019;6:122-130. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Chen J, Wang F, Liu J, Lee FS, Wang X, Yang H. Analysis of alkaloids in Coptis chinensis Franch by accelerated solvent extraction combined with ultra performance liquid chromatographic analysis with photodiode array and tandem mass spectrometry detections. Anal Chim Acta. 2008;613:184-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 34. | Tan L, Li C, Chen H, Mo Z, Zhou J, Liu Y, Ma Z, Xu Y, Yang X, Xie J, Su Z. Epiberberine, a natural protoberberine alkaloid, inhibits urease of Helicobacter pylori and jack bean: Susceptibility and mechanism. Eur J Pharm Sci. 2017;110:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 35. | Li C, Huang P, Wong K, Xu Y, Tan L, Chen H, Lu Q, Luo C, Tam C, Zhu L, Su Z, Xie J. Coptisine-induced inhibition of Helicobacter pylori: elucidation of specific mechanisms by probing urease active site and its maturation process. J Enzyme Inhib Med Chem. 2018;33:1362-1375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 36. | Zhou JT, Li CL, Tan LH, Xu YF, Liu YH, Mo ZZ, Dou YX, Su R, Su ZR, Huang P, Xie JH. Inhibition of Helicobacter pylori and Its Associated Urease by Palmatine: Investigation on the Potential Mechanism. PLoS One. 2017;12:e0168944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 37. | Jung J, Choi JS, Jeong CS. Inhibitory Activities of Palmatine from Coptis chinensis Against Helicobactor pylori and Gastric Damage. Toxicol Res. 2014;30:45-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Shen YJ. People’s Health Press. Zhonghua Zhongyi Yaolixue Zazhi. 2000;200-208. |
| 39. | Huynh DL, Ngau TH, Nguyen NH, Tran GB, Nguyen CT. Potential therapeutic and pharmacological effects of Wogonin: an updated review. Mol Biol Rep. 2020;47:9779-9789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 40. | Lai MY, Hsiu SL, Tsai SY, Hou YC, Chao PD. Comparison of metabolic pharmacokinetics of baicalin and baicalein in rats. J Pharm Pharmacol. 2003;55:205-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 157] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 41. | Dinda B, Dinda S, DasSharma S, Banik R, Chakraborty A, Dinda M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur J Med Chem. 2017;131:68-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 380] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 42. | Chen ME, Su CH, Yang JS, Lu CC, Hou YC, Wu JB, Hsu YM. Baicalin, Baicalein, and Lactobacillus Rhamnosus JB3 Alleviated Helicobacter pylori Infections in Vitro and in Vivo. J Food Sci. 2018;83:3118-3125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 43. | Ji X, Huang B, Wang G, Zhang C. The ethnobotanical, phytochemical and pharmacological profile of the genus Pinellia. Fitoterapia. 2014;93:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 44. | Chen JH, Cui GY, Liu JY, Tan RX. Pinelloside, an antimicrobial cerebroside from Pinellia ternata. Phytochemistry. 2003;64:903-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 45. | Zuo F, Zhou ZM, Zhang Q, Mao D, Xiong YL, Wang YL, Yan MZ, Liu ML. Pharmacokinetic study on the multi-constituents of Huangqin-Tang decoction in rats. Biol Pharm Bull. 2003;26:911-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Li S, Zhang B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chin J Nat Med. 2013;11:110-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 627] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 47. | Pachathundikandi SK, Tegtmeyer N, Backert S. Signal transduction of Helicobacter pylori during interaction with host cell protein receptors of epithelial and immune cells. Gut Microbes. 2013;4:454-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 48. | Tran CT, Garcia M, Garnier M, Burucoa C, Bodet C. Inflammatory signaling pathways induced by Helicobacter pylori in primary human gastric epithelial cells. Innate Immun. 2017;23:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Pan G, Wang X, Wang Y, Li R, Li G, He Y, Liu S, Luo Y, Wang L, Lei Z. Helicobacter pylori promotes gastric cancer progression by upregulating semaphorin 5A expression via ERK/MMP9 signaling. Mol Ther Oncolytics. 2021;22:256-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 50. | Fattahi S, Amjadi-Moheb F, Tabaripour R, Ashrafi GH, Akhavan-Niaki H. PI3K/AKT/mTOR signaling in gastric cancer: Epigenetics and beyond. Life Sci. 2020;262:118513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 248] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 51. | Ohtani M, Ge Z, García A, Rogers AB, Muthupalani S, Taylor NS, Xu S, Watanabe K, Feng Y, Marini RP, Whary MT, Wang TC, Fox JG. 17 β-estradiol suppresses Helicobacter pylori-induced gastric pathology in male hypergastrinemic INS-GAS mice. Carcinogenesis. 2011;32:1244-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 52. | Hosoda K, Shimomura H, Hayashi S, Yokota K, Hirai Y. Steroid hormones as bactericidal agents to Helicobacter pylori. FEMS Microbiol Lett. 2011;318:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Fong P, Wang QT. Protective effect of oral contraceptive against Helicobacter pylori infection in US adult females: NHANES 1999-2000. Epidemiol Infect. 2021;149:e120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 54. | Kang MH, Eyun SI, Park YY. Estrogen-related receptor-gamma influences Helicobacter pylori infection by regulating TFF1 in gastric cancer. Biochem Biophys Res Commun. 2021;563:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 55. | Gravina AG, Zagari RM, De Musis C, Romano L, Loguercio C, Romano M. Helicobacter pylori and extragastric diseases: A review. World J Gastroenterol. 2018;24:3204-3221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 244] [Cited by in RCA: 196] [Article Influence: 28.0] [Reference Citation Analysis (6)] |
| 56. | Goni E, Franceschi F. Helicobacter pylori and extragastric diseases. Helicobacter. 2016;21 Suppl 1:45-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |