Published online Nov 26, 2022. doi: 10.12998/wjcc.v10.i33.12440
Peer-review started: September 13, 2022
First decision: September 26, 2022
Revised: October 13, 2022
Accepted: October 20, 2022
Article in press: October 20, 2022
Published online: November 26, 2022
Processing time: 70 Days and 12.9 Hours
Dyskeratosis congenita is a rare disease characterized by bone marrow failure and a clinical triad of oral leukoplakia, nail dystrophy, and abnormal skin pigmen
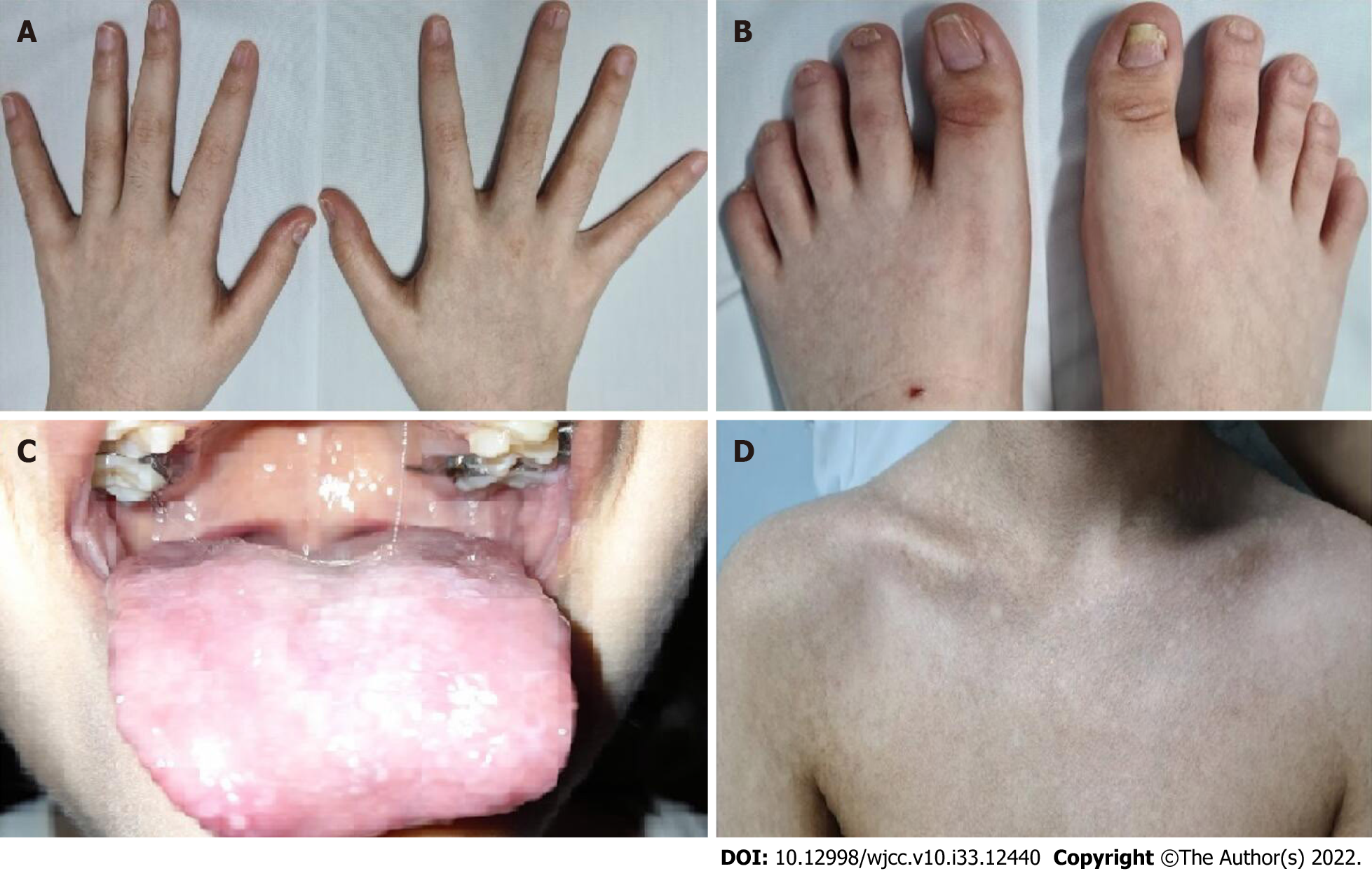
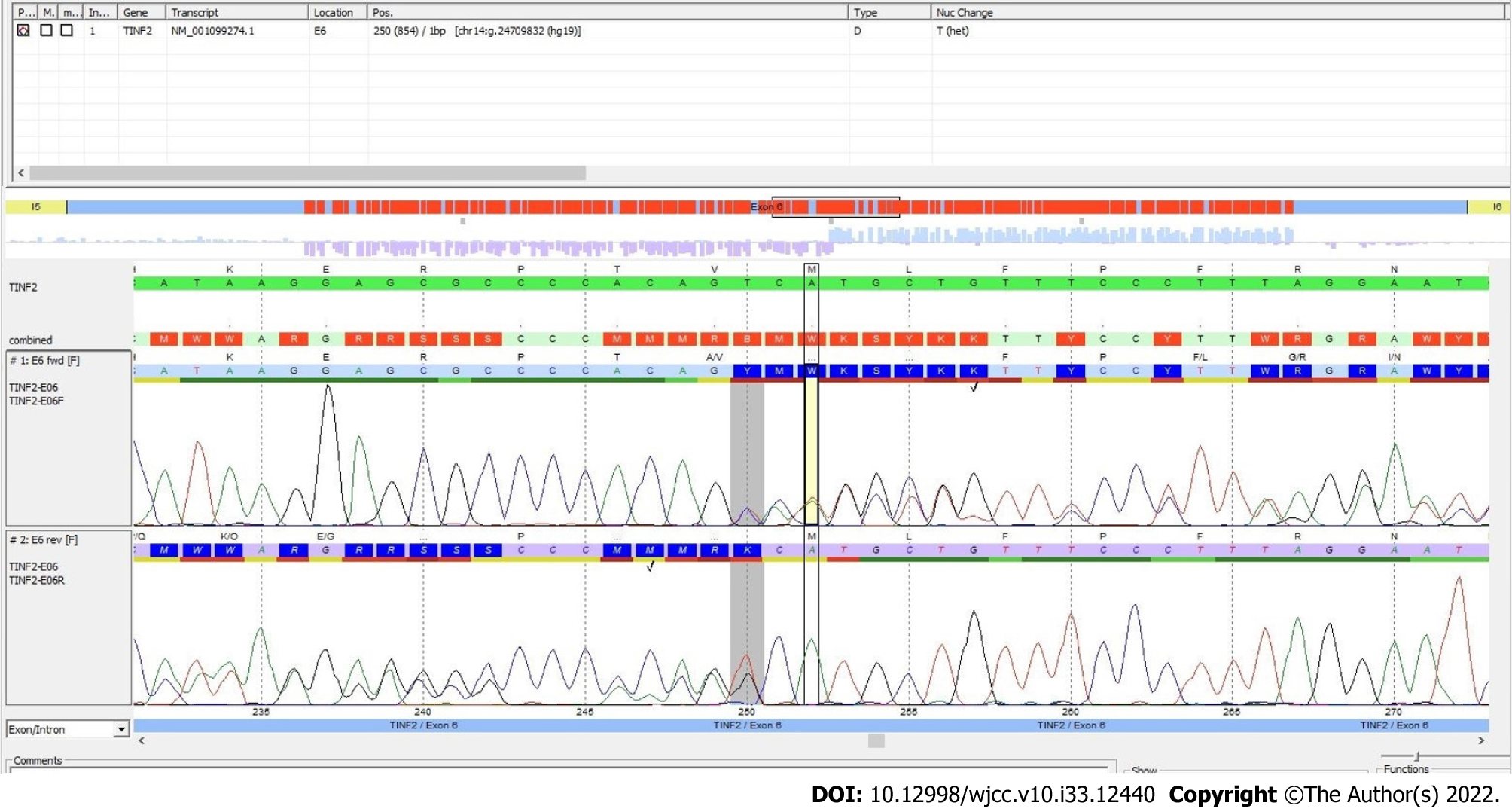
Here, we report a female patient who presented thrombocytopenia, anemia, reticulate hyperpigmentation, dystrophy in fingernails and toenails, and leukoplakia on the tongue. A histopathological study of the skin showed dyskeratocytes; however, a bone marrow biopsy revealed normal cell morphology. The patient was diagnosed with dyskeratosis congenita, but her family history did not reveal significant antecedents. Whole-exome sequencing showed a novel heterozygous punctual mutation in exon 6 from the TINF2 gene, namely, NM_001099274.1:
The disease in this patient was caused by a germline novel mutation of TINF2 in one of her parents.
Core Tip: Dyskeratosis congenita, characterized by a clinical triad of oral leukoplakia, nail dystrophy, and abnormal skin pigmentation, is a rare disease caused by mutations in genes governing telomere maintenance, including TINF2. We performed whole-exome sequencing in a female pediatric patient who presented with dyskeratosis congenita, and subsequently, a novel heterozygous mutation in exon 6 of the TINF2 gene was detected: NM_001099274.1:c.854delp.(Val285Alafs*32). An analysis of telomere length demonstrated short telomeres relative to the girl’s age. Patients with TINF2 mutations have more severe disease, so their detection is necessary to provide timely treatment.
- Citation: Picos-Cárdenas VJ, Beltrán-Ontiveros SA, Cruz-Ramos JA, Contreras-Gutiérrez JA, Arámbula-Meraz E, Angulo-Rojo C, Guadrón-Llanos AM, Leal-León EA, Cedano-Prieto DM, Meza-Espinoza JP. Novel TINF2 gene mutation in dyskeratosis congenita with extremely short telomeres: A case report. World J Clin Cases 2022; 10(33): 12440-12446
- URL: https://www.wjgnet.com/2307-8960/full/v10/i33/12440.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i33.12440
Dyskeratosis congenita is a rare genetic disease whose prevalence in the general population has been estimated at nearly 1/1000000[1]. This disorder is characterized by bone marrow failure and a typical clinical triad comprised of oral leukoplakia, nail dystrophy, and abnormal skin pigmentation. The risk of aplastic anemia, myelodysplastic syndrome, and leukemia is elevated in patients with this condition[2,3]. Other clinical findings in dyskeratosis congenita include pulmonary fibrosis, liver cirrhosis, and premature hair graying[4]. The genetics of dyskeratosis congenita involve mutations in genes that govern the maintenance of telomeres; therefore, this disorder is marked, molecularly, by a progressive shortening of telomeres[5,6]. The genes associated with dyskeratosis congenita are DKC1, TERC, TERT, TINF2, RTEL1, PARN, ACD, NOP10, NHP2, TERT, USB1, and WRAP53[2,3,7] (Table 1). Pathogenic variants in any of these genes have been identified in most individuals who meet diagnostic criteria for dyskeratosis congenita[3]. Because of this locus heterogeneity, there is a wide clinical variation among patients with this syndrome[8]. Here, we report the case of a girl with dyskeratosis congenita who carries a previously undescribed germline mutation in the TINF2 gene and an extremely short telomere length.
| Gene | Chromosome | Inheritence pattern | Frequency, % | Main mutation types |
| DKC1 | Xq28 | XLR | Approximately 25 | Missense |
| TINF2 | 14q12 | AD | Approximately 12 | Missense |
| TERC | 3q26 | AD | Approximately 5 | Point and deletions |
| TERT | 5p15 | AD, AR | Approximately 5 | Missense |
| USB1 | 16q21 | AR | Approximately 2 | Frameshift and nonsense |
| RTEL1 | 20q13 | AR, AD | Approximately 2 | Missense |
| CTC1 | 17p13 | AR | Approximately 1 | Missense and frameshift |
| NHP2 | 5q35 | AR | < 1 | Missense |
| NOP10 | 15q14 | AR | < 1 | Missense |
| WRAP53 | 17p13 | AR | < 1 | Missense |
| ACD | 16q22 | AD, AR | < 1 | Missense and frameshift |
| PARN | 16p13 | AR, AD | < 1 | Frameshift |
A 13-year-old Mexican female patient, height 151.0 cm and weight 48.0 kg, was found to have thrombocytopenia, anemia, abnormal skin pigmentation, dystrophic nails, and leukoplakia on the tongue.
The patient was the product of the second pregnancy of healthy nonconsanguineous parents (both parents were 31 years old at the time of birth). At the age of 5 years, she was detected to have thrombocytopenia and anemia (platelets 26000/mm3 and hemoglobin 9.0 g/dL); peripheral blood cell smears showed normal morphology and no evidence of blasts. She was thought to have primary immune thrombocytopenia. Prednisone (1 mg/kg/d) was administered as therapy for 21 d. After the administration of prednisone, platelet and hemoglobin counts have fluctuated from 32000/mm3 to 110000/mm3 and 9.5 g/dL to 11.7 g/dL, respectively.
When the patient was 9 years old, she was suspected to have systemic lupus erythematosus and mixed connective tissue disease due to her nail dystrophy and neck pigmentation abnormalities, but an antinuclear antibody test was negative. Methotrexate was administered as prophylaxis regardless. Around this time, an esophagogram was performed due to her difficulty swallowing since childhood; this revealed esophageal stenosis requiring two endoscopies for dilation. At the age of 11, dyskeratosis congenita was suspected due to the progression of reticulate pigmentation to the entire upper trunk, the presence of leucoplakia on the tongue, and the evolution of fingernails and toenails dystrophy; a molecular study was subsequently performed.
The patient had no history of other significant diseases.
The patient had a healthy older brother, and family history did not indicate any significant morbidities.
At the age of 6, the patient presented with microcephaly, reticulate pigmentation in the neck, neckline, and axillae, dystrophic nails on hands and feet (Figure 1A and B), and lacrimal obstruction in the right eye. At the age of 11, she showed leukoplakia on the tongue (Figure 1C), and her reticulate pigmen
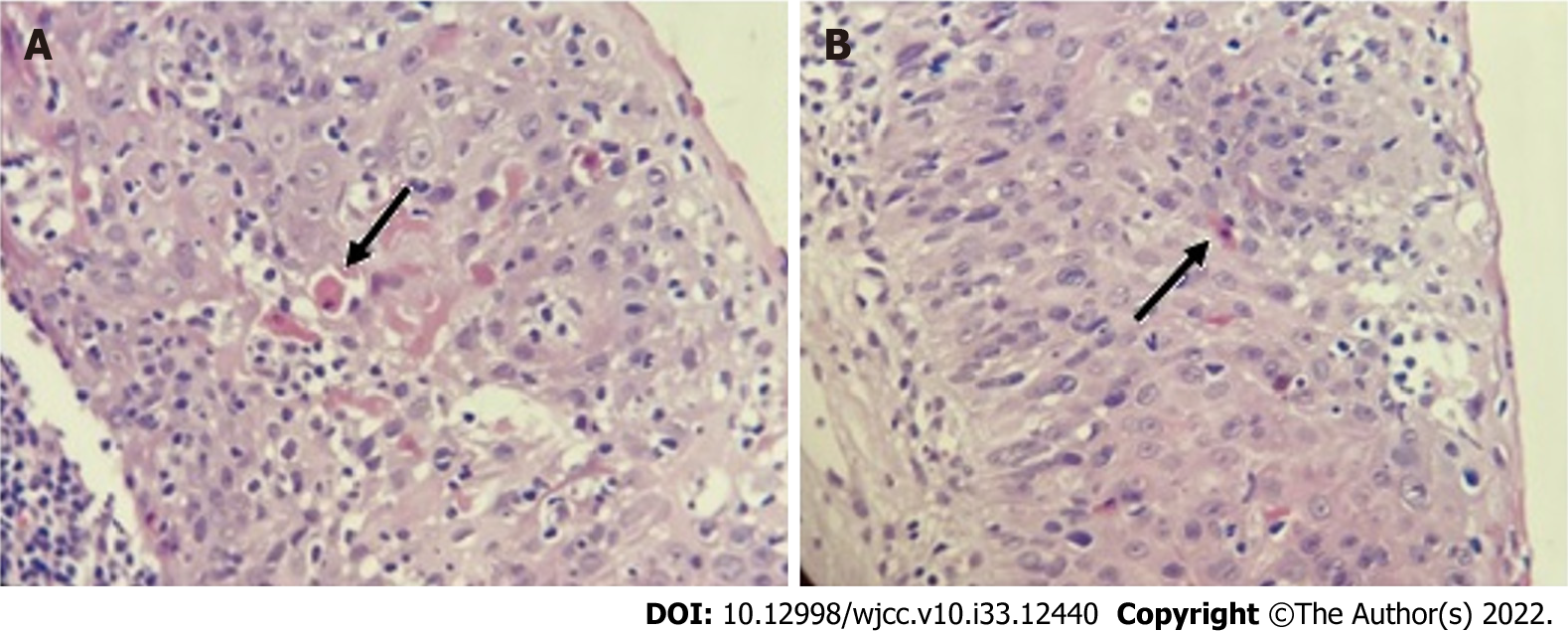
A histopathological study of the skin showed dyskeratocytes (Figure 2). Although previous peripheral blood smears showed normal leukocyte counts and the absence of blasts throughout the disease, a bone marrow biopsy was performed, which revealed normal cellularity.
Whole-exome sequencing analysis was performed according to the manufacturer’s protocol. DNA was enzymatically fragmented and hybridized with CentoXomeTM (CENTOGENE, Rostock, Germany). Libraries were generated with Illumina-compatible adapters and sequenced on the Illumina platform (Illumina, Inc., San Diego, CA, United States). Sequenced readings were aligned to the hg19 version of the human genome (Genome Reference Consortium GRCh37) using validated software (Rostock, Germany). All variants reported in the Human Gene Mutation Database (HGMD®), ClinVar, and CentoMD, as well as those in which the frequency of the least common allele was less than 1% in the Single Nucleotide Polymorphism database (dbSNP) and the Genome Aggregation Database (gnomAD) were considered. After analysis, a novel mutation, Chr14(GRCh37):g.24709832del NM_0010992
To assess telomere length, genomic DNA was extracted from peripheral blood leukocytes using the Flexigene DNA kit (QIAGEN, Hilden, Germany) and qPCR was performed using the Absolute Human Telomere Length and Mitochondrial DNA Copy Number Dual Quantification qPCR kit (ScienCell Research Laboratories, San Diego, CA, United States) on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA, United States) according to the supplier’s recommendations. The analysis showed an absolute telomere length of 2.80 ± 0.09 kb.
Based on clinical features and molecular analysis, the patient was diagnosed with dyskeratosis congenita.
Prednisone (1 mg/kg/d) is administered as therapy for 21 d, whenever platelet count drops by nearly 30000/mm3.
Hopefully, the patient has had a favorable evolution, as she has not developed aplastic anemia or bone marrow failure, although she is currently off medication.
Dyskeratosis congenita is caused more than 99% of the time by a germline mutation in one of the parents[9], as was the case of our patient, who presented with the typical triad of this disease: Dystrophy in fingernails and toenails, leukokeratosis plaques on the tongue, and reticulate skin pigmentation. Mutations in the TINF2 gene (which encodes the TIN2 protein, a component of the shelterin telomere protection complex)[2] represent the second most common cause of dyskeratosis congenita, accounting for approximately 12% of the cases, only after mutations in the DKC1 gene (approximately 25%)[1]. It is well known that patients with a mutation in the TINF2 gene have a more severe course and a higher risk of developing aplastic anemia before the age of 10 years[6]. To date, there are more than 200 punctual variations in the TINF2 gene recorded in the ClinVar database. Most of them result in missense mutations, eight in nonsense, and almost twenty are frameshift mutations (Table 2), although some are associated with Revesz syndrome, a more severe variant of dyskeratosis congenita. Most pathogenic mutations occur in exon 6, principally between codons 280 and 288[10]. The mutation detected in this patient was a deletion of a nucleotide (frameshift), which also occurred in exon 6, codon 285, and caused an amino acid change at position 285 and a stop codon 31 amino acids later.
| Location (GRCh37) | Mutation | Protein change |
| Chr14:24709067 | NM_001099274.3(TINF2):c.1292del (p.Pro431fs) | P431fs |
| Chr14:24709132 | NM_001099274.3(TINF2):c.1227del (p.Leu410fs) | L410fs |
| Chr14:24709288-24709289 | NM_001099274.3(TINF2):c.1202dup (p.Asn401fs) | N401fs |
| Chr14:24709507-24709508 | NM_001099274.3(TINF2):c.1090dup (p.Leu364fs) | L364fs |
| Chr14:24709627-24709628 | NM_001099274.3(TINF2):c.1058dup (p.Glu354fs) | E354fs |
| Chr14:24709676 | NM_001099274.3(TINF2):c.1010del (p.Gly337fs) | G337fs |
| Chr14:24709794 | NM_001099274.3(TINF2):c.892del (p.Gln298fs) | Q298fs |
| Chr14:24709836-24709837 | NM_001099274.3(TINF2):c.849dup (p.Thr284fs) | T284fs |
| Chr14:24709860 | NM_001099274.3(TINF2):c.826del (p.Arg276fs) | R276fs |
| Chr14:24710080 | NM_001099274.3(TINF2):c.606del (p.Glu202fs) | E202fs |
| Chr14:24710937-24710938 | NM_001099274.3(TINF2):c.342_343del (p.Phe114fs) | F114fs |
| Chr14:24711135-24711136 | NM_001099274.3(TINF2):c.257_258del (p.His86fs) | H86fs |
| Chr14:24711394-24711395 | NM_001099274.3(TINF2):c.144_145insTT (p.Val49fs) | V49fs |
While telomere shortening is a molecular feature of dyskeratosis congenita[5], this is dramatic in patients with TINF2 mutations[11], as their telomere lengths are significantly shorter than those of patients with DKC1 mutations[6]. Our case showed an absolute telomere length of 2.80 ± 0.09 kb, which is considered very short relative to the patient's age. As a reference, a study performed on healthy young women aged 18 to 30 years showed an absolute telomere length of 4.59 ± 0.24 kb[12]. TIN2 is important for telomere protection, and TIN2 deficiency increases the risk of telomeric DNA damage and consequent telomere shortening[13-15]. Short telomeres are known to cause premature aging and increase the risk of developing cancer[16]. Accordingly, patients with dyskeratosis congenita have a higher risk of developing bone marrow failure, acute leukemia, myelodysplastic syndrome, and squamous cell carcinoma of the head and neck[3]. However, despite her extremely short telomere length, our patient has had a favorable evolution, as she has not developed aplastic anemia or bone marrow failure, although she is currently off medication.
Since patients with mutations in the TINF2 gene have a more severe course and a higher risk of developing aplastic anemia[6], it is important to detect patients with such mutations to follow them more frequently, mainly through blood cytometry, and, if necessary, to provide some treatment, as has been done in this patient. In the meantime, she and her parents are hoping for an orphan or experimental drug that will impede the progression of the disease.
The strength of this case report is that it was approached with a clinical, genetic, and pathological focus. The main limitation is that it is a single case.
We thank the proband and her parents for their collaboration and support for the publication of this case. We also thank the biomedicine and genomic biotechnology students, Liliana Itzel Patrón Baro (fellowship PROFAPI-UAS-2022, Pro_A3_031) and Lucero García Hernández, respectively, for the technical and samples collection support. Special thanks to Centogene AG, Germany for its support for the whole-exome sequencing analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dermatology
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ishida T, Japan; Vyshka G, Albania S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Dokal I, Vulliamy T, Mason P, Bessler M. Clinical utility gene card for: Dyskeratosis congenita - update 2015. Eur J Hum Genet. 2015;23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 2. | Savage SA, Giri N, Baerlocher GM, Orr N, Lansdorp PM, Alter BP. TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. Am J Hum Genet. 2008;82:501-509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 308] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 3. | Savage SA, Niewisch MR. Dyskeratosis Congenita and Related Telomere Biology Disorders. 2009 Nov 12. In: GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993. [PubMed] |
| 4. | Wang P, Xu Z. Pulmonary fibrosis in dyskeratosis congenita: a case report with a PRISMA-compliant systematic review. BMC Pulm Med. 2021;21:279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Nelson ND, Bertuch AA. Dyskeratosis congenita as a disorder of telomere maintenance. Mutat Res. 2012;730:43-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Walne AJ, Vulliamy T, Beswick R, Kirwan M, Dokal I. TINF2 mutations result in very short telomeres: analysis of a large cohort of patients with dyskeratosis congenita and related bone marrow failure syndromes. Blood. 2008;112:3594-3600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 232] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 7. | Dyskeratosis congenita, autosomal dominant 1 in: Online Mendelian Inheritance in Man. Available from: https://omim.org/entry/127550. |
| 8. | Barbaro PM, Ziegler DS, Reddel RR. The wide-ranging clinical implications of the short telomere syndromes. Intern Med J. 2016;46:393-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | AlSabbagh MM. Dyskeratosis congenita: a literature review. J Dtsch Dermatol Ges. 2020;18:943-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | TINF2 gene in: ClinVar. Available from: https://www.ncbi.nlm.nih.gov/clinvar/?term=TINF2%5Bgene%5D&redir=gene). |
| 11. | Frescas D, de Lange T. A TIN2 dyskeratosis congenita mutation causes telomerase-independent telomere shortening in mice. Genes Dev. 2014;28:153-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Hagman M, Fristrup B, Michelin R, Krustrup P, Asghar M. Football and team handball training postpone cellular aging in women. Sci Rep. 2021;11:11733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Kim SH, Beausejour C, Davalos AR, Kaminker P, Heo SJ, Campisi J. TIN2 mediates functions of TRF2 at human telomeres. J Biol Chem. 2004;279:43799-43804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Lansdorp PM. Telomeres, aging, and cancer: the big picture. Blood. 2022;139:813-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 15. | Sasa GS, Ribes-Zamora A, Nelson ND, Bertuch AA. Three novel truncating TINF2 mutations causing severe dyskeratosis congenita in early childhood. Clin Genet. 2012;81:470-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Takai KK, Kibe T, Donigian JR, Frescas D, de Lange T. Telomere protection by TPP1/POT1 requires tethering to TIN2. Mol Cell. 2011;44:647-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |